Abstract
Background:
Eribulin mesylate is a synthetic macrocyclic ketone analogue of Halichondrin B that has demonstrated high antitumor activity in preclinical and clinical settings. This phase I study aimed to determine the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and pharmacokinetics in combination with cisplatin (CP) in patients with advanced solid tumours.
Methods:
Thirty-six patients with advanced solid tumours received eribulin mesylate 0.7–1.4 mg m−2 and CP 60–75 mg m−2. Eribulin mesylate was administered on days 1, 8, and 15 in combination with CP day 1 every 28-day cycle. The protocol was amended after dose level 4 (eribulin mesylate 1.4 mg m−2, CP 60 mg m−2) when it was not feasible to administer eribulin mesylate on day 15 because of neutropenia; the treatment schedule was changed to eribulin mesylate on days 1 and 8 and CP on day 1 every 21 days.
Results:
On the 28-day schedule, three patients had DLT during the first cycle: grade (G) 4 febrile neutropenia (1.0 mg m−2, 60 mg m−2); G 3 anorexia/fatigue/hypokalemia (1.2 mg m−2, 60 mg m−2); and G 3 stomatitis/nausea/vomiting/fatigue (1.4 mg m−2, 60 mg m−2). On the 21-day schedule, three patients had DLT during the first cycle: G 3 hypokalemia/hyponatremia (1.4 mg m−2, 60 mg m−2); G 4 mucositis (1.4 mg m−2, 60 mg m−2); and G 3 hypokalemia (1.2 mg m−2, 75 mg m−2). The MTD and recommended phase II dose was determined as eribulin mesylate 1.2 mg m−2 (days 1, 8) and CP 75 mg m−2 (day 1), on a 21-day cycle. Two patients had unconfirmed partial responses (PR) (pancreatic and breast cancers) and two had PR (oesophageal and bladder cancers).
Conclusions:
On the 21-day cycle, eribulin mesylate 1.2 mg m−2, administered on days 1 and 8, in combination with CP 75 mg m−2, administered on day 1 is well tolerated and showed preliminary anticancer activity.
Similar content being viewed by others
Main
Eribulin mesylate is a structurally simplified synthetic macrocyclic ketone analogue of halichondrin B, an antimitotic agent that exhibits potent anticancer effects in both in vitro and in vivo models of cancer (Bai et al, 1991; Towle et al, 2001; Kuznetsov et al, 2004; Dabydeen et al, 2006). Eribulin mesylate inhibits microtubule dynamics through a novel mechanism that is distinct from those of other tubulin-targeted agents. Specifically, eribulin suppresses microtubule polymerisation without affecting microtubule depolymerisation, and sequesters tubulin into non-functional aggregates (Bai et al, 1991; Jordan et al, 2005). Preclinical studies showed that eribulin has potent antiproliferative activity against several cancer cell lines, including lung, breast, pancreatic, lymphoma, prostate, melanoma, and colorectal cancer (Towle et al, 2001, 2012). Budman et al, 2004, using the SK-BR3 cells, demonstrated synergistic effect of E7389 (halichondrin B analogue) in combination with cisplatin. In addition, eribulin produced tumor regression in a variety of human tumor xenograft models (Towle et al, 2001, 2012).
A first-in-human phase I study of eribulin mesylate conducted by the California Cancer Consortium established a maximum tolerated dose (MTD) of 1.4 mg m−2, given on days 1, 8, and 15 of a 28-day cycle. Responses included two partial responses (PR) (lung, bladder) and three minor responses (lung, breast, and thyroid), with 12 patients exhibiting stable disease as the best response, lasting a median of 4 months (range 2–14 months). Two dose-limiting toxicities (DLTs) occurred at 2.0 mg m−2 per week (one grade 3 and one grade 4 febrile neutropenia) and serious non-haematologic toxicities included hypoglycemia, hypophosphatemia, and fatigue (Synold et al, 2005). A subsequent phase I trial reported by Goel et al Enroled patients with advanced solid malignancies. Thirty-two patients were treated with eribulin mesylate on days 1, 8, and 15 every 28 days. The MTD was determined to be 1.0 mg m−2 and the principal DLT was neutropenia. Most frequent drug-related adverse events were grades 1–2 fatigue, nausea, and anorexia. Interestingly, eribulin mesylate exhibited a low incidence of neuropathy (Goel et al, 2009). Tan et al reported a phase I trial of eribulin mesylate administered to patients on days 1 and 8 every 21 days. Twenty-one patients with advanced solid tumours were enroled, and the MTD of eribulin mesylate was established at 2 mg m−2. Febrile neutropenia was the dominant DLT with all three patients in the 4 mg m−2 cohort experiencing it. (Tan et al, 2009). Eribulin mesylate is approved by Food and Drug Administration for the treatment of patients with metastatic breast cancer who were previously treated with anthracycline and taxane therapy and at least two prior regimens based on phase III EMBRACE trial (Cortes et al, 2011). The current label dose for eribulin mesylate is 1.4 mg m−2 on days 1 and 8 of a 21-day cycle. Preclinical studies of the combination of cisplatin and paclitaxel, showed that paclitaxel inhibited the repair of DNA adducts formed by platinum agents (Parker et al, 1993). Several clinical trials, which have evaluated various doses and treatment schedules for paclitaxel and cisplatin, have confirmed that the combination of paclitaxel and cisplatin has an enhanced antitumor effect (Gatzemeier et al, 1998). This led to the hypothesis that combining cisplatin and a microtubule interacting agent such as eribulin mesylate, which has a different mechanism of action from paclitaxel or docetaxel, has potential for increased anticancer activity. Eisai tested the ability of eribulin mesylate to interact with existing cytotoxic drugs in combination studies with carboplatin, gemcitabine, doxorubicin, and docetaxel. The in vitro combination of eribulin mesylate and carboplatin in NSCLC cell lines H23 and H522-T1 demonstrated additive affects (Eisai Medical Research Inc., 2007).
On the basis of these findings, the present study was designed to investigate the combination of eribulin mesylate and cisplatin with the objective to determine the MTD and DLT, safety, tolerability, pharmacokinetic (PK) profile, as well as tumor response.
Patients and methods
Patients
Eligible patients enroled in the study were at least 18 years old and had histologically confirmed malignancy that was metastatic or unresectable and for which standard curative or palliative measures did not exist. Patients were allowed two or fewer prior chemotherapy regimens for advanced disease. Prior neoadjuvant or adjuvant chemotherapy was allowed if received ⩾6 months before. Patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status ⩽2, life expectancy >3 months, and adequate function of all major organs (including bone marrow, liver, kidney, and lungs). Exclusion criteria included having received chemotherapy or investigational therapy within 4 weeks (6 weeks for nitrosoureas or mitomycin C) before study initiation, prior cumulative dose of cisplatin greater than 300 mg m−2, failure to successfully complete local therapy for brain metastasis and women who were pregnant or breast-feeding. Additional exclusion criteria included a positive human immune virus test, any medically uncontrolled cardiovascular illness, and preexisting grade 2 or higher neuropathy.
The protocol was approved by the Institutional Review Board at each participating institution. The study was conducted in accordance with the Declaration of Helsinki, and all the patients gave written informed consent before treatment.
Study design and dose escalation
Eribulin mesylate was supplied by Eisai Inc., and distributed by Cancer Therapy Evaluation Program/National Cancer Institute as 1 mg per vial (0.5 mg ml−1 per 2 ml fill) solution in ethanol/water (1 : 19). Patients received the study medication as an intravenous bolus (defined as administration within 5 min) on days 1, 8 and 15 of a 28-day cycle (cohorts 1–4) and on days 1 and 8 of a 21-day cycle (cohorts 5–6). Cisplatin was administered after eribulin mesylate as an intravenous infusion on day 1 every 28 days (initial cohorts) and every 21 days (later cohorts). Concomitant use of other medications or treatments was allowed, with the exception of antitumor-directed therapies or medications or substances with known or suspected drug–drug interactions with eribulin mesylate or cisplatin. Prophylactic use of colony-stimulating factors was permitted after cycle 1 of eribulin mesylate and cisplatin treatment, according to the American Society of Clinical Oncology guidelines.
The starting dose of eribulin mesylate in this study was based on a previous phase I study in which grade 3 toxicity first occurred at 0.5 mg m−2 and the MTD was established at 1.4 mg m−2 (Synold et al, 2005) In the current phase I combination study, the starting dose of eribulin mesylate was 0.7 mg m−2 and of cisplatin was 60 mg m−2. Dose escalation was conducted using a standard 3+3 design. If none of three patients experienced a DLT, three additional patients were treated at the next dose level. If a DLT attributable to the study drugs was experienced in exactly 1/3 patients in the first cycle, three more patients (for total of 6) were treated at that dose level. If no additional DLT was observed at the expanded dose level (i.e., 1/6 experienced a DLT), the dose was escalated. Escalation stopped as soon as two or more patients experienced a DLT attributable to the study drugs in the first cycle at the given dose level. The MTD was based on toxicities observed during the first cycle and defined as the highest dose tested in which fewer than 33% of patients experienced a DLT attributable to the study drug(s), when at least six patients were treated at that dose and evaluable for toxicity.
Clinical assessment
Medical histories and demographic data were collected at screening. Physical examinations and laboratory tests were done at screening and then weekly. Imaging studies of involved cancer sites were done within 4 weeks prior to enrolment and every two cycles. Response was assessed using Response Evaluation Criteria in Solid Tumours (Therasse et al, 2000). Adverse events were evaluated and graded using the National Cancer Institute Common Toxicity Criteria, version 3.0., until 30 June 2011, and version 4.0 thereafter. Relationship to study treatment, as assessed by investigators, was also evaluated. A DLT was defined as any ⩾grade 3 treatment-related non-haematologic toxicity (excluding alopecia, controllable nausea and vomiting, and serum triglycerides <1500 mg dl−1 that recovered within 1 week), ⩾grade 4 thrombocytopenia, ⩾grade 4 febrile neutropenia requiring hospitalisation, or treatment delay of >2 weeks as a result of unresolved toxicity during the first cycle of therapy.
Pharmacokinetic methods
Patients were asked to give a total of 14 blood samples (7 ml of heparinised blood per sample) over a 7-day period after the first doses of eribulin mesylate and cisplatin. Blood samples were kept on ice and processed within 1 h of phlebotomy by centrifugation (1500 r.p.m., 10 min, 4 °C). Plasma was transferred to appropriately labelled polypropylene tubes and stored at <−70 °C until analysis.
Eribulin mesylate concentration in plasma was determined using an liquid chromatography-tandem mass spectrometry assay method developed and validated in the City of Hope Analytical Pharmacology Core facility. Briefly, after the addition of an internal standard (ER-076349) and acidification with hydrochloric acid, plasma was extracted with six volumes of dichloromethane. The organic phase was evaporated to dryness, reconstituted in mobile phase, and analysed by gradient liquid chromatographic separation on a C18 column with tandem mass spectrometry detection. Recoveries of eribulin mesylate and internal standard from plasma and urine by this method were >60%. The mass spectrometry settings were as follows: capillary voltage=2.90 kV, cone voltage=58 V, collision cell voltage =18 V, source temperature =125 °C, desolvation temperature=300 °C, cone gas flow=150 l h−1, and desolvation gas flow =550 l h−1. The mass transitions monitored for eribulin mesylate and the internal standard were 730.5→712.5 and 731.6→681.4 m/z, respectively. The intra- and inter-day precision and accuracy of the method were within±10% of target values over the entire range of the standard curve, with a lower limit of quantitation of 0.1 ng ml−1 from a starting sample volume of 0.2 ml.
Platinum concentrations in plasma for evaluation of cisplatin PKs were also determined in the Analytical Pharmacology Core facility at City of Hope, using a validated atomic absorption assay as previously described (Synold et al, 2007). The method has a limit of quantification of 10 ng ml−1 and an intra-day and inter-day precision and accuracy within±10%.
PK data analyses were performed using non-compartmental methods according to the rule of linear trapezoids. Individual PK parameter estimates (e.g., Cmax, Vss, CLsys, t1/2, and area under the curve (AUC)) were determined for eribulin mesylate and cisplatin for each patient and tabulated by dose level with summary statistics (means and coefficients of variation).
Results
Demographics and treatment
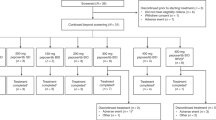
Thirty-six patients with advanced solid malignancies were enroled on the study. All the patients were eligible for toxicity and response evaluation. Demographic and baseline characteristics are summarised in (Table 1). The median patient age was 61 (range: 24–76) years. Primary tumor types included lung cancer (nine patients), gastrointestinal cancer (10 patients), head and neck (six patients), breast cancer (four patients), adrenal gland (two patients) and renal, bladder, ovary, liver, sarcoma (one patient each). Nine patients had not received prior chemotherapy and 11 patients had one prior chemotherapy regimen. The majority of patients had an Eastern Cooperative oncology group value of 0–1 (92%).
Patients received treatment in two different dosing schedules (Table 2). Twenty three patients treated on dose levels 1–4, received eribulin mesylate on days 1, 8 and 15, every 28 days, in combination with cisplatin on day 1. The protocol was amended after dose level 4 (cisplatin 60 mg m−2, eribulin mesylate 1.4 mg m−2), when it was not feasible to administer eribulin mesylate on day 15 because patients developed neutropenia. The treatment schedule was changed to eribulin mesylate administered on days 1 and 8 and cisplatin on day 1, every 21 days. Thirteen patients (dose levels 5–6) were treated on the 21-day schedule. Reasons for discontinuation of study treatment included progressive disease (23 patients), toxicity (four patients), physician discretion (five patients), and withdrawal of consent (four patients).
Dose-limiting toxicities and maximum tolerated dose
Among patients treated on the 28 day schedule with eribulin mesylate on days 1, 8 and 15, and cisplatin on day 1, a DLT of grade 4 febrile neutropenia was observed in one patient treated at dose level 2 (eribulin mesylate 1.0 mg m−2, cisplatin 60 mg m−2). One patient treated at dose level 3, (eribulin mesylate 1.2 mg m−2, cisplatin 60 mg m−2) experienced grade 3 anorexia, grade 3 hypokalemia and grade 3 fatigue. A DLT of grade 3 stomatitis and fatigue was observed in one patient at dose level 4 (eribulin mesylate 1.4 mg m−2, cisplatin 60 mg m−2) (Table 3).
Due to neutropenia, frequently limiting the ability to deliver day 15 eribulin mesylate, and a desire to avoid long delays between cisplatin treatments, the protocol was amended at this point and six patients were treated at dose level 5 on the 21-day schedule (eribulin mesylate 1.4 mg m−2, cisplatin 60 mg m−2). Two of these patients experienced DLTs (one grade 3 hypokalemia/hyponatremia and one grade 3 mucositis). Per protocol design, because two of six patients experienced a DLT at dose level 5, the eribulin mesylate dose was reduced one level to 1.2 mg m−2 and as a review of the data on the 28-day schedule already demonstrated the 21-day schedule at 1.2 mg m−2 day 1, 8, and cisplatin at 60 mg m−2 was well tolerated, the cisplatin dose was escalated to 75 mg m−2. One of six patients at dose level 6 (eribulin mesylate 1.2 mg m−2, cisplatin 75 mg m−2) had grade 3 hypokalemia. Therefore, the MTD was determined to be eribulin mesylate 1.2 mg m−2 and cisplatin 75 mg m−2. Notably, all DLTs were observed in patients exposed to at least two prior lines of systemic chemotherapy, including two prior regimens for advanced disease and prior adjuvant and neoadjuvant therapies (Table 3).
Adverse events
The most frequently reported adverse events (all grades) that were considered possibly or probably related to the study drugs were neutropenia (78%), anaemia (58%), and fatigue (39%) (Table 4). Grades 3 or 4 toxicities that were considered related to study treatment included neutropenia (25 patients), hypokalemia (seven patients), febrile neutropenia (four patients), anaemia (four patients), nausea (four patients), fatigue (four patients), hyponatremia (four patients), mucositis (three patients), anorexia (two patients), low platelets (two patients), with one patient each with diarrhoea, low albumin, elevated bilirubin, dysphagia, low magnesium, infection, infarct, low phosphate, thrombosis, and vomiting. Clinical manifestations of the neuropathy, including numbness, tingling, paraesthesia, hypostesia, or sensory loss, which were considered to be possibly or probably related to the study drugs, were observed in three patients (grade 2). There were no hypersensitivity reactions and no treatment related deaths on study.
Pharmacokinetics
Thirteen patients were evaluated for the first dose eribulin mesylate and cisplatin PKs. Eribulin PK parameters are summarised by dose in (Table 5). Because of the narrow dose range, small number of patients per group, and wide inter-patient PK variability, the mean eribulin and cisplatin Cmax and AUC0-inf did not increase in a dose-proportional manner. The mean eribulin systemic clearance (CLsys) when given at a dose of 1.2 mg m−2 together with 60 or 75 mg m−2 cisplatin, or at a dose of 1.4 mg m−2 given with 60 mg m−2 cisplatin was 1.6±0.7, 2.2±0.2, and 2.3±0.9 l h−1, respectively. The mean eribulin Vss values were 51.4±25, 68.4±27, and 77.7±36 l, respectively. The mean plasma terminal elimination half-lives were 40.8±11 l and 35.5±5, and 36.5±10 h, respectively.
Clinical outcome/antitumor activity
Among the 36 evaluable patients, there were four PR (one patient with pancreatic cancer and two prior therapies, one patient with inflammatory breast cancer who had not received prior therapy, one patient with oesophageal carcinoma and two prior cytotoxic therapies, and one patient with bladder transitional cell carcinoma and 1 prior chemotherapy. Twelve patients experienced stable disease (five patients with non-small-cell lung cancer, five with head and neck carcinomas, one with ovarian adenocarcinoma, and one with pancreatic adenocarcinoma). The median number of weeks on treatment for all 36 patients was 15 (range 2–32), and the median number of cycles at the MTD was 4.5 (2–8), corresponding to 19 weeks (6–28). The median number of weeks on treatment received by patients with PR or SD was 23 (range 11–32) (Table 6).
Discussion
This study has established a recommended phase II dose of eribulin mesylate 1.2 mg m−2 given as an intravenous bolus on days 1 and 8 in combination with cisplatin 75 mg m−2 on day 1 of a 21-day cycle in patients with advanced solid tumours. The observed DLTs were related to febrile neutropenia, hypokalemia, fatigue, hyponatremia, anorexia, stomatitis, and mucositis. On the third dose level of the initial 28-day treatment schedule (eribulin mesylate administered on days 1, 8, and 15), neutropenia-related treatment delays resulted in three of nine patients being inevaluable for dose-escalation decisions because of the inability to administer the third dose of eribulin mesylate while maintaining cisplatin dosing on time. Neutropenia has been reported in clinical trials with eribulin mesylate, and this finding is consistent with preclinical observations of reversible bone marrow toxicity that was also dose limiting in rats and dogs (Tosca et al, 2008; Goel et al, 2009; Tan et al, 2009). Eribulin mesylate has been studied as a single agent in phase II trials in patients with breast and lung cancer, for which patients were initially treated with 1.4 mg m−2 on days 1, 8, and 15 of a 28-day cycle. In these studies, many patients were unable to received eribulin mesylate on day 15 because of neutropenia (20). Our study was amended to administer eribulin mesylate on days 1 and 8 of a 21-day cycle. Anaemia was the second most common haematologic toxicity occurring in our study, and was expected because both cisplatin and eribulin mesylate are bone marrow suppressing cytotoxic agents. The observed hypokalemia most likely occurred secondary to cisplatin-induced renal potassium loss, and responded well to potassium supplementation (Fillastre and Raguenez-Viotte, 1989). Fatigue (39%), anorexia (25%), low potassium (22%), mucositis (19%), and vomiting (17%) were the most common non-haematologic toxicities, and this side-effect profile is comparable with that reported previously with eribulin mesylate, as well as cisplatin used as a single agent or in combination (Gatzemeier et al, 1998; Cortes et al, 2011). Notably, neuropathy, an adverse event that is expected of a tubulin-targeting agent, was not a common toxicity on this study (Lee and Swain, 2006). Only three patients experienced grade 2 neuropathy, which occurred during cycles 5–8 of treatment. One patient who experienced neuropathy had received prior oxaliplatin, which possibly increased the risk. Peripheral neuropathy is a major DLT associated with platinum agents commonly used in the clinic. Among the platinum compounds, cisplatin is the most neurotoxic, inducing mainly peripheral sensory neuropathy (Amptoulach and Tsavaris, 2011). In this phase I study, combining two neurotoxic drugs did not increase incidence of neuropathy. However, the median number of cycles patients received in this study was 3, and it is possible that longer exposure to both agents would increase the incidence and severity of neurotoxicity. The observation that all DLTs occurred in patients who had received two or more prior cytotoxic regimens may suggest that the dose of this combination may need to be refined in future studies depending on the patient population. We are also presenting this information to help guide others with related phase I designs as they consider eligibility criteria and the goals of a study.
Although efficacy was not a specified end point of this study, we observed PR in two patients (one with oesophageal cancer and one with transitional bladder carcinoma). Another two patients (1 with pancreatic cancer and one with breast cancer) had unconfirmed PR. Thirteen patients who had a variety of solid malignancies achieved stable disease. Eleven patients with lung, head and neck, ovarian, and pancreatic cancer exhibited stable disease for 18 weeks or more (Table 6). These results support the development of this combination for treatment of solid tumours.
An additional objective of this study was to evaluate the PK profile of eribulin mesylate and cisplatin when used in combination. Thirteen patients were evaluated for first-dose eribulin and cisplatin PKs, and there was no apparent effect of cisplatin co-administration on the disposition of eribulin mesylate when compared to previously reported single agent data (Goel et al, 2009; Tan et al, 2009). Because renal excretion does not represent an important route of elimination for eribulin mesylate (<8% of an administered dose is recovered in urine), it is not surprising that cisplatin does not alter eribulin mesylate clearance.
In conclusion, eribulin mesylate administered as an intravenous bolus on days 1 and 8 in combination with cisplatin appears well tolerated and shows preliminary anticancer activity. Overall, the manageable tolerability profile and the encouraging activity in this dose finding study support further clinical development of this combination for treatment of solid tumours. Since most DLTs observed in this study occurred in patients who had at least two prior lines of chemotherapy, we postulate that in chemotherapy naive patients, delivering full doses of eribulin mesylate and cisplatin is feasible. Further studies of this combination in front-line therapy for bladder, breast, pancreatic, head and neck, and lung cancer are warranted.
Change history
09 December 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Amptoulach S, Tsavaris N (2011) Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract 2011: 843019.
Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E (1991) Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem 266 (24): 15882–15889.
Budman DR, Calabro A, Littlefield BA (2004) Synergistic combinations of E7389 (Halichondrin B analogue) with conventional agents: in vitro median effect analysis in cell lines with potential clinical implications. In: 27th Ann San Antonio Breast Cancer Symp [abst 6055].
Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C (2011) Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377 (9769): 914–923.
Dabydeen DA, Burnett JC, Bai R, Verdier-Pinard P, Hickford SJ, Pettit GR, Blunt JW, Munro MH, Gussio R, Hamel E (2006) Comparison of the activities of the truncated halichondrin B analogue NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol Pharmacol 70 (6): 1866–1875.
Eisai Medical Research Inc (2007) E7389 Investigator's Brochure.
Fillastre JP, Raguenez-Viotte G (1989) Cisplatin nephrotoxicity. Toxicol Lett 46 (1-3): 163–175.
Gatzemeier U, Von Powel J, Lee JS (1998) Phase III comparative study of high dose cisplatin vs. combination of paclitaxel and cisplatin in with advanced NSCLC. Proc Amer Soc Clin Oncol 17: 555a.
Goel S, Mita AC, Mita M, Rowinsky EK, Chu QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, Mani S, Takimoto CH (2009) A phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res 15 (12): 4207–4212.
Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L (2005) The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther 4 (7): 1086–1095.
Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA (2004) Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analogue E7389. Cancer Res 64 (16): 5760–5766.
Lee JJ, Swain SM (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol 24 (10): 1633–1642.
Parker RJ, Dabholkar MD, Lee KB, Bostick-Bruton F, Reed E (1993) Taxol effect on cisplatin sensitivity and cisplatin cellular accumulation in human ovarian cancer cells. J Natl Cancer Inst Monogr, (15): 83–88.
Synold TW, Morgan RJ, Newman EM, Lenz HJ, Gandara DR, Colevas AD, Lewis MD, Doroshow JH (2005) A phase I pharmacokinetic and target validation study of the novel anti-tubulin agent E7389: a California Cancer Consortium trial. J Clin Oncol 23 (16): 200S–200S.
Synold TW, Takimoto CH, Doroshow JH, Gandara D, Mani S, Remick SC, Mulkerin DL, Hamilton A, Sharma S, Ramanathan RK, Lenz HJ, Graham M, Longmate J, Kaufman BM, Ivy P National Cancer Institute Organ Dysfunction Working G (2007) Dose-escalating and pharmacologic study of oxaliplatin in adult cancer patients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res 13 (12): 3660–3666.
Tan AR, Rubin EH, Walton DC, Shuster DE, Wong YN, Fang F, Ashworth S, Rosen LS (2009) Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumours. Clin Cancer Res 15 (12): 4213–4219.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumours. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92 (3): 205–216.
Tosca PJ, Bollinger LH, Merill JW (2008) Preclinical toxicology studies for halichondrin B macrocyclic ketone analogue E3789 (NSC – 707389) in beagle dogs and rats [abstract 5422]. Proc Am Assoc Cancer Res 43: 1095.
Towle MJ, Nomoto K, Asano M, Kishi Y, Yu MJ, Littlefield BA (2012) Broad spectrum preclinical antitumor activity of eribulin (Halaven(R)): optimal effectiveness under intermittent dosing conditions. Anticancer Res 32 (5): 1611–1619.
Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, Habgood GJ, Singer LA, Dipietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA (2001) In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res 61 (3): 1013–1021.
Acknowledgements
A California Cancer Consortium trial sponsored by the National Cancer Institute Cancer Therapy Evaluation Program. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U01CA062505/UM1CA186717 (City of Hope), P30CA33572 (City of Hope, including work performed in the Analytical Pharmacology Core), P30 CA014089 (University of Southern California) and P30 CA93373 (University of California, Davis). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was previously reported at ASCO 2013, (Abstract # 2564).
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Koczywas, M., Frankel, P., Synold, T. et al. Phase I study of the halichondrin B analogue eribulin mesylate in combination with cisplatin in advanced solid tumors. Br J Cancer 111, 2268–2274 (2014). https://doi.org/10.1038/bjc.2014.554
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.554
Keywords
This article is cited by
-
Eribulin, trastuzumab, and pertuzumab as first-line therapy for patients with HER2-positive metastatic breast cancer: a phase II, multicenter, collaborative, open-label, single-arm clinical trial
Investigational New Drugs (2019)
-
A phase I combination dose-escalation study of eribulin mesylate and gemcitabine in patients with advanced solid tumours: a study of the Princess Margaret Consortium
British Journal of Cancer (2015)