Abstract
Background:
The pro-inflammatory cytokine migration inhibitory factor (MIF) and its receptor CD74 have been proposed as possible therapeutic targets in several cancers. We studied the expression of MIF and CD74 together with calretinin in specimens of malignant pleural mesothelioma (MPM), correlating their expression levels with clinico-pathologic parameters, in particular overall survival (OS).
Methods:
Migration inhibitory factor, CD74, and calretinin immunoreactivity were investigated in a tissue microarray of 352 patients diagnosed with MPM. Protein expression intensities were semiquantitatively scored in the tumour cells and in the peritumoral stroma. Markers were matched with OS, age, gender, and histological subtype.
Results:
Clinical data from 135 patients were available. Tumour cell expressions of MIF and CD74 were observed in 95% and 98% of MPM specimens, respectively, with a homogenous distribution between the different histotypes. CD74 (P<0.001) but not MIF overexpression (P=0.231) emerged as an independent prognostic factor for prolonged OS. High expression of tumour cell calretinin correlated with the epithelioid histotype and was also predictive of longer OS (P<0.001). When compared with previously characterised putative epithelial-to-mesenchymal transition markers, CD74 correlated positively with tumoral PTEN and podoplanin expressions, but was inversely related with periostin expression.
Conclusions:
High expression of CD74 is an independent prognostic factor for prolonged OS in mesothelioma patients.
Similar content being viewed by others
Main
Malignant pleural mesothelioma (MPM) is a malignant tumour arising from the mesothelial cells lining the pleural cavity. Approximately 50–80% of MPM in men develop after exposure to mineral fibres such as asbestos and erionite (the proportion drops to 20–30% in women). Due to long incubation periods, malignant mesothelioma mortality rates will probably continue to increase until 2020 in most European countries, and until 2030–2040 in Japan (Stayner et al, 2013). Predicting the course of the asbestos epidemic in developing countries is difficult because of the paucity of data in these areas of the world.
Radical treatment protocols often combine induction platinum-based chemotherapy and surgery followed by radiotherapy to the involved hemithorax (Weder et al, 2007). However, this approach is seldom feasible (Hasani et al, 2009) as most patients are diagnosed with advanced disease when surgery is no longer possible. Even if its efficacy is limited in time, palliative chemotherapy with pemetrexed and cisplatin is usually resorted to in other cases (Janne et al, 2006).
As in numerous cancers such as colorectal, gastric, hepatic, and lung cancers, chronic inflammation with recruitment of macrophages and neutrophils and production of chemokines and cytokines in the thorax is thought to initiate MPM (Adamson et al, 1997; Hill et al, 2003; Dostert et al, 2008). Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine, released by a variety of cell types and is involved in numerous inflammatory and autoimmune diseases (Calandra and Roger, 2003). Apart from its role in inflammation, many factors show that MIF has an important role in promoting tumorigenesis. Migration inhibitory factor is overexpressed in a variety of malignant tumours, such as breast (Lue et al, 2007), colon (Legendre et al, 2003), prostate cancers (Meyer-Siegler et al, 1998), and melanoma (Shimizu et al, 1999), glioblastoma multiform (Bacher et al, 2003) and lung adenocarcinomas (Kamimura et al, 2000; Tomiyasu et al, 2002). It increases tumour growth by favouring tumour cell proliferation and blocking apoptosis; after binding to CD74, it activates the PI3K/Akt pathway. More recently, overexpression of MIF has also been shown to be associated with epithelial-to-mesenchymal transition (EMT) (Funamizu et al, 2013) in which cancer cells lose key adhesion molecules such as E-cadherin thus acquiring unrestrained cell motility and metastatic potential (Gjerdrum et al, 2010).
While MIF has been shown to be a prognostic factor in several cancers, this has not been shown yet in MPM and is the purpose of the present study. We resorted to a retrospective multicentre collection of 352 formalin-fixed, paraffin-embedded MPM specimens to identify the prevalence of MIF and its receptor CD74 in MPM.
Materials and methods
Patients population and clinical data
All cases of malignant mesothelioma were diagnosed between 1975 and 2004, and retrieved from the archives of the Zurich Pneumoconiosis Research Group, Switzerland (Director: M. Rueegger). The tissue specimens were mainly derived from post-mortem examination (77% autopsy and 23% biopsy) and had uniformly been formalin fixed and paraffin embedded. Classification for the histological subtype was made according to the World Health Organisation classification (WHO 2004) by one experienced lung pathologist (PV) and was reviewed (MH) to identify suitable areas for tissue microarray (TMA) construction.
Patient characteristics of the cohort were described in previous studies (Hinterberger et al, 2007; Schramm et al, 2010). Distribution of the histological subtype of MPM of the patient cohort is described in Table 1. Clinical data were assessed for a total of 135 out of 352 patients (94% male) with a median age of 63 years (range 40–93).
The construction of a set of three TMAs was accomplished with a custom-made, semiautomatic tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA) as described previously (Hinterberger et al, 2007).
Immunohistochemistry analysis
In all, 4-μm-thick human TMA sections from FFPE samples were analysed by immunohistochemistry using anti-MIF (gift of Thierry Roger, Lausanne), anti-CD74 (HPA010592; Sigma-Aldrich, St Louis, MO, USA), and anti-calretinin antibodies (18-0211; Invitrogen AG, Basel, Switzerland) using the Ventana Discovery automated staining system (Ventana Medical Systems, Tucson, AZ, USA). Ventana reagents for the entire procedure were used. For both calretinin and CD74 antibodies, antigenicity was retrieved by heating slides in CC1 cell conditioning solution for 20 min (EDTA antigen retrieval solution pH 8.4), whereas for MIF antibody, slides were heated in the same solution for 36 min. After automatic deparaffinisation and heating, slides were incubated 30 min at 37 °C with primary antibodies respectively diluted at 1/300 (calretinin), 1/1000 (CD74), and 1/300 (MIF) in antibody diluent from Dako (Baar, Switzerland; S2022). Detection of anti-calretinin antibodies was carried out using the secondary universal biotinylated antibodies reagent and the amplified DABMap kit (Ventana Medical Systems; 760-124). Detection of anti-MIF and anti-CD74 antibodies was performed using the rabbit OmniMap kit (Ventana Medical Systems; 760-149).
Scoring
Immunohistochemical analysis was carried out by two observers (CO and VSB) unaware of patient data or core distribution within the TMA. Consensus was reached in case of a significant discrepancy between the individual scores. One to four spots per tumour specimen were evaluated. Protein expression was recorded semiquantitatively using the histoscore (HS) method. Briefly, each core was scored on a semiquantitative scale ranging from 0 to 300, with the final score resulting from the percentage of tumour cells staining positively (range 0–100) multiplied by staining intensity graded as negative, weak, moderate, or strong (range from 0 to 3). Total HS for a tumour specimen (sum of HS of four spots) ranges from 0 to 1200. The obtained product scores were used for statistical analysis. We also performed analysis on four subgroups selected from the total cohort based on no expression (noted 0; HS=0), low expression (noted 1; 0
Statistics
Clinical data of these patients were retrospectively assessed from medical archives of the different hospitals and the local cancer registries. Statistical analysis was performed using Kaplan–Meier curves for correlation of survival time with expression of calretinin, MIF, and CD74 as well as other clinical and pathological marker such as age, gender, and histological subtype. Multiple Cox regression analysis was used to assess association between survival and expression of calretinin, MIF, and CD74 adjusted for patient data (age and gender). For the Cox regression analysis, the biomarkers were analysed as none/low vs medium/high expression, because the Kaplan–Meier analysis showed that the curves for medium and high expression of MIF and accordingly CD74 were likewise and the group with no expression of MIF and accordingly CD74 was small (n=6 and n=2, respectively). We used non-parametric tests to compare two (Mann–Whitney test) or more (Kruskal–Wallis test) independent groups of continuous data. The correlations among continuous variables were assessed by Pearson’s correlation analysis.
Results
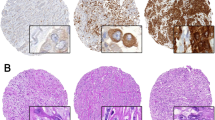
Malignant pleural mesothelioma TMAs were evaluated for calretinin, MIF, and CD74 antigens expression in tumour cells and stroma environment. Representative sections of TMA stained for each of the investigated biomarkers are shown in Figure 1. Epithelioid MPM tumours showed marked calretinin expression. Sarcomatoid malignant mesothelial cells consist of densely packed spindle cells, with high cellularity, nuclear atypia, and frequent mitotic figures. These latter elements allowed differentiating these malignant cells from fibroblasts of the tumour stroma. Sarcomatoid malignant mesothelial cells were rarely positive for calretinin labelling (Figure 1).
Calretinin, MIF, and CD74 expression in epithelioid, sarcomatoid, and biphasic mesothelioma. (A) Representative staining for calretinin, MIF, and CD74 in samples from epithelioid, sarcomatoid, and biphasic mesothelioma specimens. (B) Individual histoscore (HS) of marker expression in tumour cells for each spot.
Tumour cell expression
Percents of tumour cells expressing calretinin, MIF, and CD74 for each mesothelioma specimen are shown in Table 2. In all, 271 of the 352 patients (77%) showed positive staining of tumour cells for calretinin. Histoscore values for calretinin ranged from 0 to 1200 (median 300), and 46% were categorised as showing medium to high expression (defined as HS⩾400). In all, 334 of the 352 specimens (95%) showed positive staining of tumour cells for MIF. Histoscore values for MIF ranged from 0 to 1200 (median 655), and 66% were categorised as showing medium to high expression (defined as HS⩾400). In all, 334 of the 352 specimens (98%) showed positive staining of tumour cell surface for CD74. Histoscore value for CD74 ranged from 0 to 1200 (median 560), and 60% were categorised as showing medium to high expression (defined as HS⩾400). Expression of calretinin, MIF, and CD74 in tumour cells was independent of known asbestos exposure.
The distribution of calretinin, MIF, and CD74 expression by histology is shown in Figure 2. Calretinin expression in the tumour was dependent on histotype (Figure 2A). Epithelioid tumours had significantly higher calretinin expression levels (median HS 695, range 0–1200, n=123) compared with mixed mesotheliomas (median HS 270, range 0–1200, n=183; P<0.0001) and sarcomatoid mesotheliomas (median HS 0, range 0–450, n=46; P<0.0001).
Scattered dot plots of calretinin (A), MIF (B), and CD74 (C) expression levels in tumour cells of different histological type of mesothelioma specimens. The central horizontal line represents the median histoscore in each group. Error bars represent interquartile range (first–third quartile). The number of mesothelioma specimens (n) is indicated for each expression subgroup. ***P<0.001; ****P<0.0001.
We observed a similar level of MIF (Figure 2B) and CD74 (Figure 2C) in tumour cells of all histological types of mesothelioma (epithelioid, sarcomatoid, and biphasic).
Analysis of correlation between the expression of the three markers and overall survival (OS) of patients were performed on 135 patients for whom clinical data were available. High calretinin and CD74 expression levels in the tumour were positively associated with longer survival (P<0.001) (Figure 3A and C). Migration inhibitory factor expression level in tumour cells did not correlate with patient survival (Figure 3B). Patients with no expression of calretinin in tumour cells (score=0; n=28, 21%) had a significantly lower OS (6.5 months, 95% CI 4.0–8.9) than patients with low (11.3 months, 95% CI 10.2–12.3), medium (13.5 months, 95% CI 9.9–17.2) or high calretinin (16.5 months, 95% CI 8.3–24.8; P<0.001) expression levels, respectively (Figure 3A).
Patients with low expression of CD74 in tumour cells (score=1; n=45, 33%) had a significantly lower OS (8.2 months, 95% CI 5.6–10.9) than patients with medium (14.0 months, 95% CI 9.4–18.5), or high CD74 expression levels, respectively (14.7 months, 95% CI 10.1–19.3; P<0.001) (Figure 3C). Cox regression analysis of the joint influence of the predictors (gender, age, calretinin expression, CD74 expression, and MIF expression) showed that calretinin (medium/high vs none/low) expression, CD74 (medium/high vs none/low) expression, gender (female vs male), and age (>63 years vs⩽63 years) are independent prognosticators (Table 3). Histotype was excluded because of the association of histotype and calretinin expression. When histotype was included in the analysis, calretinin was no longer an independent prognosticator, but it had no influence on the effect of CD74 (data not shown).
Stroma cell expression
All proteins (calretinin, MIF, and CD74) analysed on the TMA were characterised by lower to no expression in the stroma compared with tumour tissue (Figure 1; Table 4). Stromal calretinin and CD74 stainings were independent of histotype while MIF expression in the stroma cells of tumour specimens was higher in epithelioid and biphasic tumours compared with sarcomatoid mesothelioma (P=0.001 and P<0.001, respectively).
Expression of all markers (calretinin, MIF, and CD74) in stroma cells of the mesothelioma specimens did not correlate with patient survival.
Correlation among markers
In previous studies, putative EMT marker periostin, podoplanin (also noted D2-40, a mucin-like glycoprotein highly expressed in MPM) and PTEN have been characterised in mesothelioma samples from the same 352 patients using these same three TMA (Hinterberger et al, 2007; Opitz et al, 2008; Schramm et al, 2010). To enquire whether calretinin, CD74, and MIF are associated with these markers, a correlation analysis was carried out. This correlation showed that most of the markers were positively correlated with one another except periostin that showed negative correlations (Table 5). We observed a positive correlation between CD74 and calretinin, MIF, D2-40, and PTEN (Supplementary Figure S1). Migration inhibitory factor was also positively correlated with calretinin, CD74, D2-40, and PTEN (Supplementary Figure S2). CD74 was negatively correlated with periostin while no different periostin expression was observed in the four MIF expression level subgroups.
Discussion
This study identified the MIF-receptor CD74 as an independent prognostic factor for OS in MPM patients. Growing interest focuses on the role of inflammatory signals in the initiation and development of mesothelioma. Among inflammatory mediators, pro-inflammatory cytokine MIF is a particularly interesting candidate. Indeed, MIF has been shown to have an important role in favouring tumorigenesis, and several research groups have shown a correlation between MIF and prognosis in hepatocellular carcinomas, gastric, ovarian, breast, colon, prostate, and non-small cell lung cancers (Meyer-Siegler et al, 1998; Kamimura et al, 2000; Tomiyasu et al, 2002; Legendre et al, 2003; Hira et al, 2005; Lue et al, 2007; Xia et al, 2009). The effect of MIF seems mediated via its binding to CD74 which expression is also linked with several forms of cancer (Datta et al, 2000; Meyer-Siegler et al, 2005, 2006; Binsky et al, 2007; McClelland et al, 2009; Nagata et al, 2009; Verjans et al, 2009).
In this study, we used a TMA with MPM tissue of the three histological types of MPM (epithelioid, sarcomatoid, and biphasic) in 352 patients to identify possible relations between MIF and/or MIF-receptor CD74 expressions, and cancer prognosis.
Calretinin staining was performed first to differentiate epithelioid and sarcomatoid areas in MPM samples. Calretinin is expressed in the epithelial type of MPM and in the epithelial component of mixed tumours (sarcomatoid mesotheliomas and the sarcomatoid component of mixed tumours do not express calretinin). Our results confirmed Kao et al’s findings (Kao et al, 2011) in which calretinin positivity is associated with improved survivals.
This study is the first to determine the prevalence and clinico-pathological significance of MIF and its receptor CD74 in MPM. Migration inhibitory factor and CD74 overexpression in tumour cells is a molecular trait found in 95% and 98% of MPM, respectively. High levels of the MIF-receptor CD74 expression in tumour cells strongly correlated with longer survivals, whatever the histological subtype. Furthermore, MIF expression levels correlated with those of CD74, suggesting that sensitivity of tumour cells to MIF (mediated through its receptor CD74) is important in malignancies and patient survival and could be the preferential pathway implicated in MPM. The MIF/CD74 couple contributes to carcinogenesis in several manners. In some cancers (gastric, hepatocellular, prostate, ovarian, breast, colon, and non-small cell lung cancers), high expression levels of MIF and/or CD74 correlate with poor prognoses (Meyer-Siegler et al, 2002; Tomiyasu et al, 2002; Hagemann et al, 2005; Hira et al, 2005; Xu et al, 2008; Xia et al, 2009). On the contrary, in nasopharyngeal carcinoma or in squamous cell carcinoma of the head and the neck, MIF expression level was associated with improved outcome (Suzuki et al, 2005; Li et al, 2012b). Finally, in breast cancer, patients with the highest levels of cytoplasmic MIF had a better prognosis, while exogenous stimulation of breast cancer cell lines with MIF increased cell proliferation and local invasion (Verjans et al, 2009). The contribution of MIF and CD74 to cancerogenesis seems to vary with the type of cancer and stage of the disease. This may be explained by the different pathways activated by this ligand/receptor complex. Once secreted, MIF binds to CD74 that interacts with CD44, a polymorphic transmembrane protein with kinase activating properties, forming a receptor complex. This complex can then activate several pathways such as the extracellular signal-regulated kinases (ERK)1 and 2 in the mitogen-activated protein kinase pathway, and the phosphoinositide-3-kinase (PI3K)/AKT/SRC signal transduction cascade (Leng et al, 2003; Shi et al, 2006; Lue et al, 2007) leading to upregulation of cell proliferation, and decrease in cell apoptosis, as well as enhancement of cell migration (Meyer-Siegler et al, 2006; Lee et al, 2012c). Migration inhibitory factor/CD74 can also activate the AMPK pathways (Miller et al, 2008; Heinrichs et al, 2011; Iwata et al, 2012). Activation of the AMPK pathway in some cancer cells has been shown to decrease cell proliferation, cell viability, and their metastatic potential (Park et al, 2012; Chang et al, 2013; Kaur et al, 2013), whereas inactivation of this pathway contributes to carcinogenesis in hepatic nodular foci (Miyoshi et al, 2009). Furthermore, underexpression of AMPK-α2 was found to be associated with an undifferentiated cellular phenotype and poor prognosis (Lee et al, 2012b), while patients with high expression of AMPK had a better prognosis in ovarian carcinoma and non-small cell lung cancer (Li et al, 2012a; William et al, 2012).
Contribution of each of these pathways could vary between the different cell types and differentiation state of the cells. Mesothelial cells are plastic cells responding to environmental cues (damaged cells, inflammatory conditions, etc.) with acquisition of mesenchymal features. This plasticity could explain the heterogeneity of MPM with its different histologic subtypes—epithelial, biphasic, and sarcomatoid. The sarcomatoid and epithelioid histotypes resemble the EMT–MET transdifferentiation, being associated with the epithelial and mesenchymal differentiation state of the cell, respectively. Our analyses suggest that the epithelial differentiation state is characterised by high levels of calretinin, MIF/CD74, podoplanin (D2-40) and the inhibitor of Akt signalling pathway PTEN and low level of periostin. Although beyond the scope of present study, it would be interesting to see if the pathway activated by the MIF/CD74 couple (AMPK vs PI3K pathways) depends on differentiation of the cancer cells. Recent studies indicate that MIF/CD74 activation of AMPK pathway inhibits TGF-β-induced EMT (Cufi et al, 2010; Heinrichs et al, 2011; Lee et al, 2013), and suppresses cell proliferation and migration in tumour cells (Kim et al, 2012; Lee et al, 2012a). In other words, activation of this pathway may represent an attempt to prevent EMT.
In summary, these data indicate that MIF and its receptor CD74 are expressed in MPM and that CD74 is an independent prognostic factor. During the MPM tumorigenesis process, malignant epithelial cells progressively change their phenotype into a mesenchymal phenotype. The following sequence could be proposed: in a first step, calretinin, podoplanin, and PTEN expressions decrease, second CD74 and MIF expressions are reduced, and third periostin expression is increased. These genes, in addition to the biomarkers routinely used for diagnosis, could help the pathologists to better characterise MPM histologies and the clinicians to identify patients with a worse prognosis. One of the disadvantages of this study is the retrospective nature of the clinical data. Therefore, correlation of the protein marker expression with tumour stage, therapy, and OS is somewhat limited. We are currently carrying out in vitro studies on human mesothelioma cell lines to characterise the role of MIF, CD74, and MIF/CD74 complexes in malignant mesothelial cell function and proliferation.
Change history
15 April 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adamson IY, Prieditis H, Young L (1997) Lung mesothelial cell and fibroblast responses to pleural and alveolar macrophage supernatants and to lavage fluids from crocidolite-exposed rats. Am J Respir Cell Mol Biol 16: 650–656.
Bacher M, Schrader J, Thompson N, Kuschela K, Gemsa D, Waeber G, Schlegel J (2003) Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme. Am J Pathol 162: 11–17.
Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, Leng L, Goldenberg DM, Shvidel L, Berrebi A, Bucala R, Shachar I (2007) IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA 104: 13408–13413.
Calandra T, Roger T (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3: 791–800.
Chang HW, Lee YS, Nam HY, Han MW, Kim HJ, Moon SY, Jeon H, Park JJ, Carey TE, Chang SE, Kim SW, Kim SY (2013) Knockdown of beta-catenin controls both apoptotic and autophagic cell death through LKB1/AMPK signaling in head and neck squamous cell carcinoma cell lines. Cell Signal 25: 839–847.
Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA (2010) Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle 9: 4461–4468.
Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM (2000) Expression of MHC class II-associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 8: 210–215.
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677.
Funamizu N, Hu C, Lacy C, Schetter A, Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, Lee DH, Subleski J, Chan T, Weiss JM, Back TC, Yanaga K, Hanna N, Alexander HR, Maitra A, Hussain SP (2013) Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J Cancer 132 (4): 785–794.
Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT, Micklem DR, Akslen LA, Glackin C, Lorens JB (2010) Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA 107: 1124–1129.
Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR (2005) Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol 175: 1197–1205.
Hasani A, Alvarez JM, Wyatt JM, Bydder S, Millward M, Byrne M, Musk AW, Nowak AK (2009) Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 4: 1010–1016.
Heinrichs D, Knauel M, Offermanns C, Berres ML, Nellen A, Leng L, Schmitz P, Bucala R, Trautwein C, Weber C, Bernhagen J, Wasmuth HE (2011) Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74. Proc Natl Acad Sci USA 108: 17444–17449.
Hill GD, Mangum JB, Moss OR, Everitt JI (2003) Soluble ICAM-1, MCP-1, and MIP-2 protein secretion by rat pleural mesothelial cells following exposure to amosite asbestos. Exp Lung Res 29: 277–290.
Hinterberger M, Reineke T, Storz M, Weder W, Vogt P, Moch H (2007) D2-40 and calretinin—a tissue microarray analysis of 341 malignant mesotheliomas with emphasis on sarcomatoid differentiation. Mod Pathol 20: 248–255.
Hira E, Ono T, Dhar DK, El-Assal ON, Hishikawa Y, Yamanoi A, Nagasue N (2005) Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer 103: 588–598.
Iwata T, Taniguchi H, Kuwajima M, Taniguchi T, Okuda Y, Sukeno A, Ishimoto K, Mizusawa N, Yoshimoto K (2012) The action of D-dopachrome tautomerase as an adipokine in adipocyte lipid metabolism. PLoS One 7: e33402.
Janne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, Mintzer DM, Ye Z, Monberg MJ, Obasaju CK (2006) Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol 1: 506–512.
Kamimura A, Kamachi M, Nishihira J, Ogura S, Isobe H, Dosaka-Akita H, Ogata A, Shindoh M, Ohbuchi T, Kawakami Y (2000) Intracellular distribution of macrophage migration inhibitory factor predicts the prognosis of patients with adenocarcinoma of the lung. Cancer 89: 334–341.
Kao SC, Klebe S, Henderson DW, Reid G, Chatfield M, Armstrong NJ, Yan TD, Vardy J, Clarke S, van Zandwijk N, McCaughan B (2011) Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 6: 1923–1929.
Kaur M, Deep G, Jain AK, Raina K, Agarwal C, Wempe MF, Agarwal R (2013) Bitter melon juice activates cellular energy sensor AMP-activated protein kinase causing apoptotic death of human pancreatic carcinoma cells. Carcinogenesis 34: 1585–1592.
Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS, Lim JS (2012) Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem Pharmacol 83: 385–394.
Lee CR, Chun JN, Kim SY, Park S, Kim SH, Park EJ, Kim IS, Cho NH, Kim IG, So I, Kim TW, Jeon JH (2012a) Cyclosporin A suppresses prostate cancer cell growth through CaMKKbeta/AMPK-mediated inhibition of mTORC1 signaling. Biochem Pharmacol 84: 425–431.
Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM, Hardie DG, Ng IO, Ching YP (2012b) AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res 72: 4394–4404.
Lee CY, Su MJ, Huang CY, Chen MY, Hsu HC, Lin CY, Tang CH (2012c) Macrophage migration inhibitory factor increases cell motility and up-regulates alphavbeta3 integrin in human chondrosarcoma cells. J Cell Biochem 113: 1590–1598.
Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, Lee SK (2013) AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am J Physiol Renal Physiol 304: F686–F697.
Legendre H, Decaestecker C, Nagy N, Hendlisz A, Schuring MP, Salmon I, Gabius HJ, Pector JC, Kiss R (2003) Prognostic values of galectin-3 and the macrophage migration inhibitory factor (MIF) in human colorectal cancers. Mod Pathol 16: 491–504.
Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R (2003) MIF signal transduction initiated by binding to CD74. J Exp Med 197: 1467–1476.
Li C, Liu VW, Chiu PM, Chan DW, Ngan HY (2012a) Over-expressions of AMPK subunits in ovarian carcinomas with significant clinical implications. BMC Cancer 12: 357.
Li J, Mo HY, Xiong G, Zhang L, He J, Huang ZF, Liu ZW, Chen QY, Du ZM, Zheng LM, Qian CN, Zeng YX (2012b) Tumor microenvironment MIF directs the accumulation of IL-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J Biol Chem 287: 35484–35495.
Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Luscher B, Bernhagen J (2007) Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene 26: 5046–5059.
McClelland M, Zhao L, Carskadon S, Arenberg D (2009) Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol 174: 638–646.
Meyer-Siegler K, Fattor RA, Hudson PB (1998) Expression of macrophage migration inhibitory factor in the human prostate. Diagn Mol Pathol 7: 44–50.
Meyer-Siegler KL, Bellino MA, Tannenbaum M (2002) Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer 94: 1449–1456.
Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL (2006) Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol 177: 8730–8739.
Meyer-Siegler KL, Iczkowski KA, Vera PL (2005) Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC Cancer 5: 73.
Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH (2008) Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 451: 578–582.
Miyoshi H, Deguchi A, Nakau M, Kojima Y, Mori A, Oshima M, Aoki M, Taketo MM (2009) Hepatocellular carcinoma development induced by conditional beta-catenin activation in Lkb1+/- mice. Cancer Sci 100: 2046–2053.
Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, Kitamura M, Takahashi H, Eguchi H, Ohigashi H, Ishikawa O, Tomita Y (2009) CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol 16: 2531–2538.
Opitz I, Soltermann A, Abaecherli M, Hinterberger M, Probst-Hensch N, Stahel R, Moch H, Weder W (2008) PTEN expression is a strong predictor of survival in mesothelioma patients. Eur J Cardiothorac Surg 33: 502–506.
Park JJ, Seo SM, Kim EJ, Lee YJ, Ko YG, Ha J, Lee M (2012) Berberine inhibits human colon cancer cell migration via AMP-activated protein kinase-mediated downregulation of integrin beta1 signaling. Biochem Biophys Res Commun 426: 461–467.
Schramm A, Opitz I, Thies S, Seifert B, Moch H, Weder W, Soltermann A (2010) Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur J Cardiothorac Surg 37: 566–572.
Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R (2006) CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 25: 595–606.
Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J (1999) High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun 264: 751–758.
Stayner L, Welch LS, Lemen R (2013) The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 34: 205–216.
Suzuki F, Nakamaru Y, Oridate N, Homma A, Nagahashi T, Yamaguchi S, Nishihira J, Furuta Y, Fukuda S (2005) Prognostic significance of cytoplasmic macrophage migration inhibitory factor expression in patients with squamous cell carcinoma of the head and neck treated with concurrent chemoradiotherapy. Oncol Rep 13: 59–64.
Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K (2002) Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin Cancer Res 8: 3755–3760.
Verjans E, Noetzel E, Bektas N, Schutz AK, Lue H, Lennartz B, Hartmann A, Dahl E, Bernhagen J (2009) Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer 9: 230.
Weder W, Stahel RA, Bernhard J, Bodis S, Vogt P, Ballabeni P, Lardinois D, Betticher D, Schmid R, Stupp R, Ris HB, Jermann M, Mingrone W, Roth AD, Spiliopoulos A (2007) Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 18: 1196–1202.
William WN, Kim JS, Liu DD, Solis L, Behrens C, Lee JJ, Lippman SM, Kim ES, Hong WK, Wistuba II, Lee HY (2012) The impact of phosphorylated AMP-activated protein kinase expression on lung cancer survival. Ann Oncol 23: 78–85.
Xia HH, Yang Y, Chu KM, Gu Q, Zhang YY, He H, Wong WM, Leung SY, Yuen ST, Yuen MF, Chan AO, Wong BC (2009) Serum macrophage migration-inhibitory factor as a diagnostic and prognostic biomarker for gastric cancer. Cancer 115: 5441–5449.
Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S (2008) Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett 261: 147–157.
Acknowledgements
Dr P Vogt and the Zürich Pneumoconiosis research group at the Swiss Federal Institute of Technology Zürich (ETHZ) are acknowledged for providing archival material. We thank the following Institutes of Pathology for providing archival paraffin blocks: University Hospital Bern (Professor Perren), University Hospital Basel (Professor Bubendorf), Cantonal Hospital St Gallen (Professor W Jochum), Cantonal Hospital Luzern (Professor J Diebold), City Hospital Triemli (Professor P Komminoth), Cantonal Hospital Aarau (Professor Grobholz), Cantonal Hospital Baden (Professor G Singer), Cantonal Hospital Winterthur (Dr R Flury). The MIF antibody was kindly provided by Dr Thierry Roger (Infectious Diseases Service, Department of Medicine, Centre hospitalier universitaire vaudois and University of Lausanne). We thank Nathalie Lin-Marq (Faculty of Medicine, Histology Core Facility, University of Geneva) for immunohistochemistry staining and Martina Friess (Division of Thoracic Surgery, University Hospital Zürich) for statistical analysis. This study was supported by Fondation Boninchi (for JR and VESB) and Ligue Pulmonaire Genevoise (for VESB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Otterstrom, C., Soltermann, A., Opitz, I. et al. CD74: a new prognostic factor for patients with malignant pleural mesothelioma. Br J Cancer 110, 2040–2046 (2014). https://doi.org/10.1038/bjc.2014.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.117
Keywords
This article is cited by
-
CD74 is associated with inflamed tumor immune microenvironment and predicts responsiveness to PD-1/CTLA-4 bispecific antibody in patients with solid tumors
Cancer Immunology, Immunotherapy (2024)
-
Prognostic role of CD74, CD10 and Ki-67 immunohistochemical expression in patients with diffuse malignant peritoneal mesothelioma: a retrospective study
BMC Cancer (2023)
-
CD74+ macrophages are associated with favorable prognosis and immune contexture in hepatocellular carcinoma
Cancer Immunology, Immunotherapy (2022)
-
LOX family and ZFPM2 as novel diagnostic biomarkers for malignant pleural mesothelioma
Biomarker Research (2020)
-
The biological function and significance of CD74 in immune diseases
Inflammation Research (2017)