Abstract
Background:
Anti-HER2/neu therapy is well-established in breast and gastric carcinoma. The increased understanding of this pathway led to the identification of new promising drugs in addition to trastuzumab, offering further perspectives. The role of HER2/neu in colorectal carcinoma is controversially discussed, as discrepant data has been reported.
Methods:
Here, we retrospectively assessed the prevalence of HER2/neu positivity in a large series of colorectal carcinoma, testing HER2/neu status according to current recommendations. We correlated the results to clinico-pathological data and patient survival.
Results:
Overall, in 1645 primary colorectal carcinoma cases, 1.6% of the cases were HER2/neu positive. HER2/neu positivity significantly correlated with higher UICC stages (P=0.017) and lymph node metastases (P=0.029). In the subgroup of sigmoideal and rectal carcinomas, positive HER2/neu status was associated with T-category (P=0.041) and higher UICC stages (P=0.022). Although statistically not significant, HER2/neu-positive colorectal carcinomas displayed a tendency to poorer overall survival.
Conclusions:
These results illustrate the importance of testing HER2/neu by approved diagnostic techniques and scoring systems. We assume that although the prevalence of HER2/neu positivity in colorectal carcinoma is low, HER2/neu testing in advanced, nodal-positive colorectal carcinoma is reasonable, offering a potential target in high risk colorectal carcinoma.
Similar content being viewed by others
Main
Colorectal carcinoma (CRC) is one of the most frequent cancers and still a major cause of cancer mortality, although the treatment regime has continuously improved (Ramsoekh et al, 2007). About 30–50% of all patients with CRC will develop metastases, which are the major culprit of CRC that lowers the 5-year survival to <10% (Parker et al, 1997). In the era of personalised medicine with targeted therapy, monoclonal antibodies have become key molecules in the treatment of metastatic CRC (mCRC). Since 2004, the US Food and Drug Administration (FDA) has approved cetuximab, panitumumab and bevacizumab for the treatment of mCRC, which are humanised monoclonal antibodies directed against the epidermal growth factor receptor (EGFR; cetuximab and panitumumab) or the vascular endothelial growth factor (bevacizumab) (McIntire and Redston, 2012). With regard to cetuximab and panitumumab, treatment response largely depends on the absence of oncogenic mutations in key signalling molecules, for example, KRAS, NRAS and BRAF (Custodio and Feliu, 2013), and the drug can only be administered after molecular tests exclude the presence of somatic RAS-mutations in mCRC (companion diagnostics). Trastuzumab is another member of this group of monoclonal antibodies, targeting the HER2/neu protein (human EGFR2). Different from cetuximab and panitumumab, treatment response to trastuzumab highly depends on the HER2/neu protein overexpression and gene amplification, which is detectable in about 15–25% of breast carcinomas and is associated with a more aggressive phenotype and poor prognosis (Ross et al, 2009). The HER2/neu status is assessed immunhistochemically by surgical pathologists in combination with in situ hybridisation using tumour tissue obtained by biopsy or resection. A validated breast cancer scoring system has to be applied prior to administration of trastuzumab on breast cancer patients. Trastuzumab has also been approved for the treatment of gastric cancer, further necessitating testing of HER2/neu status. Interestingly, the scoring system of HER2/neu in gastric cancer is different from the breast cancer scoring system, illustrating the need to validate companion diagnostics for each specific tumour type.
HER2/neu positivity rates vary between different carcinoma subtypes and show different expression patterns. In lung carcinomas (adeno- and squamous cell carcinomas), HER2/neu positivity is found in 2.5–43% (Grob et al, 2012). In gastric cancer, 7–34% of the tumours show overexpression or amplification of HER2/neu, and addition of trastuzumab to chemotherapy improved survival in patients with advanced gastric cancer compared with chemotherapy alone (Bang et al, 2010). The role of HER2/neu overexpression/amplification in CRC is less clear and controversial expression rates, ranging from 2.7 (Marx et al, 2010) to 47.7% (Park et al, 2007), have been published. This discrepancy may be due to several factors including small study populations, evaluation of distinct subgroups and the use of different scoring systems.
Here, we retrospectively assessed the prevalence and putative clinico-pathological significance of HER2/neu overexpression/amplification in a large series of 1645 CRC-samples. The HER2/neu status was evaluated according to the current recommendations of HER2/neu testing (Hofmann et al, 2008; Ruschoff et al, 2012; Hanna et al, 2014).
Materials and methods
Study population
Tissue samples of primary CRC cases were obtained from 1645 patients who had undergone elective surgery for CRC at the University Hospital Kiel (1995–2009). The clinico-pathological characteristics of the patients are summarised in Table 1. Briefly, the cohort consisted of 839 men and 806 women. The median age of the patients at the time of diagnosis was 71 years (range 16–98). Survival data was available from 1603 patients. Out of 1645 patients, 789 died during follow-up. Median follow-up for those patients still alive at the endpoint of analysis was 134.9 months. Only patients with histologically confirmed CRC and those in whom adequate tissue was available were included.
Furthermore, in 29 cases lymph node metastases were available for further analysis.
Histology and TNM classification
For histology, tissue samples were fixed in 10% neutralised formalin and embedded in paraffin. Deparaffinized sections were stained using haematoxylin and eosin. Tumours were classified according to the WHO classification. The pTNM stage was determined according to the seventh edition of the UICC guidelines.
Tissue microarray (TMA) construction
Formalin-fixed and paraffin-embedded tissue samples were used to generate TMAs as described previously (Kononen et al, 1998). Briefly, three separated, morphologically representative regions of the paraffin ‘donor’ block were chosen (triplets). Tissue cylinders of 1 mm diameter were punched from these areas and precisely arrayed into a new ‘recipient’ paraffin block using a customer built instrument (Beecher Instruments, Silver Spring, MD, USA). After completing the block construction, 4 μm sections of the resulting tumour TMA block were cut for further analysis.
HER2/neu status
The HER2/neu status was assessed by immunohistochemistry (IHC) using a monoclonal anti-HER2/neu-antibody (clone SP3, Thermo Fisher Scientific, Fremont, CA, USA) and the autostainer Bond Max System (Leica-Menarini, Berlin, Germany). Analysis of HER2/neu gene amplification was performed by chromogenic in situ hybridisation (CISH) using the ZytoDot 2C SPEC HER2/CEN17 Probe Kit (ZytoVision GmbH, Bremerhaven, Germany). Both, IHC and CISH, were assessed by one pathologist (BIH) blinded to clinical data.
HER2/neu expression was determined on TMA according to the consensus panel recommendations on HER2/neu scoring (Hofmann et al, 2008; Ruschoff et al, 2012; Hanna et al, 2014): HER/2neu-immunostaining was assessed according to the gastric cancer scoring system for biopsies (TMAs). Scores 3+ were reported as positive, scores 2+ as equivocal and scores 0/1+ as negative. A randomly selected subgroup of 54, 0/1+ samples as well as all cases, that scored 2+ and 3+ were further immunohistochemically studied on whole tissue sections.
Tumours with scores 2+ were further tested by CISH on large sections. Furthermore, CISH was extended to all tumour samples scored 3+ and additionally to 10 randomly selected 0/1+ specimens. According to the gastric cancer scoring system, a minimum number of 20 tumour cells per sample were evaluated. A HER2/CEN17-ratio ⩾2 was considered positive, whereas a ratio <2 was considered negative. In case of heterogeneous gene amplification, CRC revealing >10% amplified tumour cells were counted as positive, as suggested in a review by Hanna et al (2014) for breast cancer.
Assessment of HER2/neu status was extended to all available lymph node metastases of 2+ and 3+ scored CRC (n=12) as well as randomly selected 0/1+ specimens (n=17).
External quality assurance
HER2/neu-testing methods, IHC as well as CISH, were certified successfully in 2013 by the quality assurance programme of the German Society of Pathology and the Bundesverband Deutscher Pathologen e.V. Furthermore, HER2/neu positivity rate of the year 2013 was monitored for breast and gastric cancer and results lay within the 99.5% confidence interval of expected results (Choritz et al, 2011).
Statistical analyses
All statistical analyses were carried out using SPSS version 20 (IBM Corp., Armonk, NY, USA). To determine the significance of correlation between HER2/neu status and clinico-pathological parameters, we used Fisher’s exact test except for variables of ordinal scale (T, N, UICC stage and grading) where we used Kendall’s τ-test. We applied the Kaplan–Meier method to calculate median survival with 95% confidence intervals. The significance of differences of median survival was calculated using the log rank test. P-values⩽0.05 were considered to be statistically significant.
Results
HER2/neu status in CRC
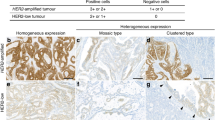
Immunohistochemical HER2/neu expression was available of all 1645 CRC cases. Thousand six hundred and one cases (97.3%) were HER2/neu negative (1548 samples scored 0, 53 samples scored 1+, Figure 1A and B). Thirty-five cases showed an equivocal result (2+; 2.1%; Figure 1C) and nine cases were HER2/neu positive (3+; 0.5%; Figure 1D). All cases scored 2+ and 3+ , and 54 randomly selected 0/1+ cases were additionally tested on whole tissue sections. No significant change in scoring, that is, from 0/1+ to 2+ or 3+, was detected. However, four cases showed a heterogeneous HER2/neu expression (‘black-and-white’-staining pattern; Figure 1E and F), revealing HER2/neu-positive areas next to areas completely devoid of HER2/neu expression.
HER2/neu immunoreactivity. Colorectal carcinoma samples displaying absent (score 0; A), weak (score 1+, B), moderate (score 2+, C) or strong (score 3+, D) HER2/neu membrane staining according to the magnification rule of current HER2/neu-testing guidelines in gastric cancer. Whole tissue section of a CRC sample showing a ‘black-and-white’ HER2/neu expression and corresponding TMA punch areas (E, F). Scale bars: A–D, 50 μm; E, 500 μm; F, 200 μm.
All tumours with equivocal or positive HER2/neu IHC results underwent HER2/neu CISH. As a control, 10 randomly selected 0/1+ cases were also evaluated by CISH. Overall, 26 of 45 (57.8%) were reported positive, that is, all nine 3+ cases and 17 of 35 equivocal tumours (2+) showed HER2/neu amplification (Figure 2A and B). The four immunohistochemically heterogeneous cases showed a heterogeneous gene amplification, ranging from 30% (n=3) to 50% of the tumour cells (n=1; Figure 2C and D), and were therefore counted as HER2/neu positive. In summary, 1.6% of the whole CRC cohort showed a positive HER2/neu status (26 of 1645; Table 2).
HER2/neu in situ hybridisation. Analysis of HER2/neu amplification by dual-color chromogenic in situ hybridisation (CISH) using HER2/neu (green) and chromosome 17 enumeration probes (red) revealing colorectal carcinoma negative (A) and positive (B) for HER2/neu amplification. Genetically heterogeneous sample examined by IHC (C) and CISH (D). Scale bar: A–D, 50 μm.
HER2/neu status in corresponding lymph node metastases
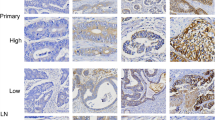
Of 29 primary CRC cases, we evaluated the HER2/neu status of corresponding lymph node metastases in order to see if the results of the metastases were concordant with the primary tumour. This included all available lymph node metastases of HER2/neu-positive CRC (n=12) as well as 17 HER2/neu-negative cases. All lymph node metastases evaluated showed an identical HER2/neu profile to that of primary tumour (12 positive, 17 negative; Figure 3A–D).
Correlation of HER2/neu status with clinico-pathological data
HER2/neu positivity was significantly correlated with higher UICC stages (P=0.020; four groups I/II/III/IV: P=0.017; two groups I/II vs III/IV: P=0.017) and lymph node metastases (P=0.033; N0 vs N+: P=0.029; Table 3). Although statistically not significant, HER2/neu-positive tumours tended to be more frequent in colon sigmoideum/rectum than in ascending/descending colon (18 vs 6; P=0.063). Therefore we decided to re-evaluate the clinico-pathological correlations in the subgroup of colon sigmoideum/rectum tumours separately. In this subgroup, HER2/neu positivity was associated with local tumour growth (T-category; P=0.041) as well as higher UICC stages (P=0.014; four groups I/II/III/IV: P=0.022). No further correlations of HER2/neu positivity and clinico-pathological parameters were detected.
Survival analysis
Univariate analysis revealed that HER2/neu status had no significant impact on patient overall survival (P=0.208), but HER2/neu-positive CRCs tended to have a worse survival compared with -negative CRCs (Figure 4). However, patient prognosis was highly significantly associated with T-category, N-category, M-category, grading, lymph and venous vessel invasion as well as UICC stage.
Discussion
In this study we investigated the prevalence of HER2/neu overexpression and amplification in a cohort of 1645 primary CRC. We found a positive HER2/neu status in 1.6% of all cases, with a significant association with higher UICC stages and positive nodal status. In the subgroup of colon sigmoideum and rectum, we detected that HER2/neu positivity was significantly correlated with local tumour growth (T-category) as well as higher UICC stages. Although statistically not significant, HER2/neu-positive CRCs showed a tendency to poorer overall survival. To our knowledge, this is the first study evaluating a large series of primary CRC with validated methods and a scoring system recommended by an expert panel (Hofmann et al, 2008; Ruschoff et al, 2012; Wolff et al, 2013; Hanna et al, 2014).
In CRC, studies on HER2/neu status revealed controversial results regarding frequency of HER2/neu positivity and its association with clinico-pathological data. HER2/neu positivity rates ranged from 2.7% (Marx et al, 2010) to 47.7% (Park et al, 2007). There are several explanations, for example different scoring systems as counting the percentages of positive tumour cells (Kluftinger et al, 1992) or applying a five-graded scoring system (Drebber et al, 2011). In studies with high HER2/neu positivity rates, tumours scored as 2+ (>10% of tumour cells showing moderate immunoreactivity) were counted as positive cases disregarding the HER2/CEP17 ratio or HER2 copy number in ISH (Kavanagh et al, 2009; Marx et al, 2010). A further difficulty in comparing immunohistochemical studies is the multiplicity of available antibodies. Five different antibodies were used in six different studies, that is, p185 (Kluftinger et al, 1992), 4B5 (Kavanagh et al, 2009; Conradi et al, 2013), HercepTest (Marx et al, 2010; Drebber et al, 2011) and Z4881 (Park et al, 2007). In addition, different preanalytical methods, variable laboratory-based procedures as fixation methods, antigen retrieval and incubation time influences testing results essentially (Blok et al, 2013). Taken all together, this emphasises the importance of external quality assurance programs, leading to a significant improvement of performance (Wasielewski et al, 2008; Choritz et al, 2011; Moelans et al, 2011). Furthermore, small study populations and cohorts of particular subgroups, for example, neoadjuvantly treated CRC (Conradi et al, 2013) may further contribute to the inconsistency of results.
Although we found a statistically significant association of HER2/neu positivity with higher UICC stages and nodal status, as well as T-category and nodal status in the subgroup of sigmoideal and rectal tumours, several studies failed to find an association of positive HER2-status with clinico-pathological parameters (Kountourakis et al, 2006; Schuell et al, 2006; Marx et al, 2010). Other authors in turn reported that HER2/neu positivity was associated with high grade tumours, higher UICC stages and positive nodal status (Kountourakis et al, 2006; Conradi et al, 2013). But again it is important to note that all of these studies used different scoring systems and/or different antibodies than what is currently recommended, which makes comparative conclusions difficult if not impossible.
In an analysis of 225 resection specimens of advanced rectal carcinomas after neoadjuvant radiochemotherapy, Conradi et al, (2013) reported a better cancer-specific survival (CSS; P=0.03) after 5 years in HER2/neu-positive tumours than in HER2/neu-negative cases. Since in this study 5% of the rectal carcinomas, which were diagnosed as HER2/neu positive before radiochemotherapy in biopsy samples, have converted to HER2/neu-negative tumours in the resection specimen–mostly due to tumour heterogeneity–HER2/neu-positive tumour cells probably have had a better response to the neoadjuvant treatment. Therefore HER2-positive tumours may reveal a better 5-year CSS than HER2/neu-negative ones.
HER2/neu positivity had no statistically significant impact on patients’ overall survival, but HER2/neu-positive tumours displayed a tendency to poorer courses, which is in line with other tumours, for example, breast cancer (Ross et al, 2009).
Unfortunately, we have no detailed information about the different applied therapy regimen, which may be a limitation of the presented study. But this bias is related to all subgroups, the HER2/neu-positive and the -negative ones. And the tendency of poorer overall survival of HER2/neu-positive CRC also reflects the association with advanced CRC, independent of treatment.
In the era of personalised medicine, a multitude of new biomarkers have been published and the choice of treatment increasingly depends on the validated analysis of predictive biomarkers (companion diagnostics). Nevertheless, apparently identical tumour subtypes respond differently to the same drug, which may be–among many others–an effect of intratumoral heterogeneity. HER2/neu heterogeneity implies tumour cell subpopulations with different HER2/neu status in the same or different regions of one individual primary tumour as well as discordance between the primary tumour and its metastases. This could lead to different HER2/neu status in core biopsies compared with surgical specimens. Current guidelines for HER2/neu assessment are different concerning the definition of HER2/neu positivity between IHC and ISH. For IHC, tumours that amounts to >10% intense membrane staining are counted as HER2/neu positive, which somewhat covers the problem of tumour heterogeneity at the level of protein expression. For ISH methods, no consistent guidelines exist (Wolff et al, 2013). In a review by Hanna et al, (2014), the authors therefore recommend that after counting 20–60 tumour cells in heterogeneous cases, an amplification in >10% of tumour cells should be considered as HER2/neu positivity. To at least minimise sampling errors using the TMA technique, we examined three core biopsies of three differently located tumour regions. In our cohort of 26 HER2/neu-amplified CRCs, four tumours showed a heterogeneous pattern with amplification in ⩾30% of tumour cells. Consequently, these samples were counted as HER2/neu positive. Tumour heterogeneity of these cases was already captured by TMA punch biopsies, but further evaluated and approved by whole tissue sections. In 29 cases, corresponding lymph node metastases were further examined, in which all cases showed identical HER2/neu status like the primary tumour. Nonetheless, further studies are needed to evaluate the potential effect of an anti-HER2/neu-therapy on tumours with heterogeneous gene expression.
In summary, in our large series of CRCs, we classified 1.6% as HER2/neu positive using current guidelines for HER2/neu testing. HER2/neu overexpression/amplification was associated with higher UICC stages and positive nodal status. Thus, future exploitations of a targeted therapy directed against HER2/neu should focus on advanced and node-positive CRCs.
Change history
11 November 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376 (9742): 687–697.
Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF (2013) Cytoplasmic overexpression of HER2: a key factor in colorectal cancer. Clin Med Insights Oncol 7: 41–51.
Choritz H, Busche G, Kreipe H (2011) Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch 459 (3): 283–289.
Conradi LC, Styczen H, Sprenger T, Wolff HA, Rodel C, Nietert M, Homayounfar K, Gaedcke J, Kitz J, Talaulicar R, Becker H, Ghadimi M, Middel P, Beissbarth T, Ruschoff J, Liersch T (2013) Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol 37 (4): 522–531.
Custodio A, Feliu J (2013) Prognostic and predictive biomarkers for epidermal growth factor receptor-targeted therapy in colorectal cancer: beyond KRAS mutations. Crit Rev Oncol Hematol 85 (1): 45–81.
Drebber U, Madeja M, Odenthal M, Wedemeyer I, Monig SP, Brabender J, Bollschweiler E, Holscher AH, Schneider PM, Dienes HP, Vallbohmer D (2011) beta-catenin and Her2/neu expression in rectal cancer: association with histomorphological response to neoadjuvant therapy and prognosis. Int J Colorectal Dis 26 (9): 1127–1134.
Grob TJ, Kannengiesser I, Tsourlakis MC, Atanackovic D, Koenig AM, Vashist YK, Klose H, Marx AH, Koops S, Simon R, Izbicki JR, Bokemeyer C, Sauter G, Wilczak W (2012) Heterogeneity of ERBB2 amplification in adenocarcinoma, squamous cell carcinoma and large cell undifferentiated carcinoma of the lung. Mod Pathol 25 (12): 1566–1573.
Hanna WM, Ruschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2014) HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol 27 (1): 4–18.
Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52 (7): 797–805.
Kavanagh DO, Chambers G, O'Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I (2009) Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer 9: 1.
Kluftinger AM, Robinson BW, Quenville NF, Finley RJ, Davis NL (1992) Correlation of epidermal growth factor receptor and c-erbB2 oncogene product to known prognostic indicators of colorectal cancer. Surg Oncol 1 (1): 97–105.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4 (7): 844–847.
Kountourakis P, Pavlakis K, Psyrri A, Rontogianni D, Xiros N, Patsouris E, Pectasides D, Economopoulos T (2006) Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J 12 (3): 229–236.
Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Atanackovic D, Bokemeyer C, Fiedler W, Terracciano L, Sauter G, Izbicki JR (2010) Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol 41 (11): 1577–1585.
McIntire M, Redston M (2012) Targeted therapies and predictive markers in epithelial malignancies of the gastrointestinal tract. Arch Pathol Lab Med 136 (5): 496–503.
Moelans CB, van Diest PJ, Milne AN, Offerhaus GJ (2011) Her-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int 2011: 674182.
Park DI, Kang MS, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Han WK, Kim H, Ryu SH, Sepulveda AR (2007) HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int J Colorectal Dis 22 (5): 491–497.
Parker SL, Tong T, Bolden S, Wingo PA (1997) Cancer statistics, 1997. CA Cancer J Clin 47 (1): 5–27.
Ramsoekh D, van Leerdam ME, van Ballegooijen M, Habbema JD, Kuipers EJ (2007) Population screening for colorectal cancer: faeces, endoscopes or X-rays? Cell Oncol 29 (3): 185–194.
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14 (4): 320–368.
Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2012) HER2 testing in gastric cancer: a practical approach. Mod Pathol 25 (5): 637–650.
Schuell B, Gruenberger T, Scheithauer W, Zielinski C, Wrba F (2006) HER 2/neu protein expression in colorectal cancer. BMC Cancer 6: 123.
Wasielewski R, Hasselmann S, Ruschoff J, Fisseler-Eckhoff A, Kreipe H (2008) Proficiency testing of immunohistochemical biomarker assays in breast cancer. Virchows Arch 453 (6): 537–543.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of Clinical O, College of American P (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31 (31): 3997–4013.
Acknowledgements
The authors thank Marten Rönckendorf for his excellent technical assistance. The study was supported by grants from the German Ministry for Education and Research (BMBF) and grants ColoNET (CR and KB).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ingold Heppner, B., Behrens, HM., Balschun, K. et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer 111, 1977–1984 (2014). https://doi.org/10.1038/bjc.2014.483
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.483
Keywords
This article is cited by
-
Therapeutic landscape and future direction of metastatic colorectal cancer
Nature Reviews Gastroenterology & Hepatology (2023)
-
A comprehensive appraisal of HER2 heterogeneity in HER2-amplified and HER2-low colorectal cancer
British Journal of Cancer (2023)
-
HER2 overexpression/amplification status in colorectal cancer: a comparison between immunohistochemistry and fluorescence in situ hybridization using five different immunohistochemical scoring criteria
Journal of Cancer Research and Clinical Oncology (2023)
-
Clinical outcomes of chemotherapy-based therapies for previously treated advanced colorectal cancer: a systematic literature review and meta-analysis
International Journal of Colorectal Disease (2023)
-
Molekularpathologie kolorektaler Karzinome
Die Pathologie (2023)