Abstract
Background:
The association between tumour measurements and survival has been studied extensively in early-stage and locally advanced non-small cell lung cancer (NSCLC). We analysed these factors in patients with advanced NSCLC.
Methods:
Data were derived from the E4599 trial of paclitaxel-carboplatin±bevacizumab. Associations between the Response Evaluation Criteria in Solid Tumors (RECIST) baseline sum longest diameter (BSLD), response rate, progression-free survival (PFS) and overall survival (OS) were evaluated using univariate and multivariable Cox regression models.
Results:
A total of 759 of the 850 patients (89%) in the E4599 trial had measurable diseases and were included in this analysis. The median BSLD was 7.5 cm. BSLD predicted OS (hazard ratio (HR) 1.41; P<0.001) and had a trend towards association with PFS (HR 1.14; P=0.08). The median OS was 12.6 months for patients with BSLD <7.5 cm compared with 9.5 months for BSLD ⩾7.5 cm. This association persisted in a multivariable model controlling multiple prognostic factors, including the presence and sites of extrathoracic disease (HR 1.24; P=0.01). There was no association between BSLD and response rate.
Conclusion:
Tumour measurements are associated with survival in the E4599 trial. If validated in other populations, this parameter may provide important prognostic information to patients and clinicians.
Similar content being viewed by others
Main
Radiographical and pathological disease burdens have an impact on clinical outcomes in lung cancer. Primary tumour dimension is associated with survival in early-stage and locally advanced non-small cell lung cancer (NSCLC) (Ball et al, 2013). Indeed, recognition of the prognostic relevance of this factor is evident in the 7th edition of AJCC/UICC NSCLC staging (Goldstraw et al, 2007). Whereas up until that version the only primary tumour size consideration was a diameter cutpoint of 3 cm (distinguishing between T1 and T2, corresponding to clinical stages IA and IB in the absence of lymph node involvement or distant disease), the recently adopted 7th edition also incorporates thresholds of 5 cm (stage IIA) and 7 cm (stage IIB). Primary tumour diameter is also a well-established indication for the administration of adjuvant chemotherapy for stage-I NSCLC, presumably because of the greater risk associated with larger tumours. Stage-IB tumours >4 cm derive a benefit from adjuvant chemotherapy, whereas smaller tumours do not (Pignon et al, 2008; Strauss et al, 2008). In stage-III NSCLC, tumour volume is an independent predictor of overall survival (OS) (Basaki et al, 2006; Morgensztern et al, 2012a). Additionally, the size of involved lymph nodes in this population has been identified as a prognostic factor (Andre et al, 2000).
In other malignancies, radiographic and clinical tumour burdens are also prognostic in the advanced disease setting. In ovarian cancer, debulking surgery that achieves a combined residual tumour measurement of less than 1 cm is associated with improved progression-free survival (PFS) and OS (Vogl et al, 1983; Van Der Burg et al, 1995). In metastatic renal cell carcinoma, the proportion of tumour burden removed at debulking nephrectomy is associated with PFS (Barbastefano et al, 2010). In stage-III Hodgkin’s lymphoma (that is, multiple lymph node groups on both sides of the diaphragm), numbers of disease sites and size of mediastinal mass are prognostic features (Rostock, 1984). In non-Hodgkin’s lymphoma, the numbers of nodal and extranodal sites of disease are associated with survival, independent of stage, and are incorporated into standard prognostic indexes (Shipp et al, 1993; Solal-Celigny et al, 2004).
Despite extensive analyses of tumour dimensions in stages I–III NSCLC, as well as the prognostic value of tumour size in the advanced disease setting for other malignancies, there is little known about the association between radiographic tumour burden and survival in patients with advanced NSCLC. We therefore analysed the association between the Response Evaluation Criteria in Solid Tumors (RECIST) baseline sum longest diameter (BSLD) and clinical outcomes in a recent phase 3 Eastern Cooperative Oncology Group (ECOG) trial for advanced NSCLC, E4599.
Materials and methods
Patient clinical and disease data were derived from the E4599 trial of paclitaxel-carboplatin±bevacizumab for advanced nonsquamous NSCLC (Sandler et al, 2006). The E4599 trial is the most recent completed and published phase III ECOG trial in advanced NSCLC and, to date, the only published phase III ECOG trial in this population to employ RECIST to assess response.
The key eligibility criteria for the E4599 trial included the following: histologically or cytologically confirmed newly diagnosed stage IIIB (malignant effusion) or stage IV or recurrent NSCLC (AJCC v 6 staging); measurable or nonmeasurable disease by RECIST; performance status ECOG 0-1; adequate haematologic, hepatic, and renal functions. Principal exclusion criteria included the following: predominantly squamous cell histology; gross hemoptysis (defined as ⩾half teaspoon bright-red blood); central nervous system metastases; history of documented haemorrhagic diathesis or coagulopathy; therapeutic anticoagulation; regular use of aspirin (>325 mg per day), non-steroidal anti-inflammatory agents or other drugs inhibiting platelet function; radiation therapy within 21 days before enrollment or major surgery within 28 days before enrollment; clinically significant cardiovascular disease (symptomatic congestive heart failure, unstable angina pectoris or cardiac arrhythmia); and medically uncontrolled hypertension (>150/100).
In E4599, eligible patients were stratified by measurable vs nonmeasurable disease, prior radiation therapy vs no prior radiation therapy, prior weight loss of <5% vs ⩾5%, and stage IIIB (malignant effusion) vs stage IV/recurrent disease. Patients were randomly assigned to receive paclitaxel-carboplatin (PC) or becavizumab-paclitaxel-carboplatin (BPC) until disease progression, treatment intolerance or a maximum of six cycles. Patients in the BPC arm who completed six cycles of combination therapy then received bevacizumab monotherapy every 3 weeks until disease progression or treatment intolerance.
For the present study, number and size of target lesions were obtained from E4599 RECIST forms. Radiographic images and radiology reports were not evaluated. There was no central radiology review in E4599. The BSLD was dichotomised at the median value and also categorised by quartile. Sites of disease were recorded from E4599 RECIST forms.
Baseline patient demographics, disease characteristics and response were compared using the Fisher’s exact test. OS was defined as time interval in months from randomisation to death from any cause. PFS was defined as the time interval in months from randomisation to documented progression or death, whichever occurred first. Patients not experiencing an event were censored at the last date of follow-up for OS and the last date of disease assessment for PFS. Time-to-event distributions were estimated using the Kaplan–Meier method, and their comparisons were made using the log-rank test.
Multivariable Cox proportional hazards models were used to estimate hazard ratios (HRs) for OS using backward stepwise regression with P=0.2 as the model entry criteria. All P-values are two-sided, CIs are at the 95% level and no adjustments were made for multiple comparisons.
Results
Among 850 total patients enrolled in the E4599 trial, 759 (89%) had baseline measurable disease by RECIST and were included in this analysis. Seventy-six percent of these patients were aged <70 years, 45% were female and 86% were white. Additional baseline patient characteristics are listed in Table 1.
The median BSLD was 7.5 cm. Range of BSLD was 1.0–80.1 cm. Interquartile range (IQR) was 4.5–11.7 cm. Notably, treatment assignment was well balanced within BSLD categories. Among patients with BSLD of ⩽7.5 cm, 52% were assigned to PC and 48% to BPC. Among patients with BSLD >7.5 cm, 50% were assigned to PC and 50% to BPC. Baseline characteristics and sites of disease (as recorded on study RECIST forms) according to BSLD (dichotomised at median value) are listed in Table 2. Numerically, age, gender, race and performance status appeared to be balanced between BSLD categories. Compared with patients with BSLD ⩽7.5 cm, those with BSLD >7.5 cm were more likely to have stage IV/recurrent disease and less likely to have adenocarcinoma histology. Numerically, compared with patients with BSLD ⩽7.5 cm, patients with BSLD >7.5 cm were more likely to have contralateral lung, hilar and mediastinal lymph nodes, liver, and adrenal lesions but less likely to have pleural or bone disease.
In the overall E4599 study population, median OS was 12.3 months in the BPC arm vs 10.3 months in the PC arm (HR for death 0.79; P=0.003). Median PFS was 6.2 months in the BPC arm vs 4.5 months in the PC arm (HR for disease progression 0.66; P<0.001). Response rates were 35% (BPC) and 15% (PC) (P<0.001) (Sandler et al, 2006). In the current analysis, the median follow-up on patients who were still alive was 23 months. The number of patients remaining in the OS risk set at 12, 24 and 36 months were 345, 55 and 9 patients, respectively. The number of patients remaining in the PFS risk set at 6, 12 and 24 months were 303, 77 and 12 patients, respectively.
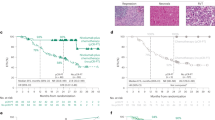
In univariate Cox models, using a BSLD cutoff value of 7.5 cm (the median for the study population), BSLD was associated with OS (Figure 1A). For patients with BSLD ⩽7.5 cm, median OS was 12.6 months (95% confidence interval (CI), 11.3–14.3 months) vs 9.5 months (95% CI, 8.7–11.0 months) for patients with BSLD >7.5 cm (HR 1.41; P<0.001). When categorised according to quartile, BSLD remained associated with OS: median OS 13.3 months (95% CI, 11.9–16.0 months) for ⩽4.5 cm, 11.5 months (95% CI, 10.0–13.5 months) for >4.5 and ⩽7.5 cm, 11.6 months (95% CI, 9.5–13.2 months) for >7.5 and ⩽11.7 cm, and 8.3 months (95% CI, 7.0–10.3 months) for >11.7 cm (P<0.001) (Figure 1B). Patients in both groups benefited from the addition of bevacizumab to chemotherapy (Figure 1C). For patients with BSLD ⩽7.5 cm, median OS was 11.5 months (95% CI, 10.3–13.2 months) in the PC arm vs 14.1 months (95% CI, 11.9–17.4 months) in the BPC arm (HR 0.81; 95% CI, 0.64–1.02; P=0.08). For patients with BSLD >7.5 cm, median OS was 8.5 months (95% CI, 7.7–10.7 months) in the PC arm vs 10.7 months (95% CI, 9.2–12.4 months) in the BPC arm (HR 0.85; 95% CI, 0.68–1.06; P=0.15).
Association between overall survival and RECIST BSLD. (A) BSLD dichotomised at median value. (B) BSLD categorised by quartile. (C) Dichotomised BSLD and treatment group. Abbreviations: BPC, bevacizumab-paclitaxel-carboplatin; BSLD, baseline sum longest diameter; PC, paclitaxel-carboplatin; RECIST, response evaluation criteria in solid tumours.
There was an association between BSLD and PFS nearing statistical significance (Figure 2A). For patients with BSLD ⩽7.5 cm, median PFS was 5.3 months (95% CI, 4.7–5.7 months) vs 5.1 months (95% CI, 4.6–5.6 months) for patients with BSLD >7.5 cm (HR 1.14; P=0.08). PFS according to BSLD and treatment group is shown in Figure 2B. For patients with BSLD ⩽7.5 cm, median PFS was 4.4 months (95% CI, 4.0–5.3 months) in the PC arm vs 6.2 months (95% CI, 5.5–6.8 months) in the BPC arm (HR 0.70; 95% CI, 0.57–0.87; P=0.001). For patients with BSLD >7.5 cm, median PFS was 4.5 months (95% CI, 3.7–5.0 months) in the PC arm vs 5.9 months (95% CI, 5.4–6.4 months) in the BPC arm (HR 0.69; 95% CI, 0.56–0.86; P=0.001).
Association between progression-free survival and RECIST BSLD. (A) BSLD dichotomised at median value. (B) Dichotomised BSLD and treatment group. BPC, bevacizumab-paclitaxel-carboplatin; Abbreviations: BSLD, baseline sum longest diameter; PC, paclitaxel-carboplatin; RECIST, response evaluation criteria in solid tumours.
There was no association between BSLD and response rates. In the PC arm, response rate was 15% for BSLD ⩽7.5 cm and 15% for BSLD >7.5 cm (P=0.89). In the BPC arm, response rate was 33% for BSLD ⩽7.5 cm and 37% for BSLD >7.5 cm (P=0.51).
We generated a multivariable model including numerous clinicopathologic factors that are considered prognostic in advanced NSCLC. The final model included treatment group, sex, ECOG performance status, weight loss (characterised as ⩾5% or not, as in the E4599 trial), recurrent disease, presence of bone metastases, presence of liver metastases, presence of adrenal metastases, treatment-by-sex interaction and BSLD (all of which were significant at the 0.05 level). In this model, BSLD remained significantly associated with OS. For BSLD >7.5 cm, the HR for death (compared with those having BSLD ⩽7.5 cm) was 1.24 (95% CI, 1.05–1.47; P=0.01). In the multivariable model, BSLD was also associated with OS when considered as a continuous variable: HR 1.03; P<0.001.
Discussion
The prognostic significance of radiographic tumour burden has been evaluated extensively in early-stage and locally advanced NSCLC, as well as in the advanced disease setting for other malignancies. However, this clinical parameter has been studied only to a limited degree in advanced NSCLC (Wang et al, 2009). To our knowledge, the present study is the first to analyse the association between radiographic tumour burden, patient and disease characteristics, and numerous clinical outcomes in this population. In this series of 759 patients with measurable disease by RECIST treated in the E4599 phase 3 trial of PC vs BPC, we found that tumour size, characterised according to RECIST BSLD, was significantly associated with OS, had a trend towards association with PFS but was not associated with response rate. Specifically, patients with BSLD ⩽7.5 cm (the median value in the study population) had a median OS more than 3 months longer than patients with BSLD >7.5 cm. These findings were observed in both the PC and the BPC treatment groups. As might be expected, patients with longer BSLD were more likely to have recorded lesions in regional lymph nodes, liver and adrenal gland. They were also more likely to have stage IV/recurrent compared with stage IIIB (malignant effusion) disease. Nevertheless, when controlling for multiple prognostic variables, including the presence and sites of extrathoracic disease, a significant association between BSLD and OS was maintained (P=0.01).
Similar findings have been reported in numerous malignancies. In advanced nasopharyngeal carcinoma, clinical tumour volume (calculated by image reconstruction using the product of tumour area and slice thickness on axial CT scan) has been correlated with local control, metastasis-free rate and survival (Lee et al, 2010). In advanced gastric cancer, large primary tumour size is an independent risk factor for survival (Li et al, 2009). In lung cancer, a recent variation of these observations can be found in studies of tumour metabolic activity. A number of studies have demonstrated an independent association between FDG-PET metabolic tumour volume (at the level of the primary tumour, nodal metastases, distant metastases, and total body disease) and OS, even after controlling for other prognostic criteria, such as stage, performance status and age (Lee et al, 2007, 2012; Liao et al, 2012).
The present study adds to an earlier analysis of tumour size and survival in advanced NSCLC, in which baseline and change in tumour size were analysed as predictors of survival in four NSCLC registration trials (Wang et al, 2009). We expand on these findings by investigating other efficacy end points (response rate and PFS), assessing multiple clinical and disease characteristics and incorporating numerous prognostic factors in a multivariable model.
The use of RECIST tumour dimensions, rather than direct measurements from radiographic studies, distinguishes the current study from most other reports. As the case for any treatment response guideline, RECIST inherently modifies the clinical and radiographic extent of disease. Following RECIST 1.0 (the version used in the E4599 trial), patients can have a maximum of five target lesions per organ and ten target lesions in total (Therasse et al, 2000). To be designated as a target lesion, a lesion must meet the criteria to be considered measurable, which are as follows: ⩾10 mm in longest diameter (with spiral CT) or ⩾20 mm in longest diameter (with conventional techniques). Several radiographic manifestations of cancer are considered nonmeasurable, including bone lesions, leptomeningeal disease, ascites, pleural/pericardial effusions, cystic lesions and previously radiated lesions (unless there is evidence of post-treatment radiographic progression or a post-radiation biopsy confirms viable malignancy).
Despite these restrictions, RECIST has been shown to be reproducible and to correlate with other clinical end points in advanced NSCLC. Furthermore, RECIST data in our population are consistent with those reported in other studies. In an analysis of 32 North Central Cancer Treatment Group (NCCTG) trials encompassing 2374 total patients, the median number of target lesions recorded was two (Hillman et al, 2009) – identical to the median number of target lesions in the current study. One lesion at baseline was reported in 49% of patients, two lesions in 28% of patients, three lesions in 12% of patients, four lesions in 6% of patients and five lesions in 5% of patients. The use of the two largest lesions provided a high degree of concordance with the use of all reported lesions for the end points of response rate and time to progression. Erasmus et al (2003) evaluated intra- and inter-observer variability in the unidimensional measurements of 40 lung tumours in 33 patients. In general, there was close agreement. Across five readers, recorded tumour sizes ranged from 1.0 to 9.0 cm. The measurement of the smallest recorded tumour varied by 0.5 cm, whereas the measurement of the largest recorded tumour varied by 1.2 cm. It has also been demonstrated that the accuracy of measuring pulmonary lesions does not significantly differ according to medical specialty (radiology, thoracic surgery, radiation oncology, pulmonary and medical oncology), familiarity with lesion measurement or years since medical degree (Grossi et al, 2004).
The biological explanation for our results is not clear. A number of preclinical observations link increasing tumour size with therapeutic resistance. As tumours grow, the proportion of cells resistant to chemotherapy increases (Goldie and Coldman (1979) hypothesis). Larger cancers may also have relatively poor blood supply and more pronounced gradients in interstitial pressure, hypoxia and acidity, which may influence tumour-cell sensitivity to therapy (Skipper, 1974) (Jain, 1990; Tredan et al, 2007). Additionally, as the distance from blood supply increases, tumour-cell proliferation rates decrease, thereby diminishing the response to cytotoxic agents (Hirst and Denekamp, 1979). However, the lack of association between tumour dimensions and radiographic response rate in the present study appears to refute these hypotheses. It is possible that the lack of association between BSLD and response rate may in part reflect how this efficacy parameter is calculated; as a percentage change, response rate is intended to normalise the differing baseline tumour sizes. Additionally, in the present study, tumour dimensions may be more closely associated with therapeutic resistance than with therapeutic sensitivity, as there was a near-significant association between BSLD and PFS.
This study has a number of limitations. The study population is restricted to patients meeting eligibility criteria for E4599 and thus does not include individuals with squamous histology or brain metastases. Although reproducibility of target lesion measurements has been studied extensively, the nature and variability of RECIST target lesion selection have not been well described; hence, it is not clear to what extent RECIST BSLD is reproducible. BSLD does not account for non-target sites of disease, such as malignant effusions, which may be associated with decreased survival in advanced NSCLC (Morgensztern et al, 2012b). Importantly, in the present study, BSLD data came from clinical-trial RECIST forms. We did not evaluate radiographic images or radiology reports, and there was no central radiology review in the original clinical trial. Whereas these study characteristics may introduce variability into the present analysis, they also suggest that our findings may be applicable to other real-world clinical scenarios. Finally, tumour measurement guidelines are subject to change over time. Already, RECIST 1.1 has been incorporated into clinical trials. This version differs from its predecessor by the number of target lesions permitted (five maximum total; two maximum per organ), lymph node assessment (must be >1.5 cm in short axis to be considered measurable), and allowance of cystic lesions and lytic bone lesions with soft tissue components as target lesions (Eisenhauer et al, 2009). The relatively small proportion of our study population with more than five target lesions (7% of patients) suggests that our findings would likely persist using the revised RECIST system. Additionally, our selected BSLD cutpoints require validation in other cohorts. Nevertheless, this study demonstrates a consistent association between this parameter and clinical outcomes – whether the data are analysed as a single cohort or according to treatment groups; whether the population is divided into two or four categories; and whether the outcome of interest is OS or PFS. Furthermore, the differences noted are clinically meaningful, with a 5-month difference in the median survival between the lowest and the highest BSLD quartiles.
In summary, this study demonstrates an association between radiographic tumour burden – defined by RECIST BSLD – and clinical outcomes in advanced NSCLC. We found that this metric had a near-significant association with PFS and a statistically and clinically significant association with OS. This association persists across various treatments, using various cutpoints, and when controlling for other prognostic patient and disease characteristics, including presence and sites of extrathoracic disease. If confirmed in other advanced NSCLC populations, these findings merit consideration in the stratification of patients for clinical trials and may provide important prognostic information to patients and clinicians.
Change history
17 September 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T (2000) Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 18: 2981–2989.
Ball D, Mitchell A, Giroux D, Rami-Porta R IASLC Staging Committee and Participating Institutions (2013) Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol 8: 315–321.
Barbastefano J, Garcia JA, Elson P, Wood LS, Lane BR, Dreicer R, Campbell SC, Rini BI (2010) Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU Int 106: 1266–1269.
Basaki K, Abe Y, Aoki M, Kondo H, Hatayama Y, Nakaji S (2006) Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int J Radiat Oncol Biol Phys 64: 449–454.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247.
Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, Munden RF (2003) Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 21: 2574–2582.
Goldie JH, Coldman AJ (1979) A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep 63: 1727–1733.
Goldstraw P, Crowley J, Chanksy K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2: 706–714.
Grossi F, Belvedere O, Fasola G, Ceschia T, Meduri S, Sibau A, Sacco C, Bazzocchi M, Talmassons G, Morelli A, Barbone F, Sobrero A (2004) Tumor measurements on computed tomographic images of non-small cell lung cancer were similar among cancer professionals from different specialties. J Clin Epidemiol 57: 804–808.
Hillman SL, An MW, O'Connell MJ, Goldberg RM, Schaefer P, Buckner JC, Sargent DJ (2009) Evaluation of the optimal number of lesions needed for tumor evaluation using the response evaluation criteria in solid tumors: a north central cancer treatment group investigation. J Clin Oncol 27: 3205–3210.
Hirst DG, Denekamp J (1979) Tumour cell proliferation in relation to the vasculature. Cell Tissue Kinet 12: 31–42.
Jain RK (1990) Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 50: 814s–819s.
Lee CC, Huang TT, Lee MS, Hsiao SH, Lin HY, Su YC, Hsu FC, Hung SK (2010) Clinical application of tumor volume in advanced nasopharyngeal carcinoma to predict outcome. Radiat Oncol 5: 20.
Lee P, Bazan JG, Lavori PW, Weerasuriya DK, Quon A, Le QT, Wakelee HA, Graves EE, Loo BW (2012) Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non-small-cell lung cancer. Clin Lung Cancer 13: 52–58.
Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, Le QT, Wakelee HA, Donington JS, Graves EE, Loo BW Jr (2007) Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys 69: 328–333.
Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu ZG, Noh SH (2009) Risk factors of survival and surgical treatment for advanced gastric cancer with large tumor size. J Gastrointest Surg 13: 881–885.
Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, Shih MC, Shimada N, Chen S, Salgia R, Appelbaum DE, Suzuki K, Chen CT, Pu Y (2012) Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 39: 27–38.
Morgensztern D, Waqar S, Subramanian J, Gao F, Trinkaus K, Govindan R (2012a) Prognostic Significance of Tumor Size in Patients with Stage III Non-Small-Cell Lung Cancer: A Surveillance, Epidemiology, and End Results (SEER) Survey from 1998 to 2003. J Thorac Oncol 7: 1479–1484.
Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R (2012b) Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol 7: 1485–1489.
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T (2008) Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26: 3552–3559.
Rostock RA (1984) New aspects of Hodgkin's disease: staging for conservative management. Crit Rev Oncol Hematol 1: 295–321.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550.
Shipp MA, Harrington DP, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, Cabanillas F, Canellos GP, Coiffier B, Connors JM, Cowan RA, Crowther D, Dahlberg S, Engelhard M, Fisher RI, Gisselbrecht C, Horning SJ, Lepage E, Lister TA, Meerwaldt JH, Montserrat E, Nissen NI, Oken MM, Peterson BA, Tondini C, Velasquez WA, Yeap BY (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329: 987–994.
Skipper HE (1974) Thoughts on cancer chemotherapy and combination modality therapy (1974). JAMA 230: 1033–1035.
Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haioun C, Leblanc M, Lister AT, Lopez-Guillermo A, Mclaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E (2004) Follicular lymphoma international prognostic index. Blood 104: 1258–1265.
Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM, Sugarbaker DJ, Schilsky RL, Vokes EE, Green MR (2008) Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26: 5043–5051.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216.
Tredan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99: 1441–1454.
Van Der Burg ME, Van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, Lacave AJ, Nardi M, Renard J, Pecorelli S (1995) The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med 332: 629–634.
Vogl SE, Pagano M, Kaplan BH, Greenwald E, Arseneau J, Bennett B (1983) Cis-platin based combination chemotherapy for advanced ovarian cancer. High overall response rate with curative potential only in women with small tumor burdens. Cancer 51: 2024–2030.
Wang Y, Sung C, Dartois C, Ramchandani R, Booth BP, Rock E, Gobburu J (2009) Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther 86: 167–174.
Acknowledgements
This study was conducted by the Eastern Cooperative Oncology Group (Robert L Comis, MD) and supported in part by the Public Health Service Grant CA23318 from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. This work was also supported by a National Cancer Institute Cancer Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement) (to DEG) and the North and Central Texas Clinical and Translational Science Initiative (NCTCTSI) (KL2RR024983) (to DEG). The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Previous presentations: Presented in part at the 2012 Chicago Multidisciplinary Symposium in Thoracic Oncology, Chicago, Illinois, September 6–8, 2012; the 2012 Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) Young Investigators’ Symposium, Hollywood, Florida, November 9–11, 2012; and the Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, May 31–June 4, 2013.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Gerber, D., Dahlberg, S., Sandler, A. et al. Baseline tumour measurements predict survival in advanced non-small cell lung cancer. Br J Cancer 109, 1476–1481 (2013). https://doi.org/10.1038/bjc.2013.472
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.472
Keywords
This article is cited by
-
Identification of optimal dosing schedules of dacomitinib and osimertinib for a phase I/II trial in advanced EGFR-mutant non-small cell lung cancer
Nature Communications (2021)
-
Accuracy Enhanced Lung Cancer Prognosis for Improving Patient Survivability Using Proposed Gaussian Classifier System
Journal of Medical Systems (2019)
-
Relationship between pretreatment levels of serum Cyfra 21.1, CEA and PET metabolic parameters in NSCLC
Annals of Nuclear Medicine (2014)
-
NSCLC: Tumorgröße auch in späteren Stadien prognostisch relevant
Im Focus Onkologie (2013)