Abstract
Background:
The aim of this study was to examine the prognostic value of four significant aberrations based on our previous studies by array-CGH to develop a prognostic Fluorescence-in situ-hybridisation (FISH) assay for clear cell renal cell carcinomas (ccRCC).
Methods:
Fluorescence-in situ-hybridisation experiments were performed on 100 ccRCCs (52 metastasised out of 48 non-metastasised). The mean/median follow up of patients was 59/54 months. Commercially available FISH probes were used for each critical chromosomal region (1q21.3, 7q36.3, 9p21.3p24.1 and 20q11.21q13.32). The total number of specific aberrations (TNSA) was calculated for each tumour based on the specific genomic alterations.
Results:
Total number of specific aberrations was the best predictor of metastasis (area under the curve (AUC)=0.814) compared with single aberrations (AUC: 0.619–0.708) and to 11 different combinations of these 4 aberrations in the receiver operating characteristic curve analysis. Total number of specific aberrations, tumour grade and tumour size were independent predictors of metastasis in the multivariate analysis (P<0.001) for the whole cohort as well as for organ-confined tumours. Total number of specific aberrations and grade could also independently predict cancer-specific mortality (CSM). Total number of specific aberrations demonstrated the highest significance in COX proportional hazard models of overall survival (OS), cancer-specific survival (CSS) and progression-free survival (PFS).
Conclusions:
We identified TNSA as an independent prognostic factor which is associated with metastasis occurrence, CSM, OS, CSS and PFS in patients with ccRCCs.
Similar content being viewed by others
Main
Clear cell renal cell carcinoma (ccRCC) represents 70–88% of surgical series (Patard et al, 2005) and has the greatest malignant potential of all RCC subtypes. At the time of diagnosis, up to 30% of RCC patients already showed metastatic disease (Gupta et al, 2008). Another 10–28% of patients with no signs of metastases will later experience local recurrence or distant metastasis after tumour surgery (Kim et al, 2012). The surgical treatment of localised RCC provides excellent results in the 5-year survival rate (Kattan et al, 2001; Leibovich et al, 2003; Kim et al, 2011), but the prognosis of patients with metastatic disease remains predominantly poor. New molecular-targeted therapies show a significantly better treatment response. So adjuvant therapy approaches could improve the prognosis of patients. However, this requires the identification of high-risk patients. Therefore, there is an essential need for new molecular prognostic parameters which considerably improve individual risk assessment for patients with ccRCC. Our previous study by high-resolution array-based comparative genomic hybridisation revealed several genomic aberrations which could be used as molecular markers for defining of aggressiveness in ccRCC (Sanjmyatav et al, 2011). The aim of this study was to examine the prognostic value of these significant aberrations using the multicolor-FISH approach in combination with established prognostic factors such as TNM stage, grade and tumour size to develop a prognostic FISH assay in the future.
Materials and methods
The study cohort consisted of patients with ccRCC who underwent surgery between 1994 and 2006. All patients had no prior systemic therapy before surgery. Hundred primary tumour tissue samples selected reached a balanced contribution concerning T-category and metastatic status (52 metastasised and 48 non-metastasised) of the patients. The clinico-pathological data of patients are listed in Table 1. The cohort included 20 patients with tumour-related death and 13 cases died as a result of other causes. Metastases were detected at time of primary diagnosis (28 patients), between 3 and 9 months (6 patients) and 14 and 86 months (18 patients) after primary diagnosis. The minimum follow up for non-metastatic cases was 24 months. Tissue samples were immediately frozen in liquid nitrogen after partial or radical nephrectomy and stored at −196 °C. All patients provided a written informed consent before the surgical treatment. The study was approved by the local ethical committee.
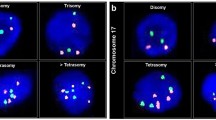
The chromosomal regions 1q21.3, 7q36.3, 9p21.3p24.1 and 20q11.21q13.32 were evaluated by FISH using commercially available probes from ZytoVision GmbH (Bremerhafen, Germany) and Kreatech Diagnostics (Amsterdam, Netherlands). Interphase nuclei from frozen tumour tissues were prepared as described in detail in a previous study (Sanjmyatav et al, 2005). Fluorescence-in situ-hybridisation- experiments were performed according to the manufacturer’s instructions. Cells were examined with an epifluorescence Axioplan microscope (Carl Zeiss, Jena, Germany). The fluorescence signals of overall 100 interphase nuclei were counted for each probe and for each case by an observer blinded to information on tumour. To calculate the cutoff value of positive FISH detection for each probe, 10 control experiments were done on normal kidney tissues using corresponding probes. Cutoff values for all probes were defined as the mean per cent of normal diploid cells in 10 normal tissues minus two-fold standard deviation. The cutoff value for positive FISH detection of each probe was adopted from our previous study (Sanjmyatav et al, 2011).
Statistical analysis was done using the software package SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). To dichotomise the variable for FISH-findings (percentage of aberrant nuclei in tumour tissue) in regard to the metastasis status of tumours and to maximise the sensitivity of detection of metastatic tumour a receiver operating characteristic curve (ROC-Curve) analysis was done with different cut-points in 5 per cent steps for each genomic aberration. A percentage point with the largest area under curve (AUC) was selected as the final cut-point (P-value: Pearson χ2-test). Binary logistic regression was performed to construct a model for the prediction of metastasis. The Kaplan–Meier method was used to analyze cancer-specific survival (CSS), overall survival (OS) and progression-free survival (PFS) of patients. Patient groups were compared using the log-rank test for censored data. The COX proportional hazard model was used to determine independent prognostic value of variables on OS, CSS and PFS. P-values less than 0.05 were considered as significant. For an exact statistical analysis the rule of thumb are 10 events per variable (EPV) in the logistic and COX proportional regressions according to Peduzzi (Peduzzi et al, 1995, 1996). Whereas Vittinghoff et al found out that systematic discounting of results, in particular statistically significant associations, from any model with 5–9 EPV does not appear to be justified (Vittinghoff and McCulloch, 2007). Thus the EPV of our logistic regression and COX-regression models were adjusted at EPV>5 by reducing the number of categorical variables. Based on T-category the cohort was devided in three groups: first group with T1a cases, second group with T1b and T2 tumours 2 and third group with T3a and T3b tumours. According to grading the study cohort was divided into two groups: G1-tumours and G2/G3 cases. This setting showed better significance in the analysis of CSS, OS, and PFS compared with dividing of the cohort into G1/G2 and G3 tumours.
Results
Metastatic risk
All four critical aberrations showed significant correlation to the metastasis status of patients at the cutoff determined by ROC analysis for each aberration and high diagnostic potential for metastasis with AUC-values in the range from 0.619 to 0.708 (Table 2). Gain of 20q11.21q13.32 showed the best correlation (P=0.00002, Fisher-exact-test) and the highest odds ratio to metastasis (OR=16.2; 95% CI: 4.5–58.8) followed by the loss of 9p21.3p24 (OR=9.3; 95% CI: 3.2–27.2). A new unified variable called as total number of specific aberrations (TNSA) was calculated from the sum of the four aberrations which were present in each tumour. Total number of specific aberrations showed the largest AUC (0.814) in the ROC analysis compared with single aberrations and to 11 different combinations of these 4 aberrations thus seems to be a better classifier regarding metastatic tumours.
A binary logistic regression model was constructed by including the TNSA and common prognostic factors such as tumour size, T-category and grade as covariates. Nodal status of tumours was not significant in univariate setting. This model could predict metastatic progression as a binary outcome (Table 3). Our regression model could identify metastatic tumours with a sensitivity of 84%, a specificity of 79% and an accuracy of 82%. The positive predictive value (PPV) was 81% and the negative predictive value (NPV) 79%. T-category was significant only in a univariate setting. Factors such as the TNSA, grade and tumour size were retained as independent predictors of metastatic risk. The odds of metastatic progression in tumours increased with each additional aberration by 3.3-fold (Table 3). Patients who had tumours with high probability to metastasise were defined as high-risk patients.
Since in organ-confined tumours (T1-T2) T-category and tumour size both represent the same feature of the tumour, only tumour size was used in all subsequent calculations. The multivariate model contained factors such as TNSA, grade and tumour size (Table 3). The regression model could predict metastatic event with a sensitivity of 75%, a specificity of 84% and an accuracy of 80% (PPV=81%, NPV=79%).
Overall survival
Significantly different OS rates were also found in patients groups divided by TNSA (P=0.000006, log-rank test), T-category (T1a/T1b-2/T3a-3b) (P=0.036) and grade (P=0.004) in Kaplan–Meier analysis (Table 4). Multivariate COX proportional hazard analysis of the whole cohort including these factors and tumour size demonstrated only TNSA and grade as independent predictors of OS (Table 5). The OS rate of patients decreases with each additional aberration in the tumour by 1.9-fold in the follow up time according to the complete model.
Kaplan–Meier analysis of organ-confined tumours revealed that only TNSA and grade divided patients into groups with significantly different OS rates (Table 4). In the multivariate analysis only TNSA was retained as significant predictor of OS (Table 5).
Cancer-specific survival
Patient groups divided by TNSA, T-categories and grade demonstrated significantly different CSS rates in the Kaplan–Meier analysis (Table 4). In the multivariate analysis the variables such as TNSA and grade were independent predictors of CSS (Table 5). With each additional aberration in tumour tissue, the hazard ratio (HR) for cancer-specific death increased by 1.9-fold in the follow-up time. Patients with grade 2 and 3 tumours showed 2.4-fold higher hazard radio to cancer-specific death in the observation time compared with the patients with grade 1 tumours.
Analogous COX model was obtained for organ-confined tumours in which the TNSA and the grade of tumours were independent predictors of CSS rate (Table 5). Organ-confined tumours showed higher hazard ratio (HR=2.2, 95% CI: 1.4–3.3) with each additional aberration in tumour tissue compared with the hazard ratio calculated for tumours of the whole cohort.
Cancer-specific mortality
Cancer-specific mortality was significantly associated with TNSA, grade and tumour size in tumours of all stages and organ-confined tumours. In a multivariate setting only TNSA and grade could independently predict CSM in both cohorts (Table 3). The regression model could predict CSM of patients of the whole cohort with a sensitivity of 46%, a specificity of 92% and an accuracy of 79% (PPV=68%, NPV=81%). The regression model for organ-confined tumours revealed a sensitivity of 50% and a specificity of 94% and an accuracy 83% (PPV=71%, NPV=86%).
Progression-free survival
While patient groups divided by T-category showed no significant differences in PFS, patient groups divided by TNSA and grade demonstrated significant differences in PFS in the Kaplan–Meier analysis of our whole cohort (Table 4). In the multivariate setting the variables TNSA and grade were retained as independent predictors of PFS in the follow-up time of our whole cohort (Table 5). In organ-confined cases the COX model also revealed the same variables such as TNSA and grade as independent predictors of recurrence-free survival.
Discussion
The main established independent prognostic factors for RCC are TNM stage, tumour size, nodal status and histological grade (Ficarra et al, 2008; Lam et al, 2008). Number of efforts was used in the past to stratify the prognosis of RCC patients and several multifactor prognostic models have been developed including these parameters (Kattan et al, 2001; Zisman et al, 2001; Frank et al, 2002; Sorbellini et al, 2005). However prediction of tumour behaviour and clinical outcome based on standard clinico-pathological features alone is quite limited and do not reflect the biology of tumours. Thus efforts to predict survival of patients with RCC moved to developing multiparameter prognostic models incorporating molecular markers into standard predictive nomograms. Kim et al (2004) used eight different molecular markers such as Ki-67, p53, gelsolin, CA9, CA12, PTEN, EpCAM, and vimentin and TNM stage, Fuhrman grade, and ECOG PS (Eastern Cooperative Oncology Group Performance Status) to predict survival of patients with ccRCC. This nomogram showed improved predictive accuracy compared with the model with established clinical prognostic variables alone. Furthermore, Parker et al (2009) developed the BioScore system which is based on molecular markers such as B7-H1, Survivin, and Ki-67 to enhance the outcome prediction of ccRCC.
Specific genomic aberrations represent critical molecular events involved in tumorigenesis. Our previous study identified several specific genomic aberrations by Array-CGH which seems to be a reliable factor for the definition of metastatic risk and clinical outcome of patients with ccRCC (Sanjmyatav et al, 2011). In this continuing study we retrospectively analysed four chromosomal regions such as 1q21.3, 7q36.3, 9p21.3p24.1 and 20q11.21q13.32 in an extended cohort of 100 ccRCC by FISH and could confirm these four specific genomic aberrations as promising factors for defining of prognosis in ccRCC patients. The new variable TNSA which unified the data of four specific genomic aberrations in primary tumours showed the best correlation to metastasis status and highest significance compared with clinico-pathologic variables such as tumour size, T-category and grade. Furthermore, the Kaplan–Meier analysis of TNSA revealed that patient groups divided by this variable demonstrate highly significant differences in OS, CSS and PFS in our cohort.
Metastasis still defines the prognosis in patients with RCC. Until now, definition of potential metastatic progression is impossible for an individual patient at the time of initial disease diagnosis. So a logistic regression model was built for definition of metastatic risk including T-category, grade, tumour size and TNSA as covariates. Total number of specific aberrations, grade and tumour size were independent predictors of metastasis risk. T-category has historically served as the best prognostic tool in RCC and provides most reliable prognostic information (Ljungberg et al, 2010). But this variable was not significant in our multivariate analysis. Most reliable prognostic information was provided by TNSA. The more specific aberrations were found in the tumour the more aggressive behaviour manifested in the tumour and the higher was the probability that this tumour metastasised.
Grade is an accepted prognostic factor in RCC (Bretheau et al, 1995; Leibovich et al, 2003) and presents as an independent predictor of metastatic risk in our multivariate model. Additionally, our model revealed that metastatic risk of ccRCC tumours depends on tumour size, too. Primary tumour size is reflected in TNM-staging and belongs to the most important prognostic factors of RCC. Recently, a study based on the National Swedish Kidney Cancer Quality Register had also revealed that metastatic risk increases with tumour size. The same study demonstrated that small tumours also (<7 cm) have an aggressive potential, resulting in lymph node and distant metastasis even in 1–2 cm tumours (Guethmundsson et al, 2011). Therefore the prognosis assessment of small tumours still remains an important task for clinicians. Our regression model allowed the identification of patients at high risk for metastasis even in an organ-confined situation based on genetic alterations of the primary tumour. This opens up a possibility to identify patients who would most likely benefit from adjuvant therapy.
Our study also revealed that TNSA and grade were independent predictors of OS, CSM, CSS and PFS not only for tumours of all T-categories but also for organ-confined tumours. The TNSA proved to be the best predictor in all multivariate settings (OS, CSS CSM and PFS) compared with clinico-pathologic variables. Analysing Kaplan–Meier curves of OS, CSS and PFS in regard to TNSA (Figure 1), it seems better to divide the cohort into two groups: patients with fewer than two aberrations and cases with at least two aberrations and to use the covariate TNSA as a categorical variable. However, calculations showed better significance value and better values for hazard ratio in regard to confidence interval in both univariate and multivariate setting for TNSA with five groups than with two groups. The four genomic aberrations, unified to TNSA, seem to have an essential role in the tumour aggressiveness and may be useful in the identification of patients whose tumours will rapidly progress after diagnosis.
To define the degree of influence of each specific aberration on OS, CSS, CSM and PFS it is necessary to analyze a larger cohort in the future to overcome limitations of this study. In upcoming studies it is necessary to analyze prospectively a large multicenter cohort of ccRCC to develop a multivariate prognostic model for ccRCC integrating each genomic aberration and well-established prognostic parameters such as T-stages, tumour size, nodal status and histological grade.
Tumour progression is a multistep process accumulating different abnormalities leading to expression and activation of genes participating in malignant progression. The four analysed genomic regions seem to harbour important genes which have a huge impact on the metastasis manifestation in RCC. We mention at this point only a few of the most important genes. The S100 family gene cluster (S100A1-S100A9, S1007A and S100A7L2) is located at 1q21.3 locus. S100A4 is a member of the S100 family of calcium-binding proteins that is directly involved in tumour metastasis, and it is being discussed as metastasis promoter (Sherbet, 2009). The tumour suppressor CDKN2AB is located on 9p21, which has an important role in the control of the cell cycle. Cycle inhibitory proteins p15INK4b and p16INK4a encoded by this locus are able to induce cell cycle arrest in G1 by inhibiting cyclin-dependent kinases CDK4 and CDK6 which normally inactivate two tumour-suppressor pathways, Rb and p53 (Sharpless et al, 2004). A number of functionally important oncogenes such as E2F1 and HCK, and apoptosis-associated genes such as BCL2L1 are localised in 20q11.21q13.2 segment. Further studies are needed to identify which genes in these regions are predominantly responsible for tumour progression and aggressiveness of ccRCC and to find out which particular pathways are activated in aggressive ccRCCs.
In conclusion, the new factor TNSA based on specific genomic alterations could independently predict metastasis occurrence, OS, CSM, CSS and PFS in patients with ccRCCs. The identification of patients at high risk for metastasis even in localised situation based on genomic alterations of the primary tumour opens up the possibility not only to select patients who would benefit from new targeted therapy regimes but also to manage a risk-adapted follow up of patients.
Change history
13 May 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bretheau D, Lechevallier E, de Fromont M, Sault MC, Rampal M, Coulange C (1995) Prognostic value of nuclear grade of renal cell carcinoma. Cancer 76 (12): 2543–2549.
Ficarra V, Galfano A, Novara G, Iafrate M, Brunelli M, Secco S, Cavalleri S, Martignoni G, Artibani W (2008) Risk stratification and prognostication of renal cell carcinoma. World J Urol 26 (2): 115–125.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H (2002) An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 168 (6): 2395–2400.
Guethmundsson E, Hellborg H, Lundstam S, Erikson S, Ljungberg B (2011) Metastatic potential in renal cell carcinomas </=7 cm: Swedish Kidney Cancer Quality Register data. Eur Urol 60 (5): 975–982.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C (2008) Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 34 (3): 193–205.
Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P (2001) A postoperative prognostic nomogram for renal cell carcinoma. J Urol 166 (1): 63–67.
Kim HL, Seligson D, Liu X, Janzen N, Bui MH, Yu H, Shi T, Figlin RA, Horvath S, Belldegrun AS (2004) Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res 10 (16): 5464–5471.
Kim SP, Crispen PL, Thompson RH, Weight CJ, Boorjian SA, Costello BA, Lohse CM, Leibovich BC (2012) Assessment of the pathologic inclusion criteria from contemporary adjuvant clinical trials for predicting disease progression after nephrectomy for renal cell carcinoma. Cancer 118 (18): 4412–4420.
Kim SP, Weight CJ, Leibovich BC, Thompson RH, Costello BA, Cheville JC, Lohse CM, Boorjian SA (2011) Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localised renal cell carcinoma. Urology 78 (5): 1101–1106.
Lam JS, Klatte T, Kim HL, Patard JJ, Breda A, Zisman A, Pantuck AJ, Figlin RA (2008) Prognostic factors and selection for clinical studies of patients with kidney cancer. Crit Rev Oncol Hematol 65 (3): 235–262.
Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, Weaver AL, Parker AS, Zincke H (2003) Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97 (7): 1663–1671.
Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC (2010) EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58 (3): 398–406.
Parker AS, Leibovich BC, Lohse CM, Sheinin Y, Kuntz SM, Eckel-Passow JE, Blute ML, Kwon ED (2009) Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer 115 (10): 2092–2103.
Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guille F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23 (12): 2763–2771.
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48 (12): 1503–1510.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49 (12): 1373–1379.
Sanjmyatav J, Junker K, Matthes S, Muehr M, Sava D, Sternal M, Wessendorf S, Kreuz M, Gajda M, Wunderlich H, Schwaenen C (2011) Identification of genomic alterations associated with metastasis and cancer specific survival in clear cell renal cell carcinoma. J Urol 186 (5): 2078–2083.
Sanjmyatav J, Rubtsov N, Starke H, Schubert J, Hindermann W, Junker K (2005) Identification of tumor entities of renal cell carcinoma using interphase fluorescence in situ hybridisation. J Urol 174 (2): 731–735.
Sharpless NE, Ramsey MR, Balasubramanian P, Castrillon DH, DePinho RA (2004) The differential impact of p16(INK4a) or p19(ARF) deficiency on cell growth and tumorigenesis. Oncogene 23 (2): 379–385.
Sherbet GV (2009) Metastasis promoter S100A4 is a potentially valuable molecular target for cancer therapy. Cancer Lett 280 (1): 15–30.
Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, McKiernan J, Russo P (2005) A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 173 (1): 48–51.
Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165 (6): 710–718.
Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, Quintana D, Gitlitz BJ, deKernion JB, Figlin RA, Belldegrun AS (2001) Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 19 (6): 1649–1657.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sanjmyatav, J., Matthes, S., Muehr, M. et al. Identification of high-risk patients with clear cell renal cell carcinoma based on interphase-FISH. Br J Cancer 110, 2537–2543 (2014). https://doi.org/10.1038/bjc.2014.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.159
Keywords
This article is cited by
-
A self-supervised vision transformer to predict survival from histopathology in renal cell carcinoma
World Journal of Urology (2023)