Abstract
Background:
Smoking is a risk factor for incident colorectal cancer (CRC); however, it is unclear about its influence on survival after CRC diagnosis.
Methods:
A cohort of 706 CRC patients diagnosed from 1999 to 2003 in Newfoundland and Labrador, Canada, was followed for mortality and recurrence until April 2010. Smoking and other relevant data were collected by questionnaire after cancer diagnosis, using a referent period of ‘2 years before diagnosis’ to capture pre-diagnosis information. Molecular analyses of microsatellite instability (MSI) status and BRAF V600E mutation status were performed in tumour tissue using standard techniques. Multivariate hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated with Cox proportional hazards regression, controlling for major prognostic factors.
Results:
Compared with never smokers, all-cause mortality (overall survival, OS) was higher for current (HR: 1.78; 95% CI: 1.04–3.06), but not for former (HR: 1.06; 95% CI: 0.71–1.59) smokers. The associations of cigarette smoking with the study outcomes were higher among patients with ⩾40 pack-years of smoking (OS: HR: 1.72; 95% CI: 1.03–2.85; disease-free survival (DFS: HR: 1.99; 95% CI: 1.25–3.19), those who smoked ⩾30 cigarettes per day (DFS: HR: 1.80; 95% CI: 1.22–2.67), and those with microsatellite stable (MSS) or MSI-low tumours (OS: HR: 1.38; 95% CI: 1.04–1.82 and DFS: HR: 1.32; 95% CI: 1.01–1.72). Potential heterogeneity was noted for sex (DFS HR: 1.68 for men and 1.01 for women: P for heterogeneity=0.04), and age at diagnosis (OS: HR: 1.11 for patients aged <60 and 1.69 for patients aged ⩾60: P for heterogeneity=0.03).
Conclusions:
Pre-diagnosis cigarette smoking is associated with worsened prognosis among patients with CRC.
Similar content being viewed by others
Main
Despite the well-established connection between cigarette smoking and pre-mature mortality, >16% of Canadians over the age of 15 smoke. Smoking is clearly associated with malignancies in the respiratory tract (Botteri et al, 2008). The IARC 2009 monograph on smoking and cancer added the colorectum to the list of smoking-associated cancer sites (IARC, 2012); however, some recent evidence suggests that smoking is differentially associated with certain tumour molecular phenotypes of colorectal cancer (CRC), such as tumours that display microsatellite instability (MSI), BRAF V600E mutation, or the CpG island methylator phenotype (CIMP) (Samowitz et al, 2006; Curtin et al, 2009; Poynter et al, 2009; Limsui et al, 2010; Ogino et al, 2011; Nishihara et al, 2013).
The influence of smoking on CRC survival is inconclusive (Curtin et al, 2009; Limsui et al, 2010; Ogino et al, 2011). Some studies (Munro et al, 2006; Phipps et al, 2011) have suggested that cigarette use is strongly associated with reduced survival among CRC patients, whereas other studies have reported no significant differences in survival rates between smokers and never smokers with CRC (Yu et al, 1997; Rohan et al, 2000; Park et al, 2006). The apparent discrepancy between studies may be attributed to the long induction period of CRC (Botteri et al, 2008), as well as the potential for modulating effects of important prognostic variables (Newcomb et al, 2007; Guastadisegni et al, 2010; Shaukat et al, 2011), many of which were not accounted for in previous studies. For example, the MSI-high phenotype and the somatic BRAF V600E mutation are strongly associated with both smoking status (Limsui et al, 2010) and CRC prognosis (Samowitz et al, 2005; Guastadisegni et al, 2010; Shaukat et al, 2010), thus a potential interaction between smoking and these tumour phenotypes should be appreciated. However, to date, only one study has explored the potential interaction between smoking and molecular tumour phenotype on mortality among CRC patients. This study showed a prominent association between smoking and CRC-specific mortality among patients whose tumours exhibited the MSI-H phenotype (Phipps et al, 2011).
We investigated the association of smoking with all-cause (overall survival, OS) and disease-free survival (DFS) in an incident cohort of 750 invasive CRC patients from the province of Newfoundland and Labrador (NL), Canada. We further assessed potential interactions of smoking with sex, age at diagnosis, tumour stage at diagnosis, MSI status, and BRAF mutation status on mortality.
Subjects and methods
Study participants
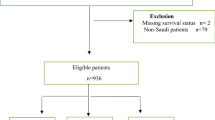
A detailed description of the study cohort has been published elsewhere (Woods et al, 2010). In brief, participants were incident CRC patients identified through the population-based Newfoundland and Labrador Colorectal Cancer Registry (NFCCR). Eligibility criteria included patients who were newly diagnosed with pathologically confirmed, invasive CRC (ICD-9 codes: 153.0–153.9, 154.0–154.3, and 154.8 or ICD-10 codes: 18.0–18.9, 19.9, and 20.9) from 1999 to 2003, and aged 20–75 years at the time of diagnosis. Seven hundred and fifty consenting patients (64% of all eligible patients) completed and returned detailed epidemiologic questionnaires (a personal history questionnaire (PHQ), a food frequency questionnaire (FFQ), and a family history questionnaire (FHQ)). Patients were also asked to donate a blood sample and for permission to access their archived tumour tissue and medical records. Exclusions from this analysis were made if patients had unknown clinical outcome or smoking status (n=41), or provided insufficient information on other critical prognostic factors (n=3). Thus, the final cohort consisted of 706 eligible participants. Ethics approval for this study was received from the Human Investigation Committee of Memorial University of Newfoundland.
Exposure assessment and baseline information collection
The PHQ and the FFQ were administered at baseline only, with a referent period of ‘2 years before diagnosis’ to capture pre-diagnosis information. The epidemiologic questionnaires included items regarding age, sex, marital status, education attainment, medical history, bowel screening history, physical activity, reproductive factors (women only), and alcohol and tobacco use. As baseline, participants were asked whether they had smoked at least one cigarette a day for 3 months or longer in their life. Participants who responded ‘yes’ were then asked about the age at which they started smoking, their usual number of cigarettes smoked per day, the duration (years/months) during which they smoked and, where applicable, the relevant information for when they quit smoking. For this analysis, cigarette smoking was represented by categories of smoking status (never, current, or former—with the referent period of 2 years before CRC diagnosis), years of smoking (none, <20, 20–29, and ⩾30), cigarettes daily (none, <20, 20–29, and ⩾30), years of abstention (non-smoker, <10, 10–29, and ⩾30), and lifetime cigarette pack-years (none, 20, 20–39, and ⩾40; calculated as the average number of cigarettes smoked per day divided by 20 and multiplied by the number of years smoked) (Zhao et al, 2010). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres. Clinical and pathologic data (e.g., tumour stage at diagnosis) were abstracted from pathology reports and medical records.
Study outcomes
The cohort was followed up for mortality and recurrence from the date of CRC diagnosis to April 2010. During this period, the FHQ was distributed to participants for the second time to collect information on additional cancer diagnosis and recurrence in their family. If a patient was deceased, then a close proxy was asked to participate (Woods et al, 2010). Information on vital status (i.e., death, recurrence, and metastasis) was gathered from follow-up questionnaires, local newspapers, death certificates, autopsy, pathology, radiology, surgical reports, as well as from physicians’ notes. Additional data were collected from the Dr H. Bliss Murphy Cancer Care Foundation (2012). For the purposes of this analysis, OS was the primary outcome, defined as time from CRC diagnosis to death from all causes. The secondary outcome, DFS, was measured from the date of cancer diagnosis to the date of death, recurrence, or metastasis (whichever came first). Patients who were still alive or who did not have a recurrence or a metastasis by the end of the follow-up period were censored at the time of last contact.
Molecular assessment
Molecular analyses for MSI and BRAF V600E mutation were performed using standard protocols as described previously (Loughrey et al, 2007; Raptis et al, 2007; Campbell et al, 2010). Briefly, for MSI analyses, both tumour DNA and normal DNA were amplified by PCR with a panel of 10 microsatellite markers: BAT25, BAT26, BAT40, BAT34C4, D5S346, D17S250, ACTC, D18S55, D10S197, and MYCL (Raptis et al, 2007; Campbell et al, 2010). The appearance of a discordant number of bands between tumour and normal DNA was interpreted as instability (Raptis et al, 2007; Campbell et al, 2010). Tumours were classified as MSI-high if 30% or more of the repeats were unstable and MS-stable/MSI-Low if less than 30% of the repeats demonstrated instability (Phipps et al, 2011). Exon 15 of the BRAF gene, spanning the mutational hotspot c.1799T>A (p.Val600Glu), was amplified by PCR using BRAF V600E allele-specific primers, followed by direct automatic sequencing to verify the mutations (Loughrey et al, 2007). Detailed descriptions of each assay, including the primer sequences and PCR conditions, are provided in earlier studies from this cohort (Loughrey et al, 2007; Raptis et al, 2007; Campbell et al, 2010; Woods et al, 2010).
Statistical analysis
Group comparisons were performed with Dunnett’s tests for continuous variables and Pearson’s chi-square tests of independence for categorical variables (McCleary et al, 2010). The Kaplan–Meier technique was applied to graphically delineate overall and stratified survival distributions (Thrift et al, 2011). Proportional hazards models were used to estimate the impact of smoking on mortality among CRC patients, while adjusting for covariates. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) were calculated for categories of exposure, using never smokers as the reference group. Subjects with missing data on any smoking exposure variable were only excluded for specific smoking-related variable analysis. In the selection approach of the multivariate models, we assessed an extensive list of potential confounders, including demographic variables, diet, lifestyle factors, treatment, clinicopathologic, and a series of molecular predictors. Factors were considered for inclusion in the multivariable Cox model if the log-rank test had a P-value of 0.2 or less in the univariate setting (Mantel, 1966). Only terms that entered the model at P<0.1, or altered the effect estimates by 10% or more, or improved the fit of the models were retained for the final models (Thrift et al, 2011). The final list of potential confounders included in the model was based on both backwards selection and the literature, including sex, age at diagnosis, BMI, stage at diagnosis, marital status, alcohol consumption, fruit intake, family history of CRC, reported chemoradiotherapy, and MSI status. The proportional hazards assumption was verified by checking the parallelism of the Kaplan–Meier curves and by testing the statistical significance of time-dependent covariates when included in the model (Statistical Consulting Group, 2012). Evidence of linear trends was tested by modelling ordinal variables of exposure as a continuous variable in a linear regression (Woodward, 2005; Zhao et al, 2010). Potential interactions were evaluated by including interaction terms between smoking and respective stratification variable in the model with the Wald test. Statistical significance was conducted at two-sided P<0.05. All calculations were performed with the SAS software version 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
By the end of the follow-up period, there was a maximum of 10.9 years of observation and 338 deaths from all causes. At baseline, 506 patients were ever smokers and 200 patients were non-smokers. Among those with available molecular data, MSI-high was observed in 11.4% (n=69) of 603 tumours. The BRAF V600E mutation was found in 65 (11.2%) of 576 CRCs.
Baseline characteristics by smoking status
Current smokers were slightly younger, leaner, consumed more alcohol, more likely to be men, and showed a greater proportion of MSI-high tumours compared with never smokers (21.5% vs 10.9%, P=0.03) (Table 1). Similarly, most former smokers were men, married or living as married, and reported greater alcohol consumption and less chemoradiotherapy use relative to never smokers.
Pre-diagnostic smoking and mortality
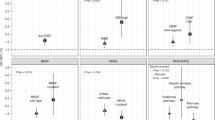
Current smoking (HR: 1.78; 95% CI: 1.04–3.06) was statistically significantly associated with higher risk of all-cause mortality in multivariable models (Table 2; Figure 1). The higher risks of mortality from smoking persisted in more detailed definitions of the exposure, including pack-years, cigarettes daily, and years of smoking, although, for the latter two variables, the risk estimates of being in the highest quartile of exposure did not quite attain statistical significance at the 0.05 level. Moreover, there was a stepwise gradient of decreasing risk of mortality with increasing years of abstention for former smokers (P for trend=0.03). Similarly, when DFS was the outcome, the HRs were elevated in the groups who had a smoking history of ⩾30 years (HR: 1.53; 95% CI: 1.01–2.34), individuals who smoked ⩾30 cigarettes per day (HR: 1.80; 95% CI: 1.22–2.67), and those with ⩾40 pack-years of smoking (HR: 1.99; 95% CI: 1.25–3.19). When the data were stratified by tumour site, smoking was associated with worse prognosis for patients diagnosed with colon cancer, but not for rectal cancer (Table 2).
Interactions between smoking and demographic or tumour characteristics
The multivariable models were repeated for smoking status between strata defined by demographic and tumour characteristics (Table 3). The P-values for heterogeneity were statistically significant between strata of sex for DFS (P=0.04) and age at diagnosis for OS (P=0.03). More specifically, the risk of mortality associated with ever smoking seemed limited to men (DFS: HR: 1.68; 95% CI: 1.16–2.44) and to patients aged ⩾60 years at CRC diagnosis (OS: HR: 1.69; 95% CI: 1.20–2.40). Although the interaction terms were not statistically significant, the impacts of smoking on mortality were more marked for patients diagnosed at earlier disease stages (OS: HR: 1.83; 95% CI: 1.07–3.14 and DFS: HR: 1.70; 95% CI: 1.04–2.78) than for those diagnosed at advanced stages (OS: HR: 1.19; 95% CI: 0.87–1.62 and DFS: HR: 1.16; 95% CI: 0.86–1.57), and for patients with microsatellite stable/MSI-low (MSS/MSI-low) tumours (OS: HR: 1.38; 95% CI: 1.04–1.82 and DFS: HR: 1.32; 95% CI: 1.01–1.72) than for those with cancers exhibiting MSI-high (OS: HR: 1.04; 95% CI: 0.28–3.95 and DFS: HR: 1.24; 95% CI: 0.37–4.14).
Discussion
We examined smoking status and gradients of smoking duration/intensity in relation to OS and DFS in a cohort of over 700 CRC patients. Pre-diagnostic smoking was associated with higher risk of all-cause mortality and poorer DFS. Evidence suggests that the association between smoking and decreased survival was restricted to patients diagnosed with colon, and not rectal, cancer. Our results are consistent with the findings from a recent study in Washington State (Phipps et al, 2011). Those authors reported that CRC patients who currently smoke have a significantly higher disease-specific (HR: 1.30; 95% CI: 1.09–1.74) and all-cause (HR: 1.51; 95% CI: 1.24–1.83) mortality than non-smokers. These authors also reported higher associations of smoking with mortality for patients diagnosed with colon cancer than for rectal cancer. Likewise, a study from the UK (Munro et al, 2006) on a cohort of CRC patients receiving curative surgery found a significantly worse cause-specific survival in active smokers compared with non-smokers (HR: 2.55; 95% CI: 1.40–4.64). Notably, current smoking, but not former smoking, was associated with poorer survival.
There are several biologic mechanisms that may explain the higher mortality among CRC patients who smoke. First, tobacco smoking may mutate the GSTM1 gene, resulting in impaired detoxification of tobacco carcinogens (McCleary et al, 2010); these carcinogens may exert growth promoting effects to residual tumour cells, either through resistance to chemotherapy or through promotion of angiogenesis (Ye et al, 2005; Munro et al, 2006). Smoking may also induce aberrant promoter DNA methylation, thus silencing regulatory genes (e.g., ECAD, p16, MGMT, and DAPK) in tumor progression (Russo et al, 2005). Possible explanations for the differential associations by subsite include the higher concentration of tobacco carcinogens in the colon and the longer contact time of tobacco constituent-carrying feces with the colon, where they are mainly stored, than the rectum (Batty et al, 2008).
In this study, cigarette smoking was associated with decreased survival only among male patients. It is important to note that the prevalence of smoking in men is higher than in women in Newfoundland and Labrador (Statistics Canada, 2013); and it is plausible that we were underpowered to detect smaller associations in women.
Our findings suggest that smoking has a negligible impact on survival for those diagnosed with advanced-stage disease, perhaps because patient outcomes are inherently poor for this patient population irrespective of smoking status. Intriguingly, smoking was observed to be significantly associated with poorer survival in patients diagnosed with early-stage disease, providing further support for the recommendation that newly diagnosed patients with less advanced CRC should consider immediate smoking cessation (Kobrinsky et al, 2003).
Smoking is strongly associated with specific somatic molecular alterations (e.g., MSI-high, CIMP, and the BRAF V600E mutation) (Curtin et al, 2009; Poynter et al, 2009; Limsui et al, 2010; Wish et al, 2010; Ogino et al, 2011; Nishihara et al, 2013). As these alterations are also related to OS, it is important to evaluate the influence of smoking on survival stratified by molecular features of tumour. However, we do not have CIMP status in this study. Our study is among the first to assess possible interactions between MSI status, BRAF V600E mutation status, and smoking on both OS and DFS for CRC patients. We found that ever smoking was associated with higher risk of mortality among patients diagnosed with MSS/MSI-low tumours, whereas smoking had little impact on patients diagnosed with MSI-high tumours (Table 3). MSI-high tumours generally have a more favourable prognosis relative to MSS/MSI-low tumours, independent of stage, grade, and other prognostic variables (Guastadisegni et al, 2010). To our knowledge, only one previous study on smoking and CRC survival has considered potential effect modification by molecular phenotypes of tumour (Phipps et al, 2011). Phipps et al (2011) reported a prominent association between smoking and disease-specific mortality in CRC patients with MSI-H tumours (HR: 3.83; 95% CI: 1.32–11.11). The reason for this discrepancy in results is unclear, but may relate to population differences or chance. These findings underscore the need for large, collaborative Molecular Pathological Epidemiology (MPE) studies of smoking and CRC aetiology to better understand the potential heterogeneous nature of smoking and colorectal carcinogenesis.
This study has both strengths and limitations. The relatively large sample size allowed us to perform stratified analyses. The availability of detailed information on personal, clinicopathologic, and molecular characteristics also allowed us to assess potential confounders, effect modifiers, and sources of potential heterogeneity. Limitations to this study include a lack of information on the cause of death for all deceased participants. The observed differences in OS and DFS could be deaths from causes other than CRC. However, the cause of death, classified according to the ICD codes, was obtained for 200 of 338 deceased patients in this cohort. Of these, the majority (86%) had died from CRC, which is in line with other studies (Jones et al, 2007; Riihimäki et al, 2012). Second, smoking is self-reported by respondents from the distant past, which leaves open the potential for recall bias; however, recent studies have generally shown strong agreement between smoking behaviours when reported over similar, and longer, time spans to the current study (Brigham et al, 2010). Self-reported cigarette consumption has been shown to be accurate in current smokers but may be under-reported in some never smokers (Martinez et al, 2004; McCleary et al, 2010); such misclassification should be non-differential and therefore bias the study results towards the null (McCleary et al, 2010). In addition, cigarette use after diagnosis was not updated in this study; hence, we were unable to assess the potential impacts of post-diagnosis changes in smoking habits on survival. Regardless, we were interested in the effect of pre-diagnosis exposures on survival among CRC patients. Finally, this study additionally involved analyses of associations stratified by tumour subtypes, thus increasing the probability of false positive findings committing a type I error due to multiple testing (Ogino et al, 2011); therefore, some results in this study should be taken as merely suggestive of potential biological or clinical associations. This underscores the need for our results to be confirmed in future large MPE studies (Ogino and Stampfer, 2010).
In conclusion, pre-diagnosis cigarette smoking was independently predictive of worse survival after CRC diagnosis. Results from this prospective, population-based study underscore the importance of cultivating healthy lifestyle habits. This study presents preliminary results concerning potential interactions between smoking, clinicopathologic features, tumour molecular phenotype, and CRC survival. Confirmation of these findings is needed in other large studies and further analyses using tobacco-specific DNA adducts as quantitative measurements of exposure are warranted.
Change history
04 March 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Batty GD, Kivimaki M, Gray L, Smith GD, Marmot MG, Shipley MJ (2008) Cigarette smoking and site-specific cancer mortality: testing uncertain associations using extended follow-up of the original Whitehall study. Ann Oncol 19 (5): 996–1002.
Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P (2008) Smoking and colorectal cancer: a meta-analysis. JAMA 300 (23): 2765–2778.
Brigham J, Lessov-Schlaggar CN, Javitz HS, Krasnow RE, Tildesley E, Andrews J, Hops H, Cornelius MD, Day NL, McElroy M, Swan GE (2010) Validity of recall of tobacco use in two prospective cohorts. Am J Epidemiol 172 (7): 828–835.
Campbell PT, Jacobs ET, Ulrich CM, Figueiredo JC, Poynter JN, McLaughlin JR, Haile RW, Jacobs EJ, Newcomb PA, Potter JD, Le Marchand L, Green RC, Parfrey P, Younghusband HB, Cotterchio M, Gallinger S, Jenkins MA, Hopper JL, Baron JA, Thibodeau SN, Lindor NM, Limburg PJ, Martinez ME (2010) Case-control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst 102 (6): 391–400.
Curtin K, Samowitz WS, Wolff RK, Herrick J, Caan BJ, Slattery ML (2009) Somatic alterations, metabolizing genes and smoking in rectal cancer. Int J Cancer 125 (1): 158–164.
Dr H. Bliss Murphy Cancer Care Foundation (2012) Building health together Available at http://www.cancercarefoundation.nl.ca/ (accessed on 18 July 2012).
Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer 46 (15): 2788–2798.
IARC (2012) Monograph on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer: Lyon, France.
Jones OM, John SK, Horseman N, Lawrance RJ, Fozard JB (2007) Cause and place of death in patients dying with colorectal cancer. Colorectal Dis 9 (3): 253–257.
Kobrinsky NL, Klug MG, Hokanson PJ, Sjolander DE, Burd L (2003) Impact of smoking on cancer stage at diagnosis. J Clin Oncol 21 (5): 907–913.
Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, Lynch CF, Anderson KE, French AJ, Haile RW, Harnack LJ, Potter JD, Slager SL, Smyrk TC, Thibodeau SN, Cerhan JR, Limburg PJ (2010) Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst 102 (14): 1012–1022.
Loughrey MB, Waring PM, Tan A, Trivett M, Kovalenko S, Beshay V, Young MA, McArthur G, Boussioutas A, Dobrovic A (2007) Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam Cancer 6 (3): 301–310.
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50 (3): 163–170.
Martinez ME, Reid M, Jiang R, Einspahr J, Alberts DS (2004) Accuracy of self-reported smoking status among participants in a chemoprevention trial. Prev Med 38 (4): 492–497.
McCleary NJ, Niedzwiecki D, Hollis D, Saltz LB, Schaefer P, Whittom R, Hantel A, Benson A, Goldberg R, Meyerhardt JA (2010) Impact of smoking on patients with stage III colon cancer: results from Cancer and Leukemia Group B 89803. Cancer 116 (4): 957–966.
Munro AJ, Bentley AH, Ackland C, Boyle PJ (2006) Smoking compromises cause-specific survival in patients with operable colorectal cancer. Clin Oncol (R Coll Radiol) 18 (6): 436–440.
Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, Limburg P, Lindor N, Potter JD, Templeton AS, Thibodeau S, Seminara D (2007) Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev 16 (11): 2331–2343.
Nishihara R, Morikawa T, Kuchiba A, Lochhead P, Yamauchi M, Liao X, Imamura Y, Nosho K, Shima K, Kawachi I, Qian ZR, Fuchs CS, Chan AT, Giovannucci E, Ogino S (2013) A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol 178 (1): 84–100.
Ogino S, Chan AT, Fuchs CS, Giovannucci E (2011) Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 60 (3): 397–411.
Ogino S, Stampfer M (2010) Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst 102 (6): 365–367.
Park SM, Lim MK, Shin SA, Yun YH (2006) Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol 24 (31): 5017–5024.
Phipps AI, Baron J, Newcomb PA (2011) Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle Colon Cancer Family Registry. Cancer 117 (21): 4948–4957.
Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, Limburg P, Young J, Le Marchand L, Potter JD, Cotterchio M, Casey G, Hopper JL, Jenkins MA, Thibodeau SN, Newcomb PA, Baron JA (2009) Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev 18 (10): 2745–2750.
Raptis S, Mrkonjic M, Green RC, Pethe VV, Monga N, Chan YM, Daftary D, Dicks E, Younghusband BH, Parfrey PS, Gallinger SS, McLaughlin JR, Knight JA, Bapat B (2007) MLH1 -93G>A promoter polymorphism and the risk of microsatellite-unstable colorectal cancer. J Natl Cancer Inst 99 (6): 463–474.
Riihimäki M, Thomsen H, Sundquist H, Hemminki K (2012) Colorectal cancer patients: what do they die of. Frontline Gastroenterol 3 (3): 143–149.
Russo AL, Thiagalingam A, Pan H, Califano J, Cheng KH, Ponte JF, Chinnappan D, Nemani P, Sidransky D, Thiagalingam S (2005) Differential DNA hypermethylation of critical genes mediates the stage-specific tobacco smoke-induced neoplastic progression of lung cancer. Clin Cancer Res 11 (7): 2466–2470.
Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, Wolff RK, Slattery ML (2006) Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst 98 (23): 1731–1738.
Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML (2005) Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res 65 (14): 6063–6069.
Shaukat A, Arain M, Anway R, Manaktala S, Pohlman L, Thyagarajan B (2011) Is KRAS mutation associated with interval colorectal cancers? Dig Dis Sci 57 (4): 913–917.
Shaukat A, Arain M, Thaygarajan B, Bond JH, Sawhney M (2010) Is BRAF mutation associated with interval colorectal cancers? Dig Dis Sci 55 (8): 2352–2356.
Statistical Consulting Group (2012) Introduction to SAS, UCLA: Academic Technology Services (accessed on 4 June 2012). Available at http://www.ats.ucla.edu/stat/examples/asa/test_proportionality.htm.
Statistics Canada (2013) Smokers, by Sex, Provinces and Territories (accessed on 11 November 2013). Available at http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/health74b-eng.htm.
Rohan TE, Jain M, Rehm JT, Ashley MJ, Bondy SJ, Ferrence RG, Miller AB (2000) Cigarette smoking and risk of death from colorectal cancer in women. Blackwell Science Ltd Colorectal Disease 2: 6.
Thrift AP, Nagle CM, Fahey PP, Russell A, Smithers BM, Watson DI, Whiteman DC (2011) The influence of pre-diagnostic demographic and lifestyle factors on oesophageal squamous cell carcinoma survival. Int J Cancer 131 (5): E759–E768.
Wish TA, Hyde AJ, Parfrey PS, Green JS, Younghusband HB, Simms MI, Fontaine DG, Dicks EL, Stuckless SN, Gallinger S, McLaughlin JR, Woods MO, Green RC (2010) Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev 19 (7): 1831–1839.
Woods MO, Younghusband HB, Parfrey PS, Gallinger S, McLaughlin J, Dicks E, Stuckless S, Pollett A, Bapat B, Mrkonjic M, de la Chapelle A, Clendenning M, Thibodeau SN, Simms M, Dohey A, Williams P, Robb D, Searle C, Green JS, Green RC (2010) The genetic basis of colorectal cancer in a population-based incident cohort with a high rate of familial disease. Gut 59 (10): 1369–1377.
Woodward M (2005) Epidemiology: Study Design and Data Analysis. Chapman & Hall/CRC: Boca Raton: LA.
Ye YN, Wu WK, Shin VY, Cho CH (2005) A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol 519 (1-2): 52–57.
Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP (1997) Smoking history and cancer patient survival: a hospital cancer registry study. Cancer Detect Prev 21 (6): 497–509.
Zhao J, Halfyard B, Roebothan B, West R, Buehler S, Sun Z, Squires J, McLaughlin JR, Parfrey PS, Wang PP (2010) Tobacco smoking and colorectal cancer: a population-based case-control study in Newfoundland and Labrador. Can J Public Health 101 (4): 281–289.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research Team Grant (CIHR-CPT79845) and Canadian Institutes of Health Research Team in Interdisciplinary Research on Colorectal Cancer Studentship (205835). Yun Zhu was supported by Master's fellowship from the Newfoundland and Labrador Centre for Applied Health Research and by a trainee award from the Beatrice Hunter Cancer Research Institute with funds provided by the Cancer Research Training Program as part of The Terry Fox Foundation Strategic Health Research Training Program in Cancer Research at CIHR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Zhu, Y., Yang, S., Wang, P. et al. Influence of pre-diagnostic cigarette smoking on colorectal cancer survival: overall and by tumour molecular phenotype. Br J Cancer 110, 1359–1366 (2014). https://doi.org/10.1038/bjc.2014.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.6
Keywords
This article is cited by
-
Smoking and colorectal cancer survival in relation to tumor LINE-1 methylation levels: a prospective cohort study
Epigenetics Communications (2022)
-
Association of rs2282679 A>C polymorphism in vitamin D binding protein gene with colorectal cancer risk and survival: effect modification by dietary vitamin D intake
BMC Cancer (2018)
-
Vitamin D receptor and calcium-sensing receptor polymorphisms and colorectal cancer survival in the Newfoundland population
British Journal of Cancer (2017)
-
Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression
Modern Pathology (2015)
-
Molecular pathological epidemiology gives clues to paradoxical findings
European Journal of Epidemiology (2015)