Abstract
Objectives:
The role of maximum standard uptake value (SUVmax) at baseline and after induction chemotherapy (CT) on positron emission tomography (PET) as an imaging biomarker has not been well established in oesophageal squamous cell carcinoma (SCC). In this retrospective analysis, we investigated the prognostic significance of various PET metrics in oesophageal SCC patients treated with induction chemotherapy followed by concurrent chemoradiotherapy (CRT).
Methods:
A total of 57 patients were treated with CRT; 52 patients received induction chemotherapy and 10 patients underwent surgery following CRT. Scans were independently analysed by a nuclear medicine physician blinded to patient outcome. Using region of interest analysis, SUVmax and metabolic tumour volume (MTV) were calculated for the index lesion and lymph node metastases in each patient. Kaplan–Meier analysis was used to evaluate overall survival (OS), disease-free survival (DFS), local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS). Cox proportional hazards regression was used to assess correlation between outcomes and PET metrics.
Results:
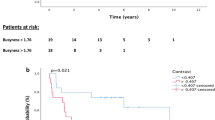
Median follow-up for those who are alive was 4.4 years, with a median survival for all patients of 2.9 years. The 3-year OS, DFS, DMFS and LRFS rates were 47, 40, 44 and 36%, respectively. Using a pre-established cutoff of a 35% decrease in SUVmax from baseline to post-induction PET, 3-year OS for responders (⩾35% decrease from baseline) was 64%, whereas non-responders (<35% decrease from baseline) had a 3-year OS of 15% (P=0.004).
Conclusions:
The pre-specified 35% decrease in SUVmax after induction chemotherapy was prognostic for OS. Baseline and post-induction PET metrics provide prognostic information for oesophageal SCC.
Similar content being viewed by others
Main
Squamous cell carcinomas (SCCs) represent a minority of new oesophageal cancers in the United States, but remain the dominant histologic subtype of oesophageal cancer worldwide and a significant cause of mortality (Zhang, 2013). Various combinations of chemotherapy, radiotherapy and surgery have been evaluated in the management of this disease. Preoperative chemotherapy or chemoradiotherapy (CRT) followed by surgical resection and definitive chemoradiation are all standard options for this disease (van Hagen et al, 2012). However, despite aggressive therapeutic approaches, outcomes remain poor. Better risk stratification using prognostic and predictive markers would allow for tailoring therapeutics to the biology of the individual tumour. One such approach is the use of 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) imaging to measure the metabolic activity in tumours, which represents a combination of proliferative activity and number of viable cells. The standard uptake value (SUV) is a semi-quantitative measure of 18FDG uptake and metabolic activity, and has been shown to be predictive of survival in many cancer types.
FDG-PET imaging is a routine component of initial workup for oesophageal cancer and is of proven value in staging, response assessment and detection of recurrence (Kato et al, 2002; Barber et al, 2012). Moreover, FDG-PET has substantial promise as a tool to assess response to therapy and as an imaging biomarker to help guide therapy in oesophageal cancer. Because PET imaging can be obtained at various time points in the treatment process, serial scans may provide additional prognostic information based on metabolic response. Baseline pretreatment maximum SUV (SUVmax) levels have been correlated with survival among patients treated with definitive chemoradiation (Suzuki et al, 2011) or surgical resection (van Westreenen et al, 2005; Blackstock et al, 2006; Omloo et al, 2008; Kato et al, 2009).
Post-CRT FDG-PET imaging has been evaluated in several studies, however, the prognostic utility of SUVmax after CRT remains controversial due to difficulties in distinguishing post-radiotherapy inflammation from residual viable tumour(Hong et al, 2005; Konski et al, 2007; Rizk et al, 2009; Brown et al, 2012).
Another approach has been to evaluate change in SUVmax from baseline to post treatment. In several studies of patients with oesophageal adenocarcinoma in Germany, where the typical treatment consisted of neoadjuvant chemotherapy, followed by surgical resection, a decline in SUV after one cycle of chemotherapy was found to be a prognostic marker. Patients who were found to be metabolic responders, based on a >35% decline in SUVmax after one cycle of chemotherapy, experienced better progression-free survival (PFS) and overall survival (OS) than metabolic non-responders (Weber et al, 2001). These data have led to further evaluation of the approach of early PET imaging assessment after induction chemotherapy and determination of the efficacy of the regimen based on metabolic response in a multicentre, cooperative group study (Cancer and Leukemia Group B trial 80803).
Nonetheless, because of differences in tumour biology and prognosis (Bollschweiler and Holscher, 2007), the data from oesophageal adenocarcinomas cannot be extrapolated to SCC. Thus, the aim of our study was to validate the 35% decline in SUVmax after chemotherapy as prognostic for outcome in oesophageal SCC patients treated with induction chemotherapy followed by CRT. In addition, we report outcomes of an aggressive approach of CRT in oesophageal SCC patients, the majority of whom also received induction chemotherapy, and investigated the use of SUVmax as well as a quantitative metabolic parameter, metabolic tumour volume (MTV), obtained at serial imaging sessions before, during and after treatment as potential imaging biomarkers for response assessment and prognostication.
Materials and Methods
Patient and treatment characteristics
After obtaining a waiver of authorisation from the Institutional Review Board, we identified 57 patients with oesophageal SCC treated with CRT with curative intent using intensity-modulated radiotherapy (IMRT) at our institution between 2007 and 2011. For staging (2002 AJCC classification), patients underwent endoscopic ultrasound and biopsy, diagnostic CT of chest and abdomen with intravenous contrast, and 18F-FDG-PET/CT.
Fifty-two patients received induction chemotherapy before CRT and 10 patients underwent surgery following CRT (see below). Chemotherapy regimens were recorded as induction or concurrent therapy and classified either as platinum/irinotecan regimen, platinum/taxane regimen, 5-fluorouracil-based regimen or other (Table 1). Five patients did not receive induction chemotherapy: four patients did not have baseline FDG-avid tumours; one patient had advanced age with multiple comorbidities, which was deemed ineligible for induction chemotherapy.
All patients underwent CT-based simulation on a Discovery ST PET/CT (GE Healthcare, Waukesha, WI, USA) for RT in the supine position, using intravenous and oral contrast to better delineate the target volume and were immobilised in an alpha cradle (except cervical oesophageal SCC patients who had a custom facemask). Four-dimensional CT scans were used to track tumour motion, and respiratory gating was used to deliver radiotherapy only during the expiratory phase of the respiratory cycle for patients with distal oesophageal and GE junction tumours. The clinical tumour volume (CTV) included the primary tumour, all involved lymph nodes on PET and CT imaging, peri-oesophageal nodes and mediastinal nodes ∼4–5 cm above and below the GTV. The coeliac nodes were included in the CTV for lower oesophageal tumours and supraclavicular nodes were included for tumours above the carina. Organs at risk included lungs, heart, spinal cord, liver and kidneys. Intensity-modulated radiotherapy was delivered with dynamic multi-leaf collimation on a Varian accelerator (Varian Medical Systems, Inc., Palo Alto, CA, USA). Median dose to the primary tumour was 5600 cGy (Table 1). A subset of 10 patients underwent Ivor Lewis oesophagectomy. Seven patients had surgery at a median of 2.65 months (range: 1.43–3.67 months) following CRT with curative intent. Another 3 patients had surgery following local recurrence at a median of 11.1 months (range: 5.13–17.5 months) after CRT.
PET imaging
Patients underwent PET/CT at three time points: (1) for pretreatment staging (n=51); (2) post-induction chemotherapy (n=41); and (3) after the end of chemoradiotherapy (n=40), using Siemens Biograph (Siemens/CPS Innovations, Knoxville, TN, USA) or Discovery LS, 690, ST or STE PET/CT scanners (GE Medical Systems). All the patients were not imaged on the same scanner but all were state-of-the-art equipment. Similar to our clinical scans, all patients fasted for 6 h before 18F-FDG injection (12 mCi). The standard uptake time was 60–80 min. For repeat scans, uptake times were within ∼10–15 min of baseline uptake times. Unenhanced CT was performed from the skull base to the upper thighs. The PET emission scans were obtained afterwards from the base of skull to the upper thighs. Images were reconstructed with and without attenuation correction. The average time from completion of IMRT to post-CRT PET was 74 days and Median of 42 days (range: 19–631). All PET/CT scans were analysed retrospectively on a Hermes workstation using software version 4.7-B (Hermes Medical Solutions AB, Stockholm, Sweden). The average liver SUV for baseline PET, post-induction PET and post-CRT PET were 2.0, 2.2 and 2.1, respectively. The SUVs were normalised to body weight ((kBq per ml activity in region)/(kBq injected activity per body mass in g)). The SUVmax is the maximum SUV in any voxel within the tumour (Wahl et al, 2009). The highest SUVmax value was in the primary tumour. We then calculated the MTV, defined as the volume of FDG-avid disease including all voxels that contain activity equal to or higher than 42% of the maximum tumour activity. There is no general agreement regarding what threshold to choose, different groups use different approaches. This value was chosen based on published data completed at our institution (Erdi et al, 1997). Table 2 summarises the PET metrics at the three different scans.
Follow-up
All patients were followed at regular intervals after completion of therapy. Follow-up generally consisted of a physical exam and laboratory testing every 3 months and imaging with CT of the chest and abdomen every 6 months for the first 2 years and then annually until 5 years after the completion of treatment. Oesophagogastroduodenoscopy (EGD) was performed every 6 months for 2 years and then annually. Patients treated with definitive CRT who did not undergo surgery usually underwent more frequent EGD to monitor for local recurrence. All patients were followed until date of death or last follow-up.
Statistical analysis
Patient characteristics presented by median and range for continuous variables, and by frequency for categorical variables. Summary statistics of PET parameters are presented by mean, s.d. and range. The OS, DFS, LRFS and DMFS rates were evaluated from the three different scan time points (baseline, post induction and post CRT) with results specifying from which scan a clinical outcome is being assessed. There was no significant delay from diagnosis to patients getting scanned. Only patients still alive at scan or having PET measurements were used in the analysis of the PET parameters.
Time to LRFS was calculated from the date of scan to the date of first event (LR or death) or last follow-up. Time to DMFS was calculated from the date of scan to the date of first event (DM or death) or until last follow-up. Overall survival was calculated from the date of scan to the date of death or last follow-up. Disease-free survival was calculated from the date of scan to the date of disease recurrence (LR, DM or death). For all four survival end points, patients alive without the event of interest were censored. Kaplan–Meier and Cox regression were used to evaluate the relationship between PET parameters and the four outcomes. Hazard ratio (HR) is equivalent to the odds of a specified clinical end point occurring with every one incremental increase in the PET parameter of interest. The percent changes in the PET parameters were calculated from baseline to post-induction chemotherapy, from baseline to post CRT and from post induction to post CRT. On the basis of the pre-established 35% SUVmax decrease from baseline to post-induction PET, we analysed this cutoff to define PET responders (⩾35%) and non-responders (<35%). All PET parameters were assessed as continuous variables.
Variables that were significant in the univariate analyses were evaluated in a multivariate model (Peduzzi et al, 1995). P-values <0.05 were considered significant. All analysis was done using R version 3.0.0 with survival package (cran.r-project.org).
To identify a potential threshold SUVmax at the various scanning time points that best separated DFS, a minimally selected log-rank test was used to find an optimal cut point for baseline and post-induction SUVmax. In this approach, selected values of the variable of interest were examined as candidates for the cut point. The value chosen was one that best separated patient outcomes according to a minimum P-value. The P-value was adjusted for multiple testing.
Results
Patient characteristics
A total of 57 patients with oesophageal SCC who underwent CRT at our institution were analysed, of whom 52 received induction chemotherapy before CRT. Of these 57 patients, 7 patients underwent surgical resection following CRT with curative intent. Patient and tumour characteristics are shown in Table 1. These patients had clinical stage T1N1 or T2-4N0-1 without evidence of metastatic disease.
Clinical outcomes
With a median follow-up of 23.6 months from end of radiotherapy, the median OS for the entire cohort of 57 patients was 2.9 years. The 3-year OS rate from date of baseline PET for the entire cohort was 47% (Figure 1A). At the time of analysis, 21 (37%) patients remained alive with median follow-up of 4.4 years from end of radiotherapy for this subset of patients. Median DFS for entire cohort of 57 patients was 1.5 years. Three-year DFS from date of baseline PET for the cohort was 40% (Figure 1B). Median DMFS time for all 57 patients was 2.0 years with 3-year rate of DMFS from initial PET of 44%. Twenty-one of the 57 patients (37%) developed DM.
Local disease recurrence was evaluated in the subset of 50 patients who did not undergo curative surgery following completion of CRT. Seventeen of the 50 patients (34%) developed LR. Median LRFS was 2.0 years with 3-year LRFS from date of baseline PET of 36%. Three of these 17 patients underwent surgical resection with a median time of 11.1 months from end of radiotherapy to surgery.
PET imaging findings
A total of 57 patients (100%) underwent a baseline PET/CT; of these 51 patients (91%) had analysable baseline imaging, while 5 had SUVmax values from the outside report. One patient underwent outside baseline imaging without an analysable or reported SUV value. All regions of interest were manually redrawn in consensus with two nuclear medicine physicians (HS and LO). Forty patients of the entire cohort (70%) also underwent post-induction PET/CT and 41 patients of the entire cohort (72%) underwent PET/CT after completion of CRT. Scan data for one patient in the CRT group was not available but the reported SUVmax value was used. Post-induction and post-CRT scans for 17 and 16 patients, respectively, were not in our PACS and their outside reports were not in the electronic medical records. A total of 30 (53%) patients had scans at all 3 time points. Table 2 shows data for SUVmax and MTV at all 3 time points.
PET prognosticators of outcome
Percent change in SUVmax and MTV
Of the 38 patients with both baseline and post-induction chemotherapy PET scans, there were 25 PET ‘responders’ and 13 PET ‘non-responders’ based on the pre-specified cutoff of 35% decrease in SUVmax from baseline. The responders had a significantly longer median survival and 3-year OS, as calculated from date of post-induction PET, in comparison with non-responders (3-year OS 64% vs 15%; P=0.004). Furthermore, PET responders had a significantly greater DFS, as calculated from date of post-induction PET (3-year DFS 52% vs 15.4%; P=0.021) and DMFS, as calculated from date of post-induction PET (3-year DMFS 60% vs 15.4%; P=0.011). Of note, the cutoff of 35% in SUVmax did not correlate with LRFS (P=0.15). Percent change in SUVmax from baseline to post-CRT PET or post-induction to post-CRT PET did not correlate with OS, LRFS, DMFS or DFS.
Percent change of MTV from baseline to post-induction therapy scan was not significant for any clinical outcomes. In addition, percent change in MTV between baseline and post-CRT scans as well as between post-induction and post-CRT scans were not associated with OS, LRFS, DMFS or DFS.
Baseline SUVmax and MTV
In univariate analysis, lower baseline SUVmax (HR 1.093; P=0.001) and MTV (HR 1.018; P=0.002) were significantly associated with better OS (Table 3). Both baseline PET metrics were also significantly associated with DFS and DMFS. Local recurrence-free survival was associated with decreased baseline SUVmax (HR 1.080; P=0.001) and MTV (HR 1.013; P=0.025) (Table 3).
On multivariate analysis for baseline PET/CT parameters, SUVmax trended towards significance for OS (HR 1.074; P=0.059) as well as DFS (HR 1.07; P=0.052) when compared with MTV. The SUVmax remained prognostic for LRFS (HR 1.091; P=0.012) and DMFS (HR 1.081; P=0.031) when compared with MTV (Table 4).
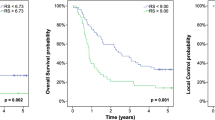
Utilising a minimally selected log-rank test, we found the optimal cutoff for baseline SUVmax that best separated DFS with a minimum P-value was 17.1 (P=0.014). The 3-year DFS, as calculated from baseline PET, was 7% for baseline SUVmax >17.1, and 52% for baseline SUVmax ⩽17.1 (Figure 2A).
Post-induction chemotherapy SUVmax and MTV
In univariate analysis, lower SUVmax (HR 1.108; P<0.001) and MTV (HR 1.02; P=0.001) were significantly associated with better OS. These same parameters were also significantly associated with DFS, LRFS and DMFS (Table 3).
On multivariate analysis, SUVmax was only significant for DMFS (HR 1.108; P=0.046) in comparison with MTV (Table 4).
The optimal post-induction SUVmax cutoff for DFS was 9.09 (P=0.019). The 3-year DFS, as calculated from post-induction PET, was 7% for post-induction SUVmax >9.09, and 56% for post-induction SUVmax ⩽9.09 (Figure 2B).
Post-CRT SUVmax and MTV
In univariate analysis of post-CRT PET parameters, only MTV was prognostic for OS (HR 1.089; P=0.046). None of the PET metrics were significant for DFS. With regards to LRFS and DMFS, only SUVmax was significant (Table 3). Owing to low number of events, multivariate analysis of post-CRT parameters was not feasible (Table 4).
Discussion
The management of oesophageal SCCs remains controversial with multiple therapeutic combinations and no established prognostic or predictive factors to guide treatment decisions. There are limited data on the use of PET imaging to prognosticate outcomes or to predict treatment response specifically in the oesophageal SCC population, however, the use of percent change in SUVmax after induction chemotherapy has been reported in several trials of patients with oesophageal and gastroesophageal junction adenocarcinomas (Weber et al, 2001; Lordick et al, 2007; Ilson et al, 2012). In this study, we validated the use of a SUVmax response of ⩾35% decline from baseline to after induction chemotherapy as a prognostic marker for improved OS and DFS in patients with oesophageal SCC.
Weber et al initially evaluated the use of serial PET imaging in patients with adenocarcinoma of the gastroesophageal junction who received neoadjuvant chemotherapy and demonstrated that ‘metabolic responders’ after 2 weeks of chemotherapy had improved histopathologic response and greater OS compared with ‘metabolic non-responders’; ‘metabolic responders’ were defined as patients with ⩾35% decline in FDG SUVmax value from baseline to 2 weeks after neoadjuvant chemotherapy (Weber et al, 2001). This observation was confirmed in the MUNICON (Metabolic response evalUatioN for Individualisation of neoadjuvant Chemotherapy in oesOphageal and oesophagogastric adeNocarcinoma) trial in which ‘metabolic non-responders’ to induction chemotherapy went to immediate surgery rather than continuing with the 3 months of preoperative chemotherapy (Lordick et al, 2007). The median DFS in the ‘metabolic non-responders’ who discontinued ineffective chemotherapy and went directly to surgery appeared to be better than in previous studies where these patients completed the 3 months of neoadjuvant chemotherapy (26 vs 18 months). Ilson et al also confirmed the PET scan response cutoff point of 35% after induction chemotherapy was associated with improved pathologic complete response (pCR) rates (32% vs 4%; P=0.009) and DFS (24 vs 7.7 months; P=0.02) (Ilson et al, 2012). In this prospective study, 55 patients with oesophageal cancer, predominantly (75%) adenocarcinomas, were treated with induction cisplatin and irinotecan (4 treatments over 5 weeks) followed by a post-induction PET scan and then continued on with chemoradiation with concurrent cisplatin, irinotecan and 50.4 Gy. Thirty-nine patients also underwent oesophagectomy.
Although these data were generated primarily in patients with oesophageal adenocarcinomas, we explored whether this cutoff of 35% decrease in SUVmax from baseline to post induction could be extrapolated to the population of oesophageal SCC patients. Indeed, the a priori defined cutoff of 35% decrease proved significance with respect to OS (P=0.0041) and DFS (P=0.021) (Figure 3A and B); thus confirming a similar prognostic ability among varying histological subtypes.
We also evaluated outcomes of an aggressive approach of induction chemotherapy followed by chemoradiation in this retrospective study of a relatively homogeneous cohort of patients with oesophageal SCC. The use of induction chemotherapy followed by combined chemoradiation is not the global standard of care for this disease and has not been compared with chemoradiotherapy alone in the context of a phase III trial. However, in this group of 57 oesophageal SCC patients treated with CRT, 52 of whom received induction chemotherapy, we demonstrate that the outcomes are good with a 3-year OS and DFS of 47% and 40%, respectively. The use of induction chemotherapy has several potential advantages as the particular chemotherapy regimen can be tested for efficacy using PET response in the primary tumour after induction therapy without the confounding effect of the concurrent radiotherapy. This allows for early changes in systemic therapy, which can then be administered during radiotherapy. The use of PET scans to direct preoperative therapy in patients with oesophageal and GEJ adenocarcinomas is being examined in the Cancer and Leukemia Group B trial 80803, now closed to accrual. In this randomised phase II study, patients undergo a baseline PET scan and are randomised to either FOLFOX (folinic acid–fluorouracil–oxaliplatin) or carboplatin and paclitaxel followed by a post-induction chemotherapy PET scan. The metabolic non-responders, based on the 35% cutoff, crossover to the alternative chemotherapy regimen during combined chemoradiation while the metabolic responders continue on with the same chemotherapy.
Apart from analysing the use of the 35% change in SUVmax from baseline to post-induction chemotherapy, we also evaluated the prognostic potential of SUVmax at single time points and the percent change from baseline or post induction to post CRT. We found that on UVA, baseline and post-induction SUVmax were important prognostic parameters for OS, DFS LRFS, and DMFS. In this analysis, we found that a baseline SUVmax of >17 and a post-induction SUVmax of >9 were both predictive of worse outcomes. Previous studies have demonstrated the prognostic and predictive values of these parameters, however they have focused predominantly on adenocarcinoma histology (Wieder et al, 2004; Hyun et al, 2010; Park et al, 2013). Because of differences in histology, one cannot extrapolate published data on AC to SCC. Analysis of percent change in SUVmax from baseline or post-induction PET to post-CRT PET did not appear to have any prognostic value.
Interestingly, post-CRT SUVmax was prognostic for LRFS and DMFS in this study. The use of a post-CRT prognostic SUVmax parameter may allow for stratification of patients who would benefit from surgery after CRT, though the prognostic ability of post-CRT SUVmax requires further validation. The benefit of surgical resection after CRT in oesophageal SCC has been questioned as two randomised trials of CRT with or without surgery failed to demonstrate a benefit for the addition of surgery (Bedenne et al, 2007; Stahl et al, 2009). Moreover, recent data from a large, randomised trial of surgery alone vs preoperative CRT followed by surgery for oesophageal cancer demonstrated a pCR rate of 49% in patients with oesophageal SCC after preoperative chemoradiation (van Hagen et al, 2012). This high pCR rate for the patients with oesophageal SCC suggests that surgery may not be necessary in a subset of SCC patients who respond well to CRT. Unfortunately, response assessment after CRT is generally limited using almost any modality, including PET, CT, endoscopic ultrasound or even biopsy, because of treatment-associated inflammation and fibrosis. Thus, further data are needed to verify the findings in this study.
In addition to our analysis of SUVmax, we assessed MTV, a volumetric PET parameter. We also identified on UVA, both baseline and post-induction MTV to be prognostic for OS, DFS, LRFS and DMFS. However, on subsequent multivariate analysis, MTV at baseline and post induction was not independently prognostic for any clinical outcomes when compared with SUVmax. In part, this loss of prognostication of MTV may reflect the inherent dependence of these volumetric parameters on the value of SUVmax and the predetermined threshold. In calculating MTV, a predetermined percentage of the primary tumour SUVmax affects the calculated volume. As such, relatively low SUVmax values may falsely incorporate non-malignant tissues in the MTV volume, thus reducing the prognostic value of MTV. Our data differ from a recent study from France, which demonstrated that MTV at baseline was a major prognostic factor for DFS and OS in a cohort of 67 oesophageal SCC patients (Lemarignier et al, 2014).
When evaluating the prognostic significance of post-CRT MTV, we discovered a significant correlation existed only for OS. In contrast, analysis of percent change in MTV between any two PET time points revealed no prognostic correlation with clinical outcomes.
Various limitations exist in this current study, including its retrospective nature and the relatively small sample size; however, the latter is a consequence of the rarity of SCC in the United States. Another potential concern is the uncertain impact of using induction chemotherapy before CRT on the outcome for SCC. The use of induction chemotherapy remains controversial in head and neck SCC and in anal SCC, has been shown to negatively impact outcomes (Ajani et al, 2008). The typical schedule of induction chemotherapy in our cohort consisted of 1–2 cycles, thus not delaying initiation of radiotherapy significantly or presumably negatively affecting outcomes. In conclusion, a cutoff of ⩾35% decrease in SUVmax from baseline to post-induction PET can be extrapolated from oesophageal adenocarcinoma to SCC and provides strong prognostic information in patients with SCC undergoing multimodality therapy. With the potential ability to tailor individual therapies based on PET response, prospective clinical trials are necessary to further validate this approach in the oesophageal SCC population.
Change history
22 December 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr, Mayer RJ, Haddock MG, Rich TA, Willett C (2008) Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 299 (16): 1914–1921.
Barber TW, Duong CP, Leong T, Bressel M, Drummond EG, Hicks RJ (2012) 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med 53 (6): 864–871.
Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C (2007) Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25 (10): 1160–1168.
Blackstock AW, Farmer MR, Lovato J, Mishra G, Melin SA, Oaks T, Aklilu M, Clark PB, Levine EA (2006) A prospective evaluation of the impact of 18-F-fluoro-deoxy-D-glucose positron emission tomography staging on survival for patients with locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 64 (2): 455–460.
Bollschweiler E, Holscher AH (2007) Prognosis of early esophageal cancer: differences between squamous cell carcinoma and adenocarcinoma. Ann Surg 245 (2): 334.
Brown C, Howes B, Jamieson GG, Bartholomeusz D, Zingg U, Sullivan TR, Thompson SK (2012) Accuracy of PET-CT in predicting survival in patients with esophageal cancer. World J Surg 36 (5): 1089–1095.
Erdi YE, Mawlawi O, Larson SM, Imbriaco M, Yeung H, Finn R, Humm JL (1997) Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 80 (12 Suppl): 2505–2509.
Hong D, Lunagomez S, Kim EE, Lee JH, Bresalier RS, Swisher SG, Wu TT, Morris J, Liao Z, Komaki R (2005) Value of baseline positron emission tomography for predicting overall survival in patient with nonmetastatic esophageal or gastroesophageal junction carcinoma. Cancer 104 (8): 1620–1626.
Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, Lee JY, Lee KH, Kim BT (2010) Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 17 (1): 115–122.
Ilson DH, Minsky BD, Ku GY, Rusch V, Rizk N, Shah M, Kelsen DP, Capanu M, Tang L, Campbell J, Bains M (2012) Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer 118 (11): 2820–2827.
Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Masuda N, Fukuchi M, Manda R, Tsukada K, Oriuchi N, Endo K (2002) Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with esophageal cancer. Am J Surg 184 (3): 279–283.
Kato H, Nakajima M, Sohda M, Tanaka N, Inose T, Miyazaki T, Fukuchi M, Oriuchi N, Endo K, Kuwano H (2009) The clinical application of 18F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer 115 (14): 3196–3203.
Konski AA, Cheng JD, Goldberg M, Li T, Maurer A, Yu JQ, Haluszka O, Scott W, Meropol NJ, Cohen SJ (2007) Correlation of molecular response as measured by 18-FDG positron emission tomography with outcome after chemoradiotherapy in patients with esophageal carcinoma. Int J Radiat Oncol Biol Phys 69 (2): 358–363.
Lemarignier C, Di Fiore F, Marre C, Hapdey S, Modzelewski R, Gouel P, Michel P, Dubray B, Vera P (2014) Pretreatment metabolic tumour volume is predictive of disease-free survival and overall survival in patients with oesophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging 41 (11): 2008–2016.
Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, Lorenzen S, Schuster T, Wieder H, Herrmann K, Bredenkamp R, Hofler H, Fink U, Peschel C, Schwaiger M, Siewert JR (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8 (9): 797–805.
Omloo J, Sloof G, Boellaard R, Hoekstra O, Jager P, Van Dullemen H, Fockens P, Plukker J, van Lanschot J (2008) Importance of fluorodeoxyglucose-positron emission tomography (FDG-PET) and endoscopic ultrasonography parameters in predicting survival following surgery for esophageal cancer. Endoscopy 40 (06): 464–471.
Park JS, Choi JY, Moon SH, Ahn YC, Lee J, Kim D, Kim K, Shim YM (2013) Response evaluation after neoadjuvant chemoradiation by positron emission tomography-computed tomography for esophageal squamous cell carcinoma. Cancer Res Treat 45 (1): 22–30.
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48 (12): 1503–1510.
Rizk NP, Tang L, Adusumilli PS, Bains MS, Akhurst TJ, Ilson D, Goodman K, Rusch VW (2009) Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol 4 (7): 875–879.
Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Konigsrainer A, Budach W, Wilke H (2009) Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27 (6): 851–856.
Suzuki A, Xiao L, Hayashi Y, Macapinlac HA, Welsh J, Lin SH, Lee JH, Bhutani MS, Maru DM, Hofstetter WL (2011) Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer 117 (21): 4823–4833.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A Group C (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366 (22): 2074–2084.
van Westreenen HL, Plukker JT, Cobben DC, Verhoogt CJ, Groen H, Jager PL (2005) Prognostic value of the standardized uptake value in esophageal cancer. AJR Am J Roentgenol 185 (2): 436–440.
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50 (Suppl 1): 122S–150S.
Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschlager G, Busch R, Siewert JR, Schwaiger M, Fink U (2001) Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19 (12): 3058–3065.
Wieder HA, Brucher BL, Zimmermann F, Becker K, Lordick F, Beer A, Schwaiger M, Fink U, Siewert JR, Stein HJ, Weber WA (2004) Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 22 (5): 900–908.
Zhang Y (2013) Epidemiology of esophageal cancer. World J Gastroenterol 19 (34): 5598–5606.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
YYJ: disclosures include current consultancy with Eli Lilly as well as current grants received from Amgen, Eli Lilly, Boehringer Ingelheim and Bayer. In addition, receipt of past compensation for lectures from AXIS CME. AW: disclosures include past consultancy with Pfizer. DI: disclosures include ongoing consultancy with Amgen and Lilly. In addition, ongoing grants received from Bayer and Amgen as well as ongoing compensation for lectures from Roche. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Chhabra, A., Ong, L., Kuk, D. et al. Prognostic significance of PET assessment of metabolic response to therapy in oesophageal squamous cell carcinoma. Br J Cancer 113, 1658–1665 (2015). https://doi.org/10.1038/bjc.2015.416
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.416
Keywords
This article is cited by
-
Utility of PET Scans in the Diagnosis and Management of Gastrointestinal Tumors
Digestive Diseases and Sciences (2022)
-
18F-FDG PET/CT as predictive and prognostic factor in esophageal cancer treated with combined modality treatment
Annals of Nuclear Medicine (2022)
-
Dose-escalated radiotherapy with PET/CT based treatment planning in combination with induction and concurrent chemotherapy in locally advanced (uT3/T4) squamous cell cancer of the esophagus: mature results of a phase I/II trial
Radiation Oncology (2021)
-
PET/CT-Based Response Evaluation in Cancer—a Systematic Review of Design Issues
Molecular Imaging and Biology (2020)
-
The value of GRASP on DCE-MRI for assessing response to neoadjuvant chemotherapy in patients with esophageal cancer
BMC Cancer (2019)