Abstract
Background:
The family of polypeptide N-acetylgalactosaminyl transferases is responsible for the altered O-linked glycosylation occurring during the development of various cancers and their progression via altering O-glycan biosynthesis. Our studies were designed to investigate the expression and prognostic values of GalNAc-T5 and improve the risk stratification in patients with gastric cancer.
Methods:
Tissue samples from a training set and a validation set of patients with gastric adenocarcinoma from China were used for analyses. GalNAc-T5 expression was retrospectively analysed by immunohistochemistry. Results were assessed for association with clinicopathological parameters and overall survival by using Kaplan–Meier analysis. Prognostic values of GalNAc-T5 expression and clinical outcomes were evaluated by Cox regression analysis. A molecular prognostic stratification scheme incorporating GalNAc-T5 expression was determined by using receiver operating characteristic analysis.
Results:
GalNAc-T5 expression was markedly reduced in gastric cancer tissues compared with non-malignant gastric mucosa. Low intratumoral GalNAc-T5 density, which was associated with tumour cell differentiation, T classification, N classification, and TNM stage in the two independent sets, was an independent prognosticator for poor prognosis of gastric cancer patients. Applying the prognostic value of intratumoral GalNAc-T5 density to the conventional clinicopathologic TNM stage system showed a better prognostic value in patients with gastric cancer.
Conclusions:
Intratumoral GalNAc-T5 expression was recognised as an independent prognostic marker for the overall survival of gastric cancer patients. Detection of GalNAc-T5 expression in gastric cancer tissues might add some prognostic information for patients with this disease and lead to a more accurate classification under the TNM stage system.
Similar content being viewed by others
Main
Despite a decreasing overall incidence, gastric cancer remains the fourth most common neoplasm and the second leading cause of cancer-related mortality worldwide with a frequency that varies widely across geographic locations (Hartgrink et al, 2009; Jemal et al, 2011). In China, gastric cancer is also a major public health issue, some 400 000 new cases are diagnosed every year and 95% of the total number of gastric malignancies is adenocarcinoma (Yang, 2006). The tumour–node–metastasis (TNM) classification of the International Union Against Cancer based on the post-operative clinicopathological status of patients with gastric cancer establishes a predictive model for accurately predicting patient survival and guiding therapy decisions (Washington, 2010). Nonetheless, some gastric cancer patients may experience recurrence and ultimately die from the disease, even with a favourable prognostic background (Lim et al, 2005). This considerable heterogeneity of gastric cancer presents at the molecular level and has a genetic predisposition to it (Stock and Otto, 2005). Therefore, molecular approaches for stratifying patients with gastric cancer, through incorporation of molecular information, including post-translational modification, into the conventional clinicopathologic TNM stage system will improve current prognostic stratification and provide important clinically relevant insights into predicting which patients are prone to develop recurrence and mortality after surgery.
Glycosylation is the most common post-translational modification of proteins (Dennis et al, 1999). Two major types of protein glycosylation, N-linked and O-linked, exist in mammalian cells (Dube and Bertozzi, 2005). Glycosylation alterations, including both under- and overexpression of naturally occuring glycans, as well as neoexpression of glycans normally restricted to embryonic tissues, is a well-described hallmark of many human cancers, prominently including gastric cancer, and has been shown to have multiple effects on many cellular properties, including cell differentiation, proliferation, apoptosis, transformation, migration, and invasion (Fuster and Esko, 2005; Raman et al, 2005). O-glycosylation may affect structural and functional properties of secreted and membrane-bound proteins and peptides, and have a major role in the tumorigenesis of gastric cancer (Kobayashi et al, 2009). A critical aspect of O-glycosylation, the serine and threonine residues at which target proteins are glycosylated with N-acetylgalactosamine (GalNAc), is catalysed by a large family of polypeptide GalNAc-transferases (GalNAc-Ts) that are normally located in the Golgi complex (Ten Hagen et al, 2003). Thus, through regulating the initial step of O-glycosylationed protein biosynthesis and determining sites of O-glycosylation on proteins, GalNAc-Ts are important for understanding normal and carcinoma-associated O-glycosylation (Brockhausen, 2006). Prior studies have revealed that strong GalNAc-T3 expression in gastric cancer tissues was significantly correlated with good prognosis of gastric cancer patients (Onitsuka et al, 2003). Besides, according to the previous literatures, it had been demonstrated that GalNAc-T10 was a useful indicator of tumour differentiation in gastric cancer (Gao et al, 2013). The expression of GalNAc-T6 was reported to be an immunohistochemical marker associated with venous invasion in gastric carcinoma (Gomes et al, 2009). However, to date, no study has assessed GalNAc-T5 expression in gastric cancer, and the correlation between GalNAc-T5 expression and the prognosis of patients with gastric cancer remains largely unclear and needs to be further investigated.
In the current study, we evaluated the expression of GalNAc-T5 in gastric cancer specimens and their correlation with the clinicopathologic features of the patients. The results indicated that low intratumoral GalNAc-T5 expression was a significant negative prognostic predictor for patients with gastric cancer. Moreover, integration of intratumoral GalNAc-T5 expression into the conventional clinicopathologic TNM stage system could refine prognostic stratification of patients with gastric cancer.
Materials and methods
Clinical specimens
We prospectively recruited consecutive patients with gastric cancer, collected the clinicopathologic data and the specimens, and retrospectively analysed the samples in detail for markers correlating with survival and their role in refining gastric cancer prognostic stratification (Wang et al, 2013). Two independent sets comprising of 223 patients, who had undergone total or partial gastrectomy for gastric adenocarcinoma at the Zhongshan Hospital of Fudan University (Shanghai, China), were enrolled in the study. Patients were excluded if they had previously been exposed to any targeted therapy, chemotherapy, radiotherapy, or intervention therapy for gastric cancer. Specimens of the training set (n=97) were obtained between January 2000 and December 2005, and specimens of the validation set (n=126) were obtained between January 2006 and December 2008. Detailed clinical characteristics of the two independent sets are summarised in Supplementary Table S1. There were more patients with late-stage gastric cancer (TNM III and IV, 61.11% vs 49.48%, P=0.016) and poorer overall survival (P=0.026) in the validation set compared with the training set. Besides, there were more patients with old age (P=0.002) and deep tumour invasion (T3 and T4, P=0.001) in the validation set. Such heterogeneity may help to ensure that the predictor has real-world applicability. Tissue samples of human gastric cancer, which had been formalin-fixed, paraffin-embedded, and clinically and histopathologically diagnosed, were collected at the time of surgical resection. Non-tumoral gastric tissues were obtained at least 5 cm from the tumour at the same time. After operation, routine chemotherapy had been given to the patients with advanced-stage disease, but no radiation treatment was done in any of patients included in our study. All specimens were pathologically reassessed independently by two gastroenterology pathologists blinded to the clinical data. Institutional review board approval from Zhongshan Hospital of Fudan University and written informed consent from all patients were obtained for this study.
Tissue microarray and immunohistochemistry
Tissue microarrays were constructed as previously described (Zhu et al, 2008). Primary anti-GalNAc-T5 antibody (Sigma-Aldrich, St Louis, MO, USA) was used for immunohistochemistry staining. Before immunohistochemistry staining, the expression of GalNAc-T5 was detected by RT–PCR, quantitative real-time PCR, and western blot analysis in four human gastric cancer cell lines (NCI-N87, SGC7901, AGS, and BGC823). The protein expression level of GalNAc-T5 detected by the antibody parallels its mRNA expression level in human gastric cancer cells, and the antibody is specific for it (Supplementary Figure S1). The intensity of immunohistochemistry staining of GalNAc-T5 was scored independently by two gastroenterology pathologists using the semi-quantitative immunoreactivity scoring system as previously described (Weichert et al, 2008; Wang et al, 2013). Negative controls were treated identically but with the primary antibody omitted.
Statistical analysis
Statistical analysis was performed with MedCalc Software (version 11.4.2.0; MedCalc, Mariakerke, Belgium). Numerical data were analysed using Student’s t-test, whereas categorical data were studied using χ2 or Fisher’s exact test. Cumulative survival time was calculated using the Kaplan–Meier method and analysed using the log-rank test. Numbers at risk were calculated for the beginning of each time period. The Cox proportional hazards regression model was used to perform univariate and multivariate analyses. Receiver operating characteristic analysis was used to compare the sensitivity and specificity for the prediction of overall survival by the parameters. All P-values were two sided, and differences were considered significant at values of P<0.05. Results are reported according to REMARK (Reporting Recommendations for Tumor Marker Prognostic Studies) guidelines (McShane et al, 2005).
Other materials and methods are detailed in the Supplementary Materials and Methods.
Results
Immunohistochemical findings
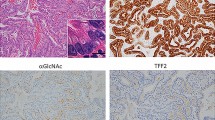
In order to ascertain whether the expression of GalNAc-T5 protein is linked to clinical progression of gastric cancer, we first evaluated GalNAc-T5 expression by immunohistochemical analyses in tumour and non-tumoral specimens from 223 patients with gastric cancer in the training and validation sets. As shown in Figure 1, GalNAc-T5 immunoreactivity was predominantly located in the cytoplasm of gastric epithelia (Figure 1B) and cancer cells (Figure 1C–F), and the intensity of the immunohistochemical staining was variable. Sporadic positive staining on the stroma cells was also observed (Figure 1B–F). Compared with higher non-tumoral GalNAc-T5 density in gastric epithelial cells (Figure 1B), intratumoral GalNAc-T5 expression in gastric cancer cells decreased gradually accompanied with disease progression from well-differentiated adenocarcinoma (Figure 1C), moderately differentiated adenocarcinoma (Figure 1D), poorly differentiated adenocarcinoma (Figure 1E) to undifferentiated adenocarcinoma (Figure 1F). Collectively, these observations suggest that GalNAc-T5 expression is decreased in gastric cancer tissues compared with the non-tumoral tissues, and decreased GalNAc-T5 expression in tumour cells might be associated with histologic progression of gastric cancer.
GalNAc-T5 expression in sections of gastric tissue. (A) Negative control. (B) Gastric cancer and adjacent peritumoral tissues. Arrowhead shows gastric peritumoral epithelial cells with strong expression of GalNAc-T5, and arrow shows gastric cancer tissue with moderate GalNAc-T5 expression. (C) Well-differentiated adenocarcinoma showing strong expression of GalNAc-T5. (D) Moderately differentiated adenocarcinoma showing moderate expression of GalNAc-T5. (E) Poorly differentiated adenocarcinoma showing moderate or weak expression of GalNAc-T5. (F) Undifferentiated adenocarcinoma showing weak expression of GalNAc-T5. (G, H) The per cent of patients with high intratumoral GalNAc-T5 expression decreased gradually accompanied with disease progression from TNM stage I to IV in (G) training set (n=97) and (H) validation set (n=126). Scale bar: 50.0 μm.
Associations between GalNAc-T5 expression and clinicopathologic parameters of patients with gastric cancer
According to the immunoreactivity scoring criterion, ∼51.55% (training set, 50 of 97) and 44.44% (validation set, 56 of 126) tumours were scored as low GalNAc-T5 expression. Immunohistochemical staining of GalNAc-T5 levels was statistically analysed to determine their relationship with various clinicopathologic features of patients with gastric cancer. As shown in Table 1, intratumoral GalNAc-T5 expression was significantly associated with tumour cell differentiation (P=0.041 and P=0.020, respectively), T classification (P=0.033 and P=0.032, respectively), N classification (P=0.013 and P=0.022, respectively), and TNM stage (P=0.018 and P=0.036, respectively) in the two independent sets. Besides, in the validation set, intratumoral GalNAc-T5 expression was also significantly related with the Lauren classification (P=0.028). In addition, the per cent of patients with high intratumoral GalNAc-T5 expression decreased gradually accompanied with disease progression from TNM stage I to IV, and there were no patients with high intratumoral GalNAc-T5 expression in the TNM stage IV in the two independent sets (Figure 1G and H). Besides, quantitative real-time PCR also confirmed that the mRNA expression level of GalNAc-T5 decreased gradually accompanied with disease progression (Supplementary Figure S2), which is consistent with the results of immunohistochemistry staining. However, non-tumoral GalNAc-T5 expression was not associated with any clinicopathologic parameters of gastric cancer patients in the training set (Supplementary Table S2).
Correlation of GalNAc-T5 expression with prognosis of patients with gastric cancer
To further investigate the prognostic value of GalNAc-T5 expression in gastric cancer patients, we compared overall survival according to intratumoral GalNAc-T5 expression, and Kaplan–Meier survival analysis was performed. Clearly, gastric cancer patients with low intratumoral GalNAc-T5 expression have a poor prognosis than those with high intratumoral GalNAc-T5 expression in the two independent sets (P<0.001 and P<0.001, respectively; Figure 2A and B; Supplementary Table S3), which indicates a crucial impact of intratumoral GalNAc-T5 expression on clinical outcome in patients with gastric cancer. However, Kaplan–Meier survival analysis showed that non-tumoral GalNAc-T5 expression was not associated with overall survival of gastric cancer patients in the training set (data not shown). In addition, in order to determine whether intratumoral GalNAc-T5 expression could stratify patients with TNM stage stratum, we evaluated the prognostic value of intratumoral GalNAc-T5 expression and performed stratified analyses of gastric cancer patients with TNM stage I+II and TNM stage III+IV, respectively. As shown in Supplementary Table S3, only the patients with TNM stage III+IV could be significantly stratified by intratumoral GalNAc-T5 expression; the prognosis of TNM stage III+IV patients with low intratumoral GalNAc-T5 expression was significantly poorer than those with high intratumoral GalNAc-T5 expression in the two independent sets (P=0.035 and P=0.008, respectively; Figure 2C–F; Supplementary Table S3).
Analyses of overall survival according to the expression of intratumoral GalNAc-T5 in gastric cancer patients. Kaplan–Meier analyses of overall survival according to intratumoral GalNAc-T5 expression in patients with gastric cancer in (A) training set, all patients (n=97), (B) validation set, all patients (n=126), (C) training set, TNM stage I+II (n=49), (D) validation set, TNM stage I+II (n=49), (E) training set, TNM stage III+IV (n=48), and (F) validation set, TNM stage III+IV (n=77). P-value was calculated using the log-rank test.
Low intratumoral GalNAc-T5 expression is an independent predictor of poor prognosis in patients with gastric cancer
In order to estimate the clinical significance of intratumoral GalNAc-T5 expression that might influence survival in the study population, univariate analyses was performed for overall survival in the two independent sets of gastric cancer patients. As shown in Table 2, low intratumoral GalNAc-T5 expression is a significant negative prognostic predictor for patients with gastric cancer in the training set (hazard ratio (HR), 2.92; 95% CI, 1.63–5.26; P<0.001) and the validation set (HR, 4.41; 95% CI, 1.90–10.26; P<0.001). In addition, T classification (P=0.002 and P=0.009, respectively), N classification (P=0.001 and P=0.002, respectively), distant metastasis (P<0.001 and P<0.001, respectively), and TNM stage (P<0.001 and P<0.001, respectively) were also statistically significant factors affecting overall survival of patients with gastric cancer in the two independent sets. To evaluate the robustness of the prognostic value of intratumoral GalNAc-T5 expression, Cox multivariate regression analyses were performed to derive risk estimates related to overall survival with the same clinicopathological parameters of the training set that show significance in univariate analyses to control for confounders. As shown in Table 3, intratumoral GalNAc-T5 expression (P=0.022 and P=0.003, respectively) and TNM stage (P<0.001 and P<0.001, respectively) were both recognised as independent prognostic factors for overall survival of gastric cancer patients in the two independent sets. Taken together, our findings indicate that intratumoral GalNAc-T5 expression may be a useful marker to predict the overall survival of patients with gastric cancer.
Extension of the TNM stage prognostic model with intratumoral GalNAc-T5 expression
To develop a more sensitive predictive tool, we constructed a prognostic model combining the two independent prognostic factors, intratumoral GalNAc-T5 expression and TNM stage, and compared its prognostic validity with the intratumoral GalNAc-T5 expression alone and TNM stage alone models by means of receiver operating characteristic analyses. Combination of intratumoral GalNAc-T5 expression and the TNM stage (AUC (95% CI), 0.840 (0.752–0.907) and 0.780 (0.698–0.849), respectively) showed a better prognostic value than did intratumoral GalNAc-T5 expression (AUC (95% CI), 0.698 (0.597–0.787) and 0.679 (0.590–0.759), respectively) or TNM stage (AUC (95% CI), 0.784 (0.688–0.861) and 0.706 (0.619–0.784), respectively) alone in the two independent sets (Figure 3).
ROC analyses for the prediction of overall survival in patients with gastric cancer. ROC analyses of the sensitivity and specificity for the prediction of overall survival by the combined TNM stage and GalNAc-T5 expression model, the TNM stage model, and the GalNAc-T5 expression model in (A) training set (n=97) and (B) validation set (n=126). P-values show the area under the ROC curves (AUC) of the combined TNM stage and GalNAc-T5 expression model vs AUCs of the TNM stage model or the GalNAc-T5 expression model.
Discussion
In general, it is often asymptomatic or causes only non-specific symptoms in its early stages of gastric cancer. By the time specific symptoms occur, the disease has often reached an advanced stage (Shou et al, 2012). Advanced gastric cancer prognosis tends to be dismal, despite aggressive therapy (Hartgrink et al, 2009). Various clinicopathologic features, including biological markers, have been proposed as prognostic indicators, although the results remain inconsistent and inconclusive to date (He et al, 2012, 2013). Defining molecular subgroups may identify patients who could benefit from aggressive and targeted therapies, and might be used to select specific treatment approaches for patients with gastric cancer (Kasaian and Jones, 2011). For this discrimination at the molecular level, in the present study, we investigated the expression and prognostic values of GalNAc-T5 in patients with gastric cancer. To our knowledge, this is the first study to identify low intratumoral GalNAc-T5 expression as an independent poor prognostic factor for overall survival of gastric cancer patients following gastrectomy, and only the patients with TNM stage III+IV could be significantly stratified by intratumoral GalNAc-T5 expression. Moreover, in this study, incorporation of intratumoral GalNAc-T5 density into the current clinicopathologic TNM stage system improved the prognostic value for overall survival. These data suggest that the intratumoral GalNAc-T5 density might have good discriminatory power as a supplementary risk factor in patients with late-stage gastric cancer and lead to a more accurate classification under the TNM stage system. Gastric cancer patients with low intratumoral GalNAc-T5 expression should have aggressive therapies and a closer follow-up. However, the results of integration of intratumoral GalNAc-T5 expression into the current prognostic model and the potential clinical practice changing should be validated in an independent and larger data set. The profound molecular roles of GalNAc-T5 in gastric cancer progression remain far from being fully elucidated and await further investigation.
Until now, clinicopathological parameters including invasion depth, lymphnode and distant metastases, and TNM stage have been considered to be the prognostic factors for gastric cancer. Tumour invasion, lymphnode metastases, and distant metastases are also primary causes for death or treatment failure among gastric cancer patients (Morabito et al, 2009). They are enormously complex and multistep processes involving regulation at the post-transcriptional level of adhesive molecules, proteolytic enzymes, and cell growth and angiogenesis factors. Altered glycoforms of these proteins with changed biological activity may have a pivotal role in these processes and contribute to the invasion and metastases of gastric cancer (Hakomori, 2002). Glycosylation alterations most often arise from changes in the expression levels of glycosyltransferases of cancerous cells (Dube and Bertozzi, 2005). One of the most common changes is an increase in the size and branching of N-glycans, which is often attributed to the increased activity of β1,6 N-acetylglucosaminyltransferase V (MGAT5). Our previous study has demonstrated that intratumoral MGAT5 expression predicted independently post-operative overall survival of patients with gastric cancer (Wang et al, 2013). In addition to abnormal N-glycosylation, alterations in the O-glycosylation patterns of some proteins have been associated with cancerous cellular transformation, and may have a critical role in determining the malignant behaviour of tumour cells through regulating biochemical and functional properties of cell-surface proteins (Slawson and Hart, 2011). The initiating step of O-glycosylation is catalysed by a large family of up to 20 distinct polypeptide GalNAc-Ts (Gill et al, 2011). GalNAc-Ts could lead to the formation of less-complex structures and an increase in the simple short determinants through catalysing the initiation of O-glycosylation at normally unoccupied potential glycosylation sites. The GalNAc-Ts are usually expressed only in the Golgi complex and it is dependent on post-translational modifications, including glycosylation. But recently a new mode of regulation has emerged where activation of Src kinase selectively redistributes Golgi-localised GalNAc-Ts to the endoplasmic reticulum (Gill et al, 2010). These results imply that the localisation of GalNAc-Ts may differ in different conditions and different cells, and may express in other organelles besides the Golgi complex. However, the exact molecular mechanisms of ectopic expression of GalNAc-Ts remain to be defined.
It has been shown that several GalNAc-Ts are useful markers for the development and progression of various tumours, such as renal cell carcinoma (Kitada et al, 2013), neuroblastoma (Berois et al, 2013), pancreatic cancer (Li et al, 2011), and gastric cancer. Prior studies had revealed that patients with strong GalNAc-T3 expression in gastric tumour tissues were significantly correlated with good prognosis (Onitsuka et al, 2003). Besides, GalNAc-T6 was reported to be an immunohistochemical marker associated with venous invasion in gastric carcinoma (Gomes et al, 2009). GalNAc-T10 had been demonstrated as a useful indicator of tumour differentiation in gastric cancer (Gao et al, 2013). Nonetheless, to date, no study has assessed GalNAc-T5 expression in gastric cancer. GalNAc-T5 accumulates in a very specific subset of cells, expresses in a highly tissue-specific manner, and glycosylates a restricted subset of peptides (Ten Hagen et al, 1998, 2003). It has been demonstrated that GalNAc-T5 could extend its catalytic domain into the lumen of the Golgi complex and/or mediate interaction with other proteins within the Golgi complex (Breton et al, 2001; Ten Hagen et al, 2003). In the present study, we first identified that GalNAc-T5 immunoreactivity was predominantly located in the cytoplasm of gastric epithelia (Figure 1B) and cancer cells (Figure 1C–F), and the intensity of the immunohistochemical staining decreased accompanied with dedifferentiation process in gastric cancer cells. Non-tumoral and well-differentiated gastric cancer tissues show strong expression of GalNAc-T5 (Figure 1B and C), whereas the less differentiated show moderate or poor expression (Figure 1D–F). These results imply that the expression status of GalNAc-T5 might be associated with the structure of gastric mucosa and the differentiated type of gastric cancer. When the normal structure was destroyed, such as carcinogenesis, the expression of GalNAc-T5 decreased. Thus, we assume that GalNAc-T5 may be an important factor that could reflect the normal form and function of gastric tissues. However, the molecular mechanisms underlying the altered expression of GalNAc-T5 remain poorly determined and await further characterisation.
Mucins are secreted or cell-surface-bound heavily O-glycosylated glycoproteins are produced by various epithelial cell types (Kufe, 2009). The changes in the biochemical characteristics of mucins are accompanied by malignant transformation of glandular epithelial cells. These profound changes include an alteration in O-glycans of mucin core peptides and an altered expression of mucin genes (Tarp and Clausen, 2008). The incomplete elongation of O-glycan saccharide chains in mucins could be induced by the downregulation of GalNAc-Ts expression, that can lead to the expression of shorter carbohydrate structures, such as Tn and sialyl-Tn-antigens (Wu et al, 2010). Tn antigen, usually masked by additional sugar residues in normal tissues, has a relatively simple structure composed of N-acetyl-D-galactosamine with a glycosidic α linkage to serine/threonine residues in glycoproteins, and it was detected in ∼90% of human carcinomas (Springer et al, 1995). A direct correlation has been shown between carcinoma aggressiveness and the density of Tn expression in the tumour (Springer, 1997). Thus, expression of the Tn determinant could be one of the results of GalNAc-Ts deregulation via changes in enzyme activity and/or in substrate specificity (Freire et al, 2005). For instance, in hepatocellular carcinoma cells, GalNAc-T2 can suppress EGF-induced malignant phenotypes through decorating the EGFR with short O-glycans, preferentially sialyl-Tn (Wu et al, 2011). In addition, recent studies focusing on the modulation of GalNAc-Ts indicated that GalNAc-Ts deregulation could affect leukocyte adhesion through modulating E-selection and P-selection counter receptors (Tenno et al, 2007). However, the exact mechanism to explain the tight relationship between GalNAc-T5 status and the progression of gastric cancer remains to be elucidated. And a better understanding of the molecular mechanism that regulates the GalNAc-T5 gene expression or enzyme activity is important for developing novel-targeted treatments for gastric cancer.
In conclusion, our results indicate that decreased intratumoral GalNAc-T5 expression predicts independently poor post-operative overall survival of patients with gastric cancer. Integration of intratumoral GalNAc-T5 density into the current clinicopathologic TNM stage system might add some prognostic information for patients with gastric cancer and could help to identify those patients in need of more aggressive treatment and closer follow-up.
Change history
15 April 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Berois N, Gattolliat CH, Barrios E, Capandeguy L, Douc-Rasy S, Valteau-Couanet D, Benard J, Osinaga E (2013) GALNT9 gene expression is a prognostic marker in neuroblastoma patients. Clin Chem 59 (1): 225–233.
Breton C, Mucha J, Jeanneau C (2001) Structural and functional features of glycosyltransferases. Biochimie 83 (8): 713–718.
Brockhausen I (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep 7 (6): 599–604.
Dennis JW, Granovsky M, Warren CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1473 (1): 21–34.
Dube DH, Bertozzi CR (2005) Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov 4 (6): 477–488.
Freire T, Bay S, von Mensdorff-Pouilly S, Osinaga E (2005) Molecular basis of incomplete O-glycan synthesis in MCF-7 breast cancer cells: putative role of MUC6 in Tn antigen expression. Cancer Res 65 (17): 7880–7887.
Fuster MM, Esko JD (2005) The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5 (7): 526–542.
Gao Y, Liu Z, Feng J, Sun Q, Zhang B, Zheng W, Ma W (2013) Expression pattern of polypeptide N-acetylgalactosaminyltransferase-10 in gastric carcinoma. Oncol Lett 5 (1): 113–116.
Gill DJ, Chia J, Senewiratne J, Bard F (2010) Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J Cell Biol 189 (5): 843–858.
Gill DJ, Clausen H, Bard F (2011) Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol 21 (3): 149–158.
Gomes J, Marcos NT, Berois N, Osinaga E, Magalhaes A, Pinto-de-Sousa J, Almeida R, Gartner F, Reis CA (2009) Expression of UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J Histochem Cytochem 57 (1): 79–86.
Hakomori S (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 99 (16): 10231–10233.
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ (2009) Gastric cancer. Lancet 374 (9688): 477–490.
He H, Chen W, Wang X, Wang C, Liu F, Shen Z, Xu J, Gu J, Sun Y (2012) Snail is an independent prognostic predictor for progression and patient survival of gastric cancer. Cancer Sci 103 (7): 1296–1303.
He H, Wang C, Shen Z, Fang Y, Wang X, Chen W, Liu F, Qin X, Sun Y (2013) Upregulated expression of C-X-C chemokine receptor 4 is an independent prognostic predictor for patients with gastric cancer. PLoS One 8 (8): e71864.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61 (2): 69–90.
Kasaian K, Jones SJ (2011) A new frontier in personalized cancer therapy: mapping molecular changes. Future Oncol 7 (7): 873–894.
Kitada S, Yamada S, Kuma A, Ouchi S, Tasaki T, Nabeshima A, Noguchi H, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Matsumoto T, Sasaguri Y (2013) Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer 109 (2): 472–481.
Kobayashi M, Lee H, Nakayama J, Fukuda M (2009) Roles of gastric mucin-type O-glycans in the pathogenesis of Helicobacter pylori infection. Glycobiology 19 (5): 453–461.
Kufe DW (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9 (12): 874–885.
Li Z, Yamada S, Inenaga S, Imamura T, Wu Y, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Sasaguri Y (2011) Polypeptide N-acetylgalactosaminyltransferase 6 expression in pancreatic cancer is an independent prognostic factor indicating better overall survival. Br J Cancer 104 (12): 1882–1889.
Lim L, Michael M, Mann GB, Leong T (2005) Adjuvant therapy in gastric cancer. J Clin Oncol 23 (25): 6220–6232.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCIEWGoCD (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93 (4): 387–391.
Morabito A, Carillio G, Longo R (2009) Systemic treatment of gastric cancer. Crit Rev Oncol Hematol 70 (3): 216–234.
Onitsuka K, Shibao K, Nakayama Y, Minagawa N, Hirata K, Izumi H, Matsuo K, Nagata N, Kitazato K, Kohno K, Itoh H (2003) Prognostic significance of UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-3 (GalNAc-T3) expression in patients with gastric carcinoma. Cancer Sci 94 (1): 32–36.
Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R (2005) Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat Methods 2 (11): 817–824.
Shou ZX, Jin X, Zhao ZS (2012) Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann Surg 256 (6): 1014–1022.
Slawson C, Hart GW (2011) O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 11 (9): 678–684.
Springer GF (1997) Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med (Berl) 75 (8): 594–602.
Springer GF, Desai PR, Ghazizadeh M, Tegtmeyer H (1995) T/Tn pancarcinoma autoantigens: fundamental, diagnostic, and prognostic aspects. Cancer Detect Prev 19 (2): 173–182.
Stock M, Otto F (2005) Gene deregulation in gastric cancer. Gene 360 (1): 1–19.
Tarp MA, Clausen H (2008) Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta 1780 (3): 546–563.
Ten Hagen KG, Fritz TA, Tabak LA (2003) All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 13 (1): 1R–16R.
Ten Hagen KG, Hagen FK, Balys MM, Beres TM, Van Wuyckhuyse B, Tabak LA (1998) Cloning and expression of a novel, tissue specifically expressed member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family. J Biol Chem 273 (42): 27749–27754.
Tenno M, Ohtsubo K, Hagen FK, Ditto D, Zarbock A, Schaerli P, von Andrian UH, Ley K, Le D, Tabak LA, Marth JD (2007) Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol Cell Biol 27 (24): 8783–8796.
Wang X, He H, Zhang H, Chen W, Ji Y, Tang Z, Fang Y, Wang C, Liu F, Shen Z, Qin J, Zhu Y, Liu H, Xu J, Gu J, Qin X, Sun Y (2013) Clinical and prognostic implications of beta1, 6-N-acetylglucosaminyltransferase V in patients with gastric cancer. Cancer Sci 104 (2): 185–193.
Washington K (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17 (12): 3077–3079.
Weichert W, Roske A, Gekeler V, Beckers T, Ebert MP, Pross M, Dietel M, Denkert C, Rocken C (2008) Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol 9 (2): 139–148.
Wu C, Guo X, Wang W, Wang Y, Shan Y, Zhang B, Song W, Ma S, Ge J, Deng H, Zhu M (2010) N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer 10: 123.
Wu YM, Liu CH, Hu RH, Huang MJ, Lee JJ, Chen CH, Huang J, Lai HS, Lee PH, Hsu WM, Huang HC, Huang MC (2011) Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res 71 (23): 7270–7279.
Yang L (2006) Incidence and mortality of gastric cancer in China. World J Gastroenterol 12 (1): 17–20.
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26 (16): 2707–2716.
Acknowledgements
This work was supported by grants from the National Natural Science Fund (31100629, 31270863, 31300671), the Program for New Century Excellent Talents in University (NCET-13-0146), the Shanghai Rising-Star Program (13QA1400300), the Key Project of Science and Technology Commission of Shanghai Municipality (09DZ1950101, 11411951000), and the Shanghai Municipal Health Bureau Fund (20124290).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
He, H., Shen, Z., Zhang, H. et al. Clinical significance of polypeptide N-acetylgalactosaminyl transferase-5 (GalNAc-T5) expression in patients with gastric cancer. Br J Cancer 110, 2021–2029 (2014). https://doi.org/10.1038/bjc.2014.93
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.93