Abstract
Background:
In certain cancers, expression of CXCL16 and its receptor CXCR6 associate with lymphocyte infiltration, possibly aiding anti-tumour immune response. In other cancers, CXCL16 and CXCR6 associate with pro-metastatic activity. In the current study, we aimed to characterise the role of CXCL16, sCXCL16, and CXCR6 in ovarian cancer (OC).
Methods:
CXCL16/CXCR6 expression was analysed on tissue microarray containing 306 OC patient samples. Pre-treatment serum sCXCL16 was determined in 118 patients using ELISA. In vitro, (primary) OC cells were treated with an ADAM-10/ADAM-17 inhibitor (TAPI-2) and an ADAM-10-specific inhibitor (GI254023x), whereupon CXCL16 levels were evaluated on the cell membrane (immunofluorescent analysis, western blots) and in culture supernatants (ELISA). In addition, cell migration was assessed using scratch assays.
Results:
sCXCL16 independently predicted for poor survival (hazard ratio=2.28, 95% confidence interval=1.29–4.02, P=0.005), whereas neither CXCL16 nor CXCR6 expression correlated with survival. Further, CXCL16/CXCR6 expression and serum sCXCL16 levels did not associate with lymphocyte infiltration. In vitro inhibition of both ADAM-17 and ADAM-10, but especially the latter, decreased CXCL16 membrane shedding and strongly reduced cell migration of A2780 and cultured primary OC-derived malignant cells.
Conclusions:
High serum sCXCL16 is a prognostic marker for poor survival of OC patients, possibly reflecting ADAM-10 and ADAM-17 pro-metastatic activity. Therefore, serum sCXCL16 levels may be a pseudomarker that identifies patients with highly metastatic tumours.
Similar content being viewed by others
Main
Epithelial ovarian cancer (OC) is the most lethal gynaecologic cancer (Jemal et al, 2009). Chances of survival are highly dependent on a timely diagnosis before the cancer has spread beyond the ovaries. However, because of the relatively asymptomatic nature of early-stage OC, the disease usually is not recognised for an extended period of time (Vine et al, 2003). Hence, 70% of OC cases are detected in a late and metastatic disease stage, by which time survival rates have dropped to only 10%–30% (Hennessy et al, 2009). Metastasis is a multifactorial process with recent evidence emerging on the importance of chemokines and chemokine receptor interactions (Kruizinga et al, 2009; Barbieri et al, 2010).
Chemokines are a family of structurally similar proteins that are classified into four subfamilies, that is, the CXC, CC, C, and CX3C subfamily, based on the configuration of their N-terminal cysteine residues (Zlotnik and Yoshie, 2000). Among these, the chemokine CXCL16 and its cognate chemokine receptor CXCR6 is of particular interest. CXCL16 is normally expressed on macrophages, dendritic cells, monocytes, and B cells and serves as an adhesion molecule for neighbouring cells expressing CXCR6. In addition, CXCL16 is a scavenger receptor for oxidised low-density lipoproteins and phosphatidyl serines (Deng et al, 2010). CXCL16 has been found to be cleaved by metalloproteinases of the ADAM family, yielding a soluble form (sCXCL16; Abel et al, 2004; Gough et al, 2004; Schramme et al, 2008). This sCXCL16 has been detected in the serum of various types of cancer patients.
The physiological role of the CXCL16/CXCR6 axis in cancer is unclear, with pro-metastatic as well as anti-tumorigenic functions being reported. In prostate cancer, high expression of CXCL16 and CXCR6 as well as high serum sCXCL16 levels were associated with a more aggressive tumour phenotype (Lu et al, 2008; Wang et al, 2008; Darash-Yahana et al, 2009) and increased formation of metastases (Hu et al, 2008; Sung et al, 2008; Ha et al, 2011). Similarly, high serum levels of sCXCL16 in colorectal cancer patients were associated with recurrent liver metastasis and poor prognosis (Matsushita et al, 2012). In contrast, in a murine model of colorectal cancer the expression of CXCL16 inhibited formation of liver metastases by recruitment of CXCR6+ T cells and NK cells (Kee et al, 2013). The latter data corroborate with the finding that CXCL16 expression in colorectal cancer patients associated with an increased number of CD4+ and CD8+ tumour infiltrating lymphocytes and a better prognosis (Hojo et al, 2007). For OC, a microarray-based study reported that CXCR6 gene expression associated with tumour infiltration of CD8+ T cells and thus with better survival (Callahan et al, 2008). In contrast, high CXCL16 expression was associated with poor survival in a univariate analysis in an immunohistochemical study in 56 OC patients (Guo et al, 2011). Thus, the CXCL16/CXCR6 axis appears to have divergent effects in cancer, with as yet apparent contrasting reports in OC.
In the current study, we set out to evaluate whether the CXCL16/CXCR6 pair and/or serum sCXCL16 levels were associated with pro-metastatic or pro-inflammatory/anti-tumorigenic properties in OC. Our results indicate that CXCL16 and CXCR6 are not predictive of survival, nor associated with T-lymphocyte infiltration. In contrast, serum sCXCL16 proved to be an independent prognostic factor for poor survival in OC patients, although not associated with T-lymphocyte infiltration. In an OC cell line and primary OC-derived malignant cells, shedding of sCXCL16 was inhibited by ADAM10/ADAM-17 and ADAM-10-specific inhibitor. Inhibition of ADAM activity reduced cell migrating potential of OC cells in a scratch assay. These data suggest that serum sCXCL16 levels may be a pseudomarker for ADAM activity, reflecting a higher risk for metastases. Thus, determination of serum sCXCL16 levels might help to stratify patients into a high-risk group that may require more aggressive treatment.
Patients and Methods
Patient selection
Since 1985, the University Medical Center Groningen keeps a computerised database of patients with malignant epithelial OC treated at this hospital at any time point during the course of their disease, prospectively collecting information on clinicopathological characteristics and follow-up.
Tissue samples for tissue microarray construction were selected if primary surgery was performed by a gynaecological oncologist from the UMCG between May 1985 and June 2006 and if paraffin-embedded tumour tissue was available. Serum is also routinely collected and stored at −80 °C until further use. For the current study, serum was selected from patients for whom tumour tissue was also available. All serum samples were taken before primary surgery.
Patients were staged according to FIGO classification (Cancer Committee of the International Federation of Gynaecology and Obstetrics, 1986). Tumours were graded and classified according to WHO criteria by a gynaecological pathologist (Scully, 2004). Adjuvant chemotherapy generally consisted of different platinum-based treatment regimens. Response to chemotherapy was evaluated according to World Health Organization criteria (1979). After treatment, patients were followed-up for at least 10 years with gradually increasing intervals.
Information on clinicopathological characteristics and follow-up of patients was obtained from a computerised database in which information of all patients with epithelial OC treated at our institute is prospectively recorded. For the present study, relevant data were transferred into a separate anonymous database. In this separate database, patient identity was protected by study-specific, unique patient codes. In case of uncertainties with respect to clinicopathological and follow-up data, the larger databases could only be checked through two data managers who have daily responsibilities for the larger database, thereby ascertaining the protection of patients’ identity. According to Dutch law no approval from our IRB was needed.
Tissue microarray (TMA) construction
Tissue microarrays were constructed as previously described (Kononen et al, 1998; Rosen et al, 2004). For 306 patients, enough tumour tissue was available to construct a TMA. The majority of patients presented with advanced stage, serous, high-grade tumours. After surgery, 56% of all patients were optimally or completely debulked. Most patients received adjuvant platinum-based chemotherapy. The median survival for all patients was 42 months. Patient characteristics are summarised in Supplementary Data 1.
In brief, paraffin-embedded tissue blocks containing tumour in ovarian and omental tissue, and corresponding haematoxylin and eosin (H&E)-stained slides were retrieved from the pathology archives. Representative areas of tumour were marked on the H&E-stained slides. Next, using these H&E slides for reference, four 0.6 mm core biopsies were taken from each tumour specimen and arrayed on a recipient paraffin block using a tissue microarrayer (Beecher instruments, Silver Spring, Maryland, USA). Adhesion of cores to the recipient block was accomplished by placing the blocks in a 37 °C incubator for 15 min. For staining, 4 μm sections were cut from each TMA block. H&E staining was performed to verify the presence of tumour in the arrayed samples.
Immunohistochemistry of TMA sections
In brief, TMA sections were dewaxed in xylene and rehydrated using graded concentrations of ethanol to distilled water. After antigen retrieval, endogenous peroxidase activity was blocked by submersion of sections in a 0.3% H2O2 solution for 30 min. Sections were incubated with anti-CXCR6 (R&D systems, Minneapolis, MN, USA, clone 56811) and anti-CXCL16 (R&D systems, AF976) for 60 min at room temperature. Sections were subsequently incubated with Dako Envision+ for 30 min at room temperature (Dako, Glostrup, Denmark). The antigen–antibody reactions were visualised with 3,3′-diaminobenzidine. Sections were counterstained with haematoxylin. Furthermore, previously published CD8, CD45R0, and FoxP3 stainings were used in statistical analyses (Leffers et al, 2009).
To obtain a high concordance rate with whole tissue slides, the standard minimum of two cores per OC patient had to be present to be included in analysis. As an additional criterion, a minimum of 20% tumour tissue had to be present on the TMA for a sample to be entered into analysis (Rosen et al, 2004). For all stainings, the scoring system proposed by Ruiter et al (1998) was used. The intensity of staining was scored as 0, 1, 2, or 3, indicating absent, weak, positive, or strong positive expression, respectively. For further analysis, the mean of the all cores available per patient was taken as final score. In total, 273 patients (89%) were evaluable for CXCL16 expression and 268 patients (87.6%) for CXCR6 expression.
Cells lines and primary patient-derived OC samples
Ovarian cancer cell line A2780 (American Type Culture Collection, ATCC, Manassas, VA, USA) was cultured in RPMI 1640 (Lonza, Basel, Switzerland), supplemented with 10% fetal calf serum (FCS). Fresh tissue and ascites samples were collected at primary surgery. Tissues were minced and adherent cells were cultured in RPMI 1640 with 10% FCS. Informed consent was obtained for the collection and storage of tumour samples. All cells were cultured at 37 °C, in a humidified 5% CO2 atmosphere.
In vitro CXCL16, ADAM-10, and ADAM-17 detection
A2780 and primary OC cells were plated (25 000 cells per well, borosilicate chambered coverglass, Lab-Tek, Nalge Nunc International, Roskilde, Denmark) and subsequently cultured in the presence or absence of ADAM inhibitor TNF-α Protease Inhibitor-2 (TAPI-2, Enzo Life Sciences, Farmingdale, NY, USA) or GI254023x (Tocris Biosciences, Bristol, UK) at 100 μ M for 48 h. Culture supernatants and cell lysates were stored for ELISA’s and western blotting.
To determine CXCL16 levels by confocal microscopy, cells were stained with anti-CXCL16 (R&D systems, AF976) and appropriate secondary antibody (Invitrogen, Carlsbad, CA, USA, Alexa Fluor 488 Donkey Anti-Goat IgG (H+L)). To determine ADAM levels, cells were first fixed with 4% PFA and then stained with anti-ADAM-10 (Abcam, Cambridge, UK; Ab1997) or anti-ADAM-17 (Abcam, Ab57484) and Alexa Fluor® 488-conjugated secondary antibodies (Invitrogen, Goat Anti-Mouse IgG (H+L) and Goat Anti-Rabbit IgG (H+L)). Fluorescent intensity was quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA) (corrected total cell fluorescence).
Western blot analysis was performed using cell lysates of non-treated and treated cells. Proteins were separated by electrophoresis on a 12% acrylamide gel and subsequently transferred to a nitrocellulose membrane. After 1 h blocking with 5% ELK, antibody staining was performed using anti-CXCL16 (R&D systems AF976), anti-ADAM-10 (Abcam, Ab1997), and anti-ADAM-17 (Abcam, Ab57484; see Supplementary Data 4A for antibody testing). Appropriate secondary HRP-conjugated antibodies were purchased from Dako, and protein bands were visualised using Luminol-based enhanced chemiluminescence (Pierce, Thermo Scientific, Waltham, MA, USA; SuperSignal West Dura). As a control for protein loading, β-actin staining was performed (Abcam).
Flow cytometric analysis was performed on A2780 cells, which were treated as above described. Subsequently, cells were harvested using cell scrapers to prevent trypsin-mediated cleavage of CXCL16. Cells were stained with anti-CXCL16 (R&D systems AF976) and appropriate secondary antibody (Invitrogen, Alexa Fluor 488 Donkey Anti-Goat IgG (H+L)) and subsequently analysed on a BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and accessory CFlow Plus analysis software (BD Biosciences).
ELISA kits were purchased from R&D Systems (Quantikine human CXCL16 immunoassay, catalog number DCX160), and used according to the manufacturer’s instructions.
For qRT-PCR, total RNA was isolated from OC cells using Trizol (Invitrogen), and quantified by Nanodrop-1000 (Nanodrop Technologies, Wilmington, DE, USA). cDNA synthesis was performed according to manufacturer’s recommendations using the iScript cDNA synthesis kit (Bio-Rad, Richmond, CA, USA). Levels of cDNA targets were quantified with the TaqMan gene expression assay, using iTaq Universal SYBR Green Supermix (Bio-Rad), commercial primer/probe sets for Adam10 (Hs00153853_m1) and Adam17 (Hs01041915_m1; Invitrogen Life Technologies, Carlsbad CA, USA) and the ABI 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). All samples were run in duplicate (CT-value, s.d.<1) and values were corrected for the expression of housekeeping genes (B2M: Hs00984230_m1 and PPIA: Hs04194521_s1).
Scratch assays and cell proliferation assays
Scratch assays were used to assess in vitro motility of OC cells. To this end, A2780 and primary OC cells were plated (500 000 cells per six well) overnight and subsequently cultured in serum-free medium for an additional 48 h to obtain a complete monolayer. Then a scratch was made using a 200-μl pipette tip, and cells were incubated in the presence or absence of TAPI-2, GI254023x, or solvent control in media containing 10% FCS. Wound healing was visualised after 24 h of incubation with microscopy (EVOS, digital inverted microscope, Advanced Microscopy Group (AMG), Bothell, WA, USA), and the distance covered by the cells was determined using ImageJ software. Of note, for long-term inhibition activity, scratch size was measured every day until 96 h.
To exclude inhibiting effects on cell growth or cell viability by ADAM inhibition, A2780 cells were seeded in a 96-wells plate (5000 cells per well) and allowed to adhere overnight. Subsequently, TAPI-2 and GI254023x or solvent controls were added and incubated for an additional 48 h. Then cell growth/cell viability was assessed using MTS assays (CellTiter 96 AQueous One Solution Cell Proliferation, Promega, Madison, WI, USA). Of note, a positive ‘100% death control’ was included whereby cells were treated with 20% ethanol. OD490 of this death control was subtracted from all conditional ODs. Cell viability was calculated as percentage of medium control.
Statistics
Immunohistochemistry scores of CXCL16 and CXCR6 were dichotomised for statistical analysis, in which scores 0 and 1 were considered low expression, whereas scores 2 and 3 were considered high expression. When ovarian tissue was not available or lost during histological processing, omental tissue was used in statistical analysis. For sCXCL16 concentrations in serum, the median (3.35 ng ml−1) was used as cutoff point, considering <3.35 ng ml−1 serum sCXCL16 as low (score 0) and >3.35 ng ml−1 as high (score 1).
Associations between chemokines and clinicopathological characteristics were tested using logistic regression. Mann–Whitney U-tests were used to determine differences between chemokine (receptor) expression and tumour infiltrating lymphocyte counts. Disease-specific survival (DSS) was defined as date of surgery until death of OC, or date of last follow-up. DSS was estimated using the Kaplan–Meier method. Univariate and multivariate analysis were performed using the Cox proportional hazards model. Only variables associated with DSS in univariate analysis were entered into multivariate analysis. Associations in CXCL16 or sCXCL16 expression between medium and TAPI-2/GI254023x-treated samples were assessed using Wilcoxon signed rank tests.
For all tests, P-values <0.05 were considered significant (where indicated: *P<0.05; **P<0.01; ***P<0.001). All P-values were two-sided. Statistical analyses were performed using PASW 18.0.3 software package for Windows (IBM, Armonk, NY, USA) or Prism software (Graphpad, La Jolla, CA, USA).
Results
CXCL16 and CXCR6 are co-expressed and do not differ between primary tumour and metastases
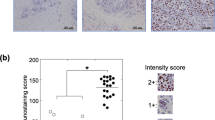
The expression of CXCL16 and CXCR6 was analysed on an in-house constructed TMA comprising material of 306 OC patients. In this TMA, CXCL16 and CXCR6 levels were scored according to low and high staining intensity (see Figure1A for representative examples). CXCL16 expression was low in 179 (65.6%) patients and high in 94 (34.4%) patients, whereas CXCR6 expression was low in 131 (48.9%) patients and high in 137 (51.1%) patients. Expression levels of CXCL16 and CXCR6 in tumour tissue were correlated (odds ratio=2.28, 95% confidence interval (CI)=1.36–3.82, P=0.002), indicating that CXCL16 and CXCR6 are often co-expressed. Of note, OC often spreads intraperitoneally, most notably to the omentum. The TMA contained paired ovarian and omental tumour samples for 111 patients, but no significant differences were detected between ovarian and omental CXCL16 and CXCR6 expression (Wilcoxon signed rank test, P=0.517, Figure 1B and C). Thus, CXCL16 and CXCR6 are often co-expressed and show no differential expression pattern between primary tumour and metastases.
CXCL16 and CXCR6 expression do not differ between primary tumour and metastases. (A) Representative examples of CXCL16 and CXCR6 staining. (I) CXCL16 weak, (II) CXCL16 strong, (III) CXCR6 weak, (IV) CXCR6 strong. (B) CXCL16 score between primary OC and omental tissue. (C) CXCR6 score between primary OC and omental tissue.
CXCL16 and CXCR6 expression do not associate with clinicopathological factors or tumour infiltrating lymphocytes
CXCL16 and CXCR6 expression did not associate with any of the well-known clinicopathological factors, such as age, stage, tumour grade, histology, and the presence of residual tumour after debulking surgery, although high expression of CXCL16 was more often seen in serous tumours (Table 1). Similarly, co-expression of CXCL16/CXCR6 was not associated with these factors (data not shown). In addition, in univariate analysis high or low CXCL16 and CXCR6 expression was not associated with survival (Figure 2A and B, respectively, Table 2). Earlier studies in colorectal cancer indicated that CXCL16/CXCR6 expression correlates with T-lymphocyte infiltration (Hojo et al, 2007). In our TMA, we previously identified that the presence of CD8+ CTLs and especially a high ratio between CD8+ CTLs and FoxP3+ Tregs predicts improved prognosis (Leffers et al, 2009). However, there was no association between CXCL16 or CXCR6 expression and tumour infiltrating CD8+ CTL, FoxP3+ Treg, or CD45R0+ memory T lymphocytes (Supplementary Data 2). Furthermore, the ratio between CD8+ CTLs and FoxP3+ Tregs did not differ between high and low CXCL16 or CXCR6 expression (Supplementary Data 3A and 3B), nor were differences detected between CXCL16:CXCR6 high/low groups (Supplementary Data 3C). This suggests that in this OC cohort, the CXCL16/CXCR6 chemokine/receptor pair does not directly modulate anti-tumour immune responses.
High serum sCXCL16 levels independently predicts poor survival in OC
The cleavage of the extracellular domain of CXCL16 into sCXCL16 has been previously reported for various types of cancer (Gough et al, 2004; Schramme et al, 2008). Therefore, serum levels of sCXCL16 were determined for 118 OC patients, of which pre-treatment serum samples were available. The concentration of sCXCL16 ranged from 0.02 to 6.66 ng ml−1, with a median of 3.35 ng ml−1. Notably, although sCXCL16 is generated by cleavage of CXCL16 from the cell membrane, no association between CXCL16 staining intensity and sCXCL16 concentration was detected (data not shown). In the evaluable OC patients, high pre-treatment levels of serum sCXCL16 associated with older age, high grade tumours, and >2 cm residual tumour after primary debulking (Table 1). More importantly, serum levels of sCXCL16 correlated with survival in OC patients, with high serum sCXCL16 being predictive of poor survival in univariate analysis (Figure 2C, Table 2). Specifically, patients with high sCXCL16 had a mean survival time of 58 months (95% CI=40.0–77.8), whereas patients with low serum sCXCL16 had an approximate three-fold increase in mean survival of 156 months (95% CI=123.5–188.6). A subsequent multivariate analysis with established prognostic factors as covariates revealed that high serum sCXCL16 levels retained its prognostic value, independent of age, stage, grade, histology, and residual tumour after debulking surgery (Table 2). Of note, when the survival analysis was performed in patients with high-stage disease (i.e., stage IIIb and IV), sCXCL16 retained its prognostic effect in both univariate (hazard ratio (HR)=2.09, 95% CI=1.25–3.49, P=0.005) and multivariate analysis (HR=2.24, 95% CI=1.25–4.03, P=0.007; data not shown). Therefore, the prognostic effect of sCXCL16 is independent and not due to an association with OC stage. Thus, sCXCL16 is an independent predictor of poor survival in OC patients.
In previous studies in colorectal cancer, serum sCXCL16 levels were found to associate with immune infiltration. However, in our cohort, the level of serum sCXCL16 did not correlate with the number of tumour infiltrating CD8+ CTL, FoxP3+ Treg, or CD45R0+ memory T lymphocytes (Supplementary Data 2), nor with the ratio between CD8+ CTLs and FoxP3+ Tregs (Supplementary Data 3D). Thus, the predictive value of serum sCXCL16 is not related to adaptive immune responses as identified in colorectal cancer.
CXCL16 shedding is inhibited by ADAM protease inhibitors
Serum CXCL16 did not associate with immune parameters in the tumours nor correlated with CXCL16/CXCR6 expression. This suggested that sCXCL16 was probably not associated with CXCL16/CXCR6 signalling itself, but may indirectly reflect other tumorigenic processes. In this respect, it has been described that CXCL16 can be cleaved by ADAM-10 and ADAM-17 thereby generating sCXCL16 (Gough et al, 2004; Schramme et al, 2008). In addition, ADAM protease activity is well known to be associated with increased metastatic behaviour. Hence, CXCL16 processing into sCXCL16 might be a pseudomarker for ADAM activity. To evaluate this possibility in vitro, A2780 OC cells were selected as model cell line, as these cells expressed CXCL16, ADAM-10, and ADAM-17 (Figure 3A, Supplementary Data 4A). Subsequently, A2780 cells were incubated with the ADAM-10/ADAM-17 inhibitor TAPI-2, as well as the ADAM-10-selective inhibitor GI254023x, as the level of expressed ADAM-10 was on average 9.8-fold higher on mRNA level compared with ADAM-17 (Supplementary Data 4C). In line with this, ADAM10 protein levels were abundant, whereas ADAM17 levels were minimally detectable (Figure 3A, see Supplementary Data 4B for complete blots). Incubation with the ADAM-10/ADAM-17 inhibitor TAPI-2, clearly upregulated the level of membrane-expressed CXCL16 as shown by immunofluorescent imaging, flow cytometry, and western blot analysis (Figure 3B and C, see Supplementary Data 4D for complete blots). In addition, GI254023x also prevented CXCL16 shedding from the cell membrane and was even more potent than TAPI-2 (Figure 3B and C). Similar to A2780, most OC cells from a small panel of primary patient-derived samples mainly expressed ADAM-10 over ADAM-17 (Figure 3D, Supplementary Data 4C). In line with this, treatment with both TAPI-2 and GI254023x highly increased CXCL16 levels on the cell surface of primary OC cells (Figure 3E and F). Correspondingly, the supernatant of A2780 and primary OC cell cultures contained high (ng ml−1) levels of sCXCL16 in standard culture conditions that was reduced upon TAPI-2 (43.9% inhibition) or GI254023x (89.7% inhibition) treatment (Figure 3G–I). Thus, sCXCL16 shedding from the membrane of OC cells is likely ADAM dependent, whereby ADAM-10 seems to be most important.
ADAM proteases regulate CXCL16 shedding. (A) Representative picture of CXCL16, ADAM-10, and ADAM-17 staining on the OC cell line A2780 as determined by fluorescent imaging (performed on PFA fixed cells) and western blot analysis. Conjugate controls were included to determine background fluorescence. (B) Levels of CXCL16 were determined on the OC cell line A2780 with or without TAPI-2/GI254023x (100 μ M) treatment, using confocal microscopy, flow cytometry, and western blot analysis. Of note, MFI of conjugate control used in flow cytometric analysis was 21 000. (C) Analysed CXCL16 levels (corrected total cell fluorescence (CTCF)) on A2780 cells (n=4) with or without TAPI-2/ GI254023x (100 μ M) treatment. Conjugate control (Alexafluor-488 Donkey anti-Goat) was used to determine background fluorescence. (D) Analysed ADAM-10 and ADAM-17 expression levels (CTCF) and background fluorescence with conjugate control on a small panel or primary derived OC cells (n=6). Depicted confocal images are a representative of ADAM-10 and ADAM-17 staining in primary OC. (E) Analysed CXCL16 levels (CTCF) on primary OC (n=11) with or without TAPI-2 treatment (100 μ M). Depicted confocal images are a representative of CXCL16 fluorescent staining on medium and TAPI-2-treated primary OC cells. (F) Analysed CXCL16 levels (CTCF) on primary OC (n=7) with or without GI254023x treatment (100 μ M). Depicted confocal images are a representative of CXCL16 staining on medium and GI254023x-treated primary OC cells. (G) sCXCL16 levels in supernatants of untreated vs TAPI-2/ GI254023x-treated (100 μ M) A2780 cells. (H) sCXCL16 levels in supernatants of untreated vs TAPI-2 (100 μ M)-treated primary OC cells (n=10). TAPI-2 inhibits 43.9% of all sCXCL16 release on average. (I) sCXCL16 levels in supernatants of untreated vs GI254023x (100 μ M)-treated primary OC cells (n=6). GI254023x inhibits 89.7% of all sCXCL16 release on average.
ADAM proteases regulate cell migrating potential of OC cells
To evaluate the functional consequences of ADAM activity, cell migration was assessed using scratch assays. In non-treated A2780 cells, the total scratch size was reduced by ∼50% within 24 h (Figure 4A), equaling an average distance covered of 105 μm (Figure 4B). In contrast, migration of TAPI-2 as well as GI254023x-treated A2780 cells was strongly inhibited (Figure 4A), covering, respectively, 60 and 50 μm of the scratch within 24 h (Figure 4B). In line with this, TAPI-2 and GI254023x reduced cell migration of primary patient-derived OC cells (Figure 4C and D). Of note, migration of primary OC showed a less structured migration pattern compared with the A2780 cell line (Figure 4A and C). The effect on cell migration by ADAM inhibition lasted up to at least 4 days (Figure 4E), and was not caused by the used solvents or a reduction in cell proliferation/cell viability (Figure 4B, D and F). These results suggest that active ADAMs positively regulate the migrating potential of OC cells.
ADAM proteases regulate migratory behaviour of ovarian cancer (OC) cells. (A) Representative picture of the scratch assay at t=0 vs t=24 h in medium or TAPI-2/ GI254023x (100 μ M)-treated A2780 cells. (B) Analysed distance covered by medium or TAPI-2/GI254023x (100 μ M)-treated A2780 cells or with solvent control (DMSO) within 24 h. (C) Representative picture of the scratch assay at t=0 vs t=24 h in medium or TAPI-2/ GI254023x (100 μ M)-treated primary OC cells. (D) Analysed distance covered by medium or TAPI-2/GI254023x (100 μ M)- or solvent control (DMSO)-treated primary OC cells within 24 h. (E) Long-term follow-up (4 days) of scratch size in medium or TAPI-2/GI254023x (100 μ M)-treated A2780 cells. (F) Effect of TAPI-2/GI254023x (100 μ M) or solvent control (DMSO) on A2780 cell proliferation/cell viability as determined by MTS assays after 48 h of incubation.
Discussion
The role of CXCL16 and CXCR6 in cancer is ambiguous and probably cancer type specific, as their expression has been associated with pro-metastatic as well as anti-tumorigenic functions. The current study, performed in a large cohort of 306 OC patients, indicated that neither expression of membrane CXCL16 nor expression of membrane CXCR6 is associated with survival. In contrast, pre-treatment serum levels of sCXCL16 independently predicted survival, showing reduced survival time in patients with high serum sCXCL16 levels. Of note, serum sCXCL16 levels did not correlate with tumour infiltration of immune cells. Furthermore, in vitro experiments showed that the inhibition of ADAM-10/17 prevented cleavage of CXCL16 from the membrane of (primary) OC cells, which is according to literature. In addition, ADAM inhibition in (primary) OC cells strongly reduced tumour cell migration. Therefore, elevated serum levels of sCXCL16 may be a pseudomarker for high ADAM activity and thereby predict a subset of patients with a more aggressive tumour.
In this cohort of OC patients, high serum sCXCL16 levels were predictive for poor survival. In addition to sCXCL16, tumour stage also associated with survival time. However, when survival analysis was performed in patients with high-stage disease (i.e., stage IIIb and IV), sCXCL16 retained its prognostic effect in both univariate and multivariate analysis. Therefore, the prognostic effect of sCXCL16 is independent and not due to an association with OC stage. This association of high serum sCXCL16 levels with poor survival in OC patients is in agreement with a previous study in colorectal cancer patients whereby high sCXCL16 serum levels also negatively correlated with survival (Matsushita et al, 2012). In addition, serum levels of sCXCL16 were elevated in prostate (Sung et al, 2008) and pancreatic (Wente et al, 2008) cancer patients compared with healthy controls. In contrast, no correlation was found between membrane CXCL16 levels and survival, although there was a modest association between CXCL16 and serous histology, an OC subtype with an unfavourable prognosis. This contrasts with a previous study in 56 OC patients in which CXCL16 associated with decreased survival (Guo et al, 2011). However, no multivariate analysis was performed and compared with our study, median follow-up was shorter. In addition, the included patients presented with more favourable clinicopathological characteristics, with a considerably better 5-year survival (70% vs 39%), which might influence outcome. On the other hand, CXCL16 expression was associated with improved prognosis in renal cell (Gutwein et al, 2009) and colorectal cancer (Hojo et al, 2007). This suggests that the effect of CXCL16 and CXCR6 on clinical outcome differs between tumour type and study design.
Previously, CXCL16 and CXCR6 were reported to be differentially expressed between primary tumor and metastases, with, for example, elevated CXCL16 and CXCR6 mRNA levels in bone and liver metastases compared with corresponding primary tumours in prostate cancer (Lu et al, 2008). Similarly, immunohistochemical analysis of breast cancer (Lin et al, 2009), nasopharyngeal carcinomas (Ou et al, 2006), and melanomas (Seidl et al, 2007) revealed CXCR6 expression in metastatic carcinoma, whereas primary tumours largely lacked CXCR6. In prostate cancer and renal and hepatocellular carcinoma, CXCL16 induced migration of CXCR6-expressing cell lines (Lu et al, 2008; Wang et al, 2008; Lin et al, 2009; Gao et al, 2012; Oldham et al, 2012). Moreover, it was demonstrated that CXCL16 treatment induced proliferation, migration, and invasive growth in CXCR6-expressing colorectal cancer cell lines (Matsushita et al, 2012). In this way, CXCL16/CXCR6 signalling contributes to detachment of tumour cells and promotes subsequent migration. In line with this, constitutive expression of CXCL16 and/or CXCR6 was found in many cancer cell lines, among which prostate (Hu et al, 2008; Lu et al, 2008; Ha et al, 2011), breast (Lin et al, 2009), nasopharyngeal (Ou et al, 2006), and pancreatic cancer (Wente et al, 2008). However, in our cohort, CXCL16 and CXCR6 expression levels did not differ between primary ovarian tumour tissue and omental metastases. The lack of such an association in OC could be due to the route of metastasis in OC. All other tumour types predominantly metastasise haematogenous or via lymph nodes. In contrast, omental metastases in OC arise from an exfoliative spread from the surface of the ovaries into the abdominal cavity. The detachment of tumour cells per se may thus be more important than the homing of tumour cells to a specific location, as is necessary in case of haematogenous metastasis.
In studies where high tumoural CXCL16 expression was associated with prolonged survival, the survival benefits were attributed to the T-lymphocyte attracting properties of CXCL16 (Hojo et al, 2007). However, we did not find an association between tumour infiltrating lymphocytes and sCXL16, CXCL16, or CXCR6. Therefore, high serum sCXCL16 levels are probably not associated with CXCL16/CXCR6 signalling itself, but may indirectly reflect other tumorigenic processes. In this respect, previous studies identified ADAM-10 and ADAM-17 to be responsible for CXCL16 cleavage (Gough et al, 2004; Schramme et al, 2008; Gutwein et al, 2009). Thus, serum levels of sCXCL16 might reflect tumour-associated ADAM activity. A similar association has previously been reported for ALCAM, an adhesion molecule also cleaved by ADAMs, with high serum sALCAM being predictive of poor survival (Carbotti et al, 2013). Indeed, in vitro inhibition of ADAM-10 and ADAM-17 enhanced CXCL16 expression on the membrane of (primary) OC cells and reduced sCXCL16 levels in culture supernatants. In addition, ADAM inhibition reduced cell migration of (primary) OC cells in a scratch assay. Of note, in all in vitro assays, the specific ADAM-10 inhibitor seemed to be more potent than the ADAM-10/ADAM-17 inhibitor. This is probably due to the expression levels of ADAMs, as A2780 cells as well as most primary OC cells mainly expressed ADAM-10 over ADAM-17. In addition, previous studies also showed that CXCL16 shedding was mainly caused by ADAM-10 and not by ADAM-17 (Gough et al, 2004; Gutwein et al, 2009). In line with this, selective ADAM-10 inhibition almost completely inhibited the release of sCXCL16, whereby 89.7% of CXCL16 shedding was blocked. Taken together, CXCL16 shedding from OC cells is likely regulated by ADAMs, especially ADAM-10, and inhibition of their activity inhibits the cell migrating potential of OC cells.
Based on these findings, high sCXCL16 levels in serum of OC patients may reflect higher ADAM activity and a more aggressive OC subtype. Indeed, expression of ADAM metalloproteinases promotes cancer cell growth, metastatic behaviour, and tumour progression (Mochizuki and Okada, 2007; Rocks et al, 2008). Of note, it has been shown that direct in vivo quantification of ADAM activity is complicated. Specifically, mRNA expression of ADAM-17 in OC was not representative of protein expression because of posttranslational modifications (Sinnathamby et al, 2011). Furthermore, measurement of ADAM activity is hampered by endogenous inhibitors, such as tissue inhibitor of metalloproteinases. Therefore, sCXCL16 may be a useful pseudomarker to predict an aggressive tumour type with higher ADAM activity in OC.
In conclusion, high pre-treatment serum sCXCL16 is a prognostic marker for poor survival in OC patients, possibly reflecting higher ADAM activity. Therefore, serum sCXCL16 levels may be a pseudomarker that identifies patients with more aggressive tumours.
Change history
18 March 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S, Ludwig A (2004) The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol 172: 6362–6372.
Barbieri F, Bajetto A, Florio T (2010) Role of chemokine network in the development and progression of ovarian cancer: a potential novel pharmacological target. J Oncol 2010: 426956.
Callahan MJ, Nagymanyoki Z, Bonome T, Johnson ME, Litkouhi B, Sullivan EH, Hirsch MS, Matulonis UA, Liu J, Birrer MJ, Berkowitz RS, Mok SC (2008) Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res 14: 7667–7673.
Cancer Committee of the International Federation of Gynaecology and Obstetrics (1986) Staging announchement: FIGO Cancer Committee. Gynecol Oncol 25: 383–385.
Carbotti G, Orengo AM, Mezzanzanica D, Bagnoli M, Brizzolara A, Emionite L, Puppo A, Centurioni MG, Bruzzone M, Marroni P, Rossello A, Canevari S, Ferrini S, Fabbi M (2013) Activated leukocyte cell adhesion molecule soluble form: a potential biomarker of epithelial ovarian cancer is increased in type II tumors. Int J Cancer 132: 2597–2605.
Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer DA, Pikarsky E, Karin M, Farber JM (2009) The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One 4: e6695.
Deng L, Chen N, Li Y, Zheng H, Lei Q (2010) CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim Biophys Acta 1806: 42–49.
Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, Yi Y, Shi JY, Shi GM, Ding ZB, Xiao YS, Zhao ZH, Zhou J, He XH, Fan J (2012) CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res 72: 3546–3556.
Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW (2004) A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol 172: 3678–3685.
Guo L, Cui ZM, Zhang J, Huang Y (2011) Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chin J Cancer 30: 336–343.
Gutwein P, Schramme A, Sinke N, bdel-Bakky MS, Voss B, Obermuller N, Doberstein K, Koziolek M, Fritzsche F, Johannsen M, Jung K, Schaider H, Altevogt P, Ludwig A, Pfeilschifter J, Kristiansen G (2009) Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur J Cancer 45: 478–489.
Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, Chung MK (2011) Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol Med Rep 4: 419–424.
Hennessy BT, Coleman RL, Markman M (2009) Ovarian cancer. Lancet 374: 1371–1382.
Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H, Takano Y, Yoshie O, Tsukada K, Saiki I (2007) High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res 67: 4725–4731.
Hu W, Zhen X, Xiong B, Wang B, Zhang W, Zhou W (2008) CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Sci 99: 1362–1369.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249.
Kee JY, Ito A, Hojo S, Hashimoto I, Igarashi Y, Tsukada K, Irimura T, Shibahara N, Nakayama T, Yoshie O, Sakurai H, Saiki I, Koizumi K (2013) Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncol Rep 29: 975–982.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4: 844–847.
Kruizinga RC, Bestebroer J, Berghuis P, de Haas CJ, Links TP, de Vries EG, Walenkamp AM (2009) Role of chemokines and their receptors in cancer. Curr Pharm Des 15: 3396–3416.
Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW (2009) Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 58: 449–459.
Lin S, Sun L, Hu J, Wan S, Zhao R, Yuan S, Zhang L (2009) Chemokine C-X-C motif receptor 6 contributes to cell migration during hypoxia. Cancer Lett 279: 108–117.
Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen X, Galson DL, Taichman RS, Zhang J (2008) CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res 6: 546–554.
Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, Inoue Y, Kusunoki M (2012) Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Ann Surg Oncol 19 (Suppl 3)): S518–S527.
Mochizuki S, Okada Y (2007) ADAMs in cancer cell proliferation and progression. Cancer Sci 98: 621–628.
Oldham KA, Parsonage G, Bhatt RI, Wallace DM, Deshmukh N, Chaudhri S, Adams DH, Lee SP (2012) T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol 61: 385–394.
Ou DL, Chen CL, Lin SB, Hsu CH, Lin LI (2006) Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. J Pathol 210: 363–373.
Rocks N, Paulissen G, El HM, Quesada F, Crahay C, Gueders M, Foidart JM, Noel A, Cataldo D (2008) Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 90: 369–379.
Rosen DG, Huang X, Deavers MT, Malpica A, Silva EG, Liu J (2004) Validation of tissue microarray technology in ovarian carcinoma. Mod Pathol 17: 790–797.
Ruiter DJ, Ferrier CM, van Muijen GN, Henzen-Logmans SC, Kennedy S, Kramer MD, Nielsen BS, Schmitt M (1998) Quality control of immunohistochemical evaluation of tumour-associated plasminogen activators and related components. European BIOMED-1 Concerted Action on Clinical Relevance of Proteases in Tumour Invasion and Metastasis. Eur J Cancer 34: 1334–1340.
Schramme A, bdel-Bakky MS, Kampfer-Kolb N, Pfeilschifter J, Gutwein P (2008) The role of CXCL16 and its processing metalloproteinases ADAM10 and ADAM17 in the proliferation and migration of human mesangial cells. Biochem Biophys Res Commun 370: 311–316.
Scully RE (2004) Histological typing of ovarian tumours. 2nd edn, 11-19. Ref Type: Generic.
Seidl H, Richtig E, Tilz H, Stefan M, Schmidbauer U, Asslaber M, Zatloukal K, Herlyn M, Schaider H (2007) Profiles of chemokine receptors in melanocytic lesions: de novo expression of CXCR6 in melanoma. Hum Pathol 38: 768–780.
Sinnathamby G, Zerfass J, Hafner J, Block P, Nickens Z, Hobeika A, Secord AA, Lyerly HK, Morse MA, Philip R (2011) ADAM metallopeptidase domain 17 (ADAM17) is naturally processed through major histocompatibility complex (MHC) class I molecules and is a potential immunotherapeutic target in breast, ovarian and prostate cancers. Clin Exp Immunol 163: 324–332.
Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, Lee JK, Amin MB, Lyles R, Johnstone PA, Marshall FF, Chung LW (2008) Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res 68: 9996–10003.
Vine MF, Calingaert B, Berchuck A, Schildkraut JM (2003) Characterization of prediagnostic symptoms among primary epithelial ovarian cancer cases and controls. Gynecol Oncol 90: 75–82.
Wang J, Lu Y, Wang J, Koch AE, Zhang J, Taichman RS (2008) CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Res 68: 10367–10376.
Wente MN, Gaida MM, Mayer C, Michalski CW, Haag N, Giese T, Felix K, Bergmann F, Giese NA, Friess H (2008) Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. Int J Oncol 33: 297–308.
World Health Organization (1979) Handbook for Reporting Results of Cancer Treatment. World Health Organization: Geneva.
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12: 121–127.
Acknowledgements
This work was supported by Dutch Cancer Society grants RUG 2009-4355/RUG2009-4542/RUG2011-5206/RUG2012-6209 (E.B), the Netherlands Organization for Scientific Research (E.B.), and the Melanoma Research Alliance (E.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Gooden, M., Wiersma, V., Boerma, A. et al. Elevated serum CXCL16 is an independent predictor of poor survival in ovarian cancer and may reflect pro-metastatic ADAM protease activity. Br J Cancer 110, 1535–1544 (2014). https://doi.org/10.1038/bjc.2014.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.55
Keywords
This article is cited by
-
Higher CXCL16 exodomain is associated with aggressive ovarian cancer and promotes the disease by CXCR6 activation and MMP modulation
Scientific Reports (2019)
-
Papillary renal cell carcinoma-derived chemerin, IL-8, and CXCL16 promote monocyte recruitment and differentiation into foam-cell macrophages
Laboratory Investigation (2017)
-
Prognostic impact of CXCL16 and CXCR6 in non-small cell lung cancer: combined high CXCL16 expression in tumor stroma and cancer cells yields improved survival
BMC Cancer (2015)