Abstract
Background:
Women treated with supradiaphragmatic radiotherapy (sRT) for Hodgkin lymphoma (HL) at young ages have a substantially increased breast cancer risk. Little is known about how menarcheal and reproductive factors modify this risk.
Methods:
We examined the effects of menarcheal age, pregnancy, and menopausal age on breast cancer risk following sRT in case–control data from questionnaires completed by 2497 women from a cohort of 5002 treated with sRT for HL at ages <36 during 1956–2003.
Results:
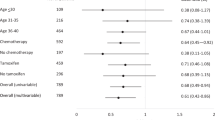
Two-hundred and sixty women had been diagnosed with breast cancer. Breast cancer risk was significantly increased in patients treated within 6 months of menarche (odds ratio (OR) 5.52, 95% confidence interval (CI) (1.97–15.46)), and increased significantly with proximity of sRT to menarche (Ptrend<0.001). It was greatest when sRT was close to a late menarche, but based on small numbers and needing reexamination elsewhere. Risk was not significantly affected by full-term pregnancies before or after treatment. Risk was significantly reduced by early menopause (OR 0.55, 95% CI (0.35–0.85)), and increased with number of premenopausal years after treatment (Ptrend=0.003).
Conclusion:
In summary, this paper shows for the first time that sRT close to menarche substantially increases breast cancer risk. Careful consideration should be given to follow-up of these women, and to measures that might reduce their future breast cancer risk.
Similar content being viewed by others
Main
Survival rates in Hodgkin lymphoma (HL) patients have improved greatly over the past few decades (Diehl et al, 2004), due to improvements in treatment techniques. As a consequence, late effects of HL treatment are becoming an increasing problem, with survivors experiencing increased risks of second malignancies (van Leeuwen et al, 2007). A major concern is the greatly increased breast cancer risk in young women treated for HL with supradiaphragmatic radiotherapy (sRT), who face cumulative risks of up to 48% of developing breast cancer by 40 years after treatment (Swerdlow et al, 2012). It is important to understand how breast cancer risk in these patients is modified by other factors. Treatment age, field of sRT, and treatment with alkylating chemotherapy (CT) or pelvic radiotherapy (RT) have been identified as factors that affect this risk (van Leeuwen et al, 2007; Inskip et al, 2009; Swerdlow et al, 2012). Menarche, pregnancy, and menopause are periods in life that have been considered critical to breast cancer risk (Hankinson et al, 2004; Colditz et al, 2006), and although a reduced risk of breast cancer has been identified in women who experience early menopause following HL treatment (Travis et al, 2003; van Leeuwen et al, 2003), little research has been carried out into how menarcheal and reproductive factors affect breast cancer risk after sRT. The high risks for women irradiated at young ages (Hancock et al, 1993; Bhatia et al, 1996; Mauch et al, 1996; Sankila et al, 1996; Aisenberg et al, 1997; Metayer et al, 2000; De Bruin et al, 2009; Swerdlow et al, 2012) imply that puberty might be a time of raised susceptibility. The rapid differentiation that occurs in the breast during a first pregnancy (Russo et al, 1992) suggests the possibility of a raised breast cancer risk following exposure during first pregnancy, at least. We have, therefore, investigated the effect of menarcheal age, pregnancy, and menopausal age in 2497 women treated with sRT for HL at ages ⩽35 years.
Patients and methods
Data collection
A cohort of 5002 women treated with sRT for HL at ages ⩽35 years during 1956–2003 in England and Wales was identified, and details of their treatment (both initial and at relapses) and all subsequent breast cancer diagnoses (including ductal carcinoma in situ) were collected. Follow-up was complete to the end of 2008 for 97% of patients. Multiple sources were used to ensure completeness of identification of breast cancers. The methods are described in detail elsewhere (Swerdlow et al, 2012). Where possible details of oestrogen receptor (ER) status were collected from clinical and registry sources. During 2003–2010 cohort members who were alive and resident in the UK were invited to complete a questionnaire, given to them by consultants when they attended clinic or sent to them by post. The questionnaire asked about lifestyle factors, medical history, and reproductive history, including age at menarche, delivery date and outcome of pregnancies, disturbances to the menstrual cycle including changes in frequency and temporary or permanent cessation, and use of oral contraceptives (OCs) and hormone replacement therapy (HRT). Blood samples were collected on a nested case–control basis from women who responded to the questionnaire, and genotyped for several single-nucleotide polymorphisms that have previously been identified as influencing breast cancer risk, as described elsewhere (Ma et al, 2012).
Medical research ethics approval was granted by the South East Multi-centre Research Ethics Committee, and patients gave signed informed consent at the time of questionnaire completion.
Statistical analysis
To investigate the effect of reproductive and menstrual factors on breast cancer risk, we analysed the data from questionnaire respondents as a case–control study, with cases being cohort members diagnosed with breast cancer, and controls being the cohort members without a known breast cancer diagnosis or prophylactic bilateral mastectomy.
Not all patients could be included in analyses of the effect of pregnancy, as some were still at childbearing age when they completed the questionnaire and hence could have experienced pregnancies after that date. Cases who completed their questionnaire before the age of 53 and were subsequently diagnosed with breast cancer were excluded from analyses involving post treatment pregnancies, as they may have experienced pregnancies between the questionnaire and the cancer occurrence that were unknown to us. All patients who completed the questionnaire at ages ⩾53 were included in these analyses, as it was considered that they were very unlikely to have experienced further pregnancies. Patients who completed the questionnaire before age 53 and were either not diagnosed with breast cancer or diagnosed before completing the questionnaire were stratum-matched on age and date of first HL treatment, so that a date comparable to a breast cancer diagnosis date could be allocated to controls, referred to as the reference date. This was used to ensure that cases and controls were analysed at a comparable point in their reproductive history. For cases, the reference date was defined as the breast cancer diagnosis date. For controls, the patients were stratified by age and date at start of HL treatment in 5-year groups and the reference date was calculated by adding the median time between start of HL treatment and breast cancer diagnosis in cases in the control’s stratum to the date that the control began treatment for HL, provided that the reference date was not later than their questionnaire completion date. For these patients, pregnancies after the reference date were omitted from analysis, and controls who could not be allocated a reference date were excluded from these analyses. The same reference date and exclusions were applied to the analysis of number of premenopausal years after the start of treatment.
Details of any disturbances in a patient’s menstrual cycle were used to define whether she had experienced premature menopause, at ages <40, or had reached menopause <5 years from start of HL treatment. Patients who had not yet reached age 40 or had been diagnosed with breast cancer before age 40 were excluded from analyses of premature menopause, and those who had completed the questionnaire within 5 years of starting treatment or had been diagnosed with breast cancer within 5 years of starting treatment were excluded from analyses of menopause within 5 years of treatment. Patients who had undergone oophorectomy or hysterectomy (as this often includes oophorectomy) were considered to be postmenopausal from the date of surgery. Menopausal status at each time point was considered unknown if the patient was using OCs or a contraceptive implant, injection or hormonal (Mirena) coil, or HRT, and had not reported going through menopause before then, and was considered unknown at all time points if the patient completed the questionnaire at age ⩾53 and did not mention having gone through menopause. Seven patients who stated that they had never experienced a natural menarche due to HL treatment at an early age were considered postmenopausal from 6 months after their final HL treatment. The stratum matching carried out for pregnancy analyses was also used in the analysis of premenopausal years following HL treatment.
Breast cancer risks were estimated using unconditional logistic regression to calculate odds ratios (OR), 95% confidence intervals (CI) and two-sided P-values (Breslow and Day, 1980). Models were adjusted for all, or where appropriate a subset, of: age and calendar year at first HL treatment, interval between start of HL treatment and questionnaire completion, calendar year of birth, whether the patient received ovarian-toxic treatment (⩾5 Gy pelvic RT or any cycles of alkylating CT), and field of sRT (mantle, other, or unknown). Trend tests were carried out on ordinal variables using a likelihood ratio test. Analyses were conducted using Stata (StataCorp, 2007), with no imputation for missing values.
Results
We received questionnaires from 2508 women from the cohort of 5002. Of the remainder, 1105 had died, 999 were mailed but did not complete a questionnaire, 35 had emigrated, and 355 were not known to have died or emigrated but were not contacted for other reasons. Of those who returned a questionnaire, 149 had had breast cancer diagnosed before completing it, 111 have had breast cancer diagnosed since completing it, and 2248 are not known to have had breast cancer. Eleven patients indicated that they had had prophylactic bilateral mastectomies, and were, therefore, excluded from analyses. Patients who were alive but did not complete a questionnaire did not differ significantly from those who did in terms of calendar year or age of treatment, or type of treatment received (not in tables). Those who had died were treated at similar ages to those who took part, but significantly more were treated in early years, and a significantly higher proportion received ovarian-toxic treatments (not in tables). There was a significantly higher proportion of breast cancer cases in the group of patients who participated than in the group who were alive but did not participate, or those who had died (not in tables).
The median age at first HL treatment among patients who completed a questionnaire was 24.3 years, and 25% were treated before age 20 (Table 1). Thirty-two percent of patients were treated with sRT alone, 61% with sRT in combination with ovarian-toxic treatment, and 7% received sRT but it was unknown whether they received other treatments. Questionnaires were completed a median of 17.2 years after first treatment.
The mean age at menarche was 13.5 years (Table 2). At menarcheal ages <15, there was no apparent effect of age at menarche on breast cancer risk; however, risks were progressively and greatly raised in patients with the oldest menarcheal ages, significantly so for ages 16 (OR 2.65, 95% CI (1.28–5.48)) and 17 (OR 3.74, 95% CI (1.08–12.98)) compared with age 13 (Table 2). The menarcheal age results were not materially altered by: adjustment for rapidity of onset of menopause after treatment; separately analysing patients treated with sRT only (n=785) and those who also received ovarian-toxic treatments (n=1565) (although higher ORs were found with older menarcheal ages in the ovarian-toxic treatment group); analysing risk of ER positive (n=100) and negative (n=33) breast cancer separately (although a higher OR was found with the oldest menarcheal ages for ER-positive cancer); analysing risk of invasive breast cancer only (n=182); repeating the analysis adding the seven patients who did not experience a natural menarche (using their age of induced menarche as a substitute for natural menarcheal age); restricting analysis to patients who received 35–40 Gy mantle RT only (n=984); or restricting analysis to those treated ⩾10 years after menarche (n=1370) (not in tables). Analysis of FGFR2 genotype, which was significantly associated with breast cancer risk in these women (Ma et al, 2012) (n=436), found no association between genotype and menarcheal age (not in tables).
The proximity of sRT to menarche had a strong effect on risk: patients who received sRT within 5 years of menarche experienced a significantly raised breast cancer risk compared with those treated ⩾10 years after menarche, with a peak 5.5-fold risk (95% CI (1.97–15.46)) in those treated within 6 months of menarche (Table 2). There was a significant trend of decreasing breast cancer risk with increasing time between menarche and sRT (Ptrend<0.001). Although the effect of treatment close to menarche was present for both those with early or normal menarche (aged <15 years) and those with late menarche (aged ⩾15 years), it was greater in the latter group; breast cancer risk reached a 15.6-fold increase (95% CI (3.92–62.22)) in those treated 0.5–2 years after a late menarche compared with those treated ⩾10 years after a normal-age menarche, which was highly significant, although based on small numbers.
Patients who had experienced a full-term pregnancy (FTP) had a similar breast cancer risk to those who had not experienced any FTPs (OR 1.17, 95% CI (0.79–1.73)) (Table 3). Patients who had experienced a FTP before receiving sRT had a non-significantly reduced breast cancer risk compared with those who had not (OR 0.77, 95% CI (0.52–1.12)), with similar results when patients treated with and without ovarian-toxic treatments were analysed separately (not in tables), and patients who had experienced a FTP after sRT had a non-significantly raised risk (OR 1.33, 95% CI (0.92–1.92)). There was no evidence that proximity of sRT to first FTP affected breast cancer risk (Table 3). Ninety-two cases were excluded from pregnancy analyses because they completed their questionnaire before the age of 53 and were subsequently diagnosed with breast cancer, and 1056 controls were excluded because they could not be allocated a reference date; results were similar if a cutoff age of 45 was used instead of 53 (not in tables).
Thirty-six percent of those who completed the questionnaire at ages ⩾40 had experienced premature menopause. These patients had a significantly reduced breast cancer risk (OR 0.65, 95% CI (0.44–0.94)) compared with those still premenopausal at age 40 (Table 4). Similar results were found when this analysis was repeated for menopausal status at age 35 (OR 0.69, 95% CI (0. 47–1.02)) or age 45 (OR 0.54, 95% CI (0.35–0.84)) (not in tables). Within the 2358 patients who completed the questionnaire ⩾5 years after HL treatment, there was a significantly reduced breast cancer risk in those who reached menopause within 5 years of starting treatment (OR 0.55, 95% CI (0.35–0.85)) (Table 4). Breast cancer risk increased significantly with increasing number of premenopausal years following treatment (Ptrend=0.003).
Analyses were repeated adjusting for use of OCs or HRT, with no material difference to results.
Discussion
Women treated with sRT for HL at young ages are known to be at greatly increased risk of breast cancer; consequently it is important to understand how this raised risk is modified by other factors. Analyses of the effect of age at sRT find greatest risks for treatment in the early teenage years (Bhatia et al, 1996; Metayer et al, 2000; Inskip et al, 2009; Swerdlow et al, 2012), or in the first two decades of life (Hancock et al, 1993; Mauch et al, 1996; Aisenberg et al, 1997; De Bruin et al, 2009); similarly, analysis of breast cancer risk in atomic bomb survivors has found a significantly decreasing risk with increasing age at exposure (Land et al, 2003). No studies, however, have examined directly whether sRT close to menarche, when the breast is developing, is particularly hazardous. We were able to categorise patients according to time between menarche and sRT, and found a clear trend for greater breast cancer risks the closer that exposure was to menarche; this applied both to patients treated before menarche and those treated after menarche. On average, pubertal breast development begins 2.3 years before menarche, continuing for 2.1 years afterwards (Marshall and Tanner, 1969); this period corresponds to the categories where we found the highest and most significantly raised breast cancer risks. Our result is, therefore, consistent with the theory that susceptibility of the breast to carcinogens is particularly high around menarche (Korenman, 1980; Russo and Russo, 1997).
It has been consistently shown that older menarcheal age confers a protective effect against breast cancer in the general population (Colditz et al, 2006). Thus, the increased breast cancer risk we found in sRT patients with a late menarche seems at first sight surprising. Risk in relation to older menarcheal age in HL patients has been investigated in one, much smaller, previous study (van Leeuwen et al, 2003), which found a somewhat raised risk with menarche at ages ⩾15 years, but with a wide CI based on only eight cases with menarche at these ages. The raised risk that we found with late menarche remained when we adjusted for menopausal age, added patients who never experienced natural menarche following HL treatment at young ages, analysed separately patients treated with sRT only and those also treated with ovarian-toxic treatments, analysed separately by ER status, and restricted to invasive cancers only. Our results thus suggest that the relationship between menarcheal age and breast cancer risk might be different in heavily irradiated women from that in the general population, although as they are based on small numbers it would be desirable to repeat these analyses in other similar cohorts.
Analysis of the proximity of sRT to menarche found that the raised risk in patients irradiated close to menarche was particularly high in those with late menarche, although risk for those with late menarche remained somewhat raised even if they received sRT ⩾10 years later. Menarche can be delayed because of poor health (Neinstein, 1985), which suggests the possibility that late menarche could have been a marker of more unwell patients who received greater radiation doses. This potential confounding did not explain the results, however, as the effect remained when we restricted the analysis to patients who received 35–40 Gy mantle RT.
It has been shown that around half of the variation in timing of menarche in the general population is attributable to genetic factors (Morris et al, 2011), and several genes associated with menarcheal age have been identified (Dvornyk and Ul-Haq, 2012). It is thought that there is also an element of genetic predisposition involved in radiosensitivity (Burrill et al, 2000; Gatti, 2001), and hence a potential explanation for our finding could be genetic linkage between loci associated with these two factors. A previous study including a subset of our patients found that FGFR2 was associated with radiation-induced breast cancer risk (Ma et al, 2012); however, analysing the same subset we found no evidence of an association between FGFR2 genotype and menarcheal age.
In the general population, later menarche is associated with greater height (Onland-Moret et al, 2005) and lower body mass index (BMI) in adulthood (Pierce and Leon, 2005); this was also true in our study. Height has a small positive association with breast cancer risk (Gunnell et al, 2001) and low BMI is associated with an increased risk of premenopausal breast cancer (van den Brandt et al, 2000). It is implausible, however, that these associations could account for the risks that we found with late menarche, as the effects are small compared with the difference in effect of menarcheal age in the irradiated women from that in the general population.
High breast density is one of the strongest known breast cancer risk factors (McCormack and dos Santos Silva, 2006). Some studies have found evidence of a positive association between breast density and menarcheal age (Sala et al, 2000; Titus-Ernstoff et al, 2006; Butler et al, 2008), although others have found no association (Vachon et al, 2000; Maskarinec et al, 2006). The raised risk that we found with late menarche could not directly be a consequence of the association between breast density and breast cancer risk, however, as this would also hold in the general population, in whom late menarche is not associated with raised breast cancer risk. Also, the size of the putative association of menarcheal age and breast density is too small.
Evidence from some small studies (Marti-Henneberg and Vizmanos, 1997; Pantsiotou et al, 2008) suggests an association between early onset of puberty and greater duration of breast growth, although others found no association (Marshall and Tanner, 1969; Largo and Prader, 1983). This suggests the possibility that the increased breast cancer risk we found in patients treated close to a late menarche might be due to more rapid pubertal breast growth in these patients, with the increased rate of cell division at this time resulting in greater susceptibility of the breast to carcinogenesis. We are unable to investigate this further, however, as data on breast development in the study subjects were not collected.
The breast undergoes rapid change during first FTP (Russo et al, 1992), and it has been suggested that this may result in increased breast cancer risk in patients treated with sRT during first FTP (Ronckers et al, 2005). Only one previous study has examined breast cancer risk after irradiation during pregnancy in HL patients; this found a significantly increased risk, although based on very small numbers and with limited follow-up (Chen et al, 2004). We found no significant effect of irradiation during first FTP on breast cancer risk, based on 12 cases irradiated. There was also no significant difference in risk between women who had ever had a FTP and those who had not, contrary to the position in the general population in whom completion of a FTP confers a long-term protective effect against breast cancer (Colditz et al, 2006). Risk was slightly reduced in patients who had had a FTP before sRT however, consistent with the theory that susceptibility of the breast to carcinogens is reduced after first FTP (Russo and Russo, 1995), and the slight increase in risk in patients who had had a FTP after sRT, likely caused by confounding by the effect of continued ovarian function after HL treatment, may explain the lack of reduced risk following FTP overall. Our results are consistent with those from two previous smaller studies, based on 51 and 37 parous cases, respectively (van Leeuwen et al, 2003; Hill et al, 2005), which also found no significant effect of pregnancies before or after HL on breast cancer risk.
Early menopause is known to have a protective effect on breast cancer risk in the general population (Trichopoulos et al, 1972; La Vecchia et al, 1992), and HL patients frequently experience early menopause as a consequence of ovarian-toxic treatment (De Bruin et al, 2008). Previous studies have found significantly reduced breast cancer risks in HL patients who experience premature menopause compared with those who maintain ovarian function post treatment (Travis et al, 2003; van Leeuwen et al, 2003), with risk increasing with number of premenopausal years after treatment (van Leeuwen et al, 2003). Our results add to the evidence, based on larger numbers: we found significant reductions in risk in both those who experienced menopause at ages <40, and those who experienced menopause <5 years after starting treatment, and a significantly increasing breast cancer risk with increasing number of premenopausal years post treatment.
As we only received questionnaires from half of the original cohort members, there is the possibility that those who responded could have been biased in some way. It seems implausible, however, that patients would have replied or not depending on combinations of their breast cancer status and their age at menarcheal/reproductive status. Similarly, it seems implausible that not including patients who had died could have introduced bias, as this would only occur if mortality were associated with the combination of breast cancer status and the reproductive factor being analysed. As our data on menarcheal ages were ascertained retrospectively, they could be subject to recall bias. Recall of menarcheal age is generally good, however, even after several decades (Bean et al, 1979; Must et al, 2002), and there seems no reason why any misclassification should have been biased, as there is no association well known to the public that would lead a patient to misreport their menarcheal age in a certain way depending on their breast cancer status.
The treatment dates of patients in our cohort cover a considerable period of time, during which many improvements to RT techniques were made. To investigate whether effects on breast cancer risk varied over time we re-ran the analyses separately by time period; however, because few breast cancers have occurred to date in the patients treated most recently, it is not possible to determine any differences at present. As many different field and dose combinations have been utilised over time we also re-ran analyses restricted to patients treated with 35–40 Gy mantle RT only, as the largest homogeneous group: results did not markedly differ from those for the whole cohort. Additionally, modern RT techniques differ in some respects from those used when the majority of patients in our study were treated. However, it is in principle impossible to assess long-term effects of current techniques until many years in the future, when sufficient time has elapsed since their introduction. Therefore, the predicted effects of modern treatment techniques must inevitably for the present be inferred from analyses of older treatments, as in our and other data (Aisenberg et al, 1997; Travis et al, 2003; van Leeuwen et al, 2003).
In conclusion, we have found that risk of breast cancer after sRT increases when sRT is given closer to menarche, and we have provided more precise data than previously available, based on larger numbers, on the effect of menopausal age on RT-related breast cancer risk. It has previously been hypothesised that the period around menarche may be a time of greater susceptibility of the breast to carcinogens (Korenman, 1980; Russo and Russo, 1997), but as far as we know there have been no previous data showing this directly in humans. Although the result was highly significant, it would be desirable to reexamine it in other cohorts. Meanwhile, these high risks should be considered when planning treatment and screening regimens for HL patients diagnosed close to menarche. Our results also suggest that research consideration is needed of the potential value of hormonal suppression of puberty, as used for instance for precocious puberty (Carel and Léger, 2008), in these patients.
Change history
11 June 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aisenberg AC, Finkelstein DM, Doppke KP, Koerner FC, Boivin JF, Willett CG (1997) High risk of breast carcinoma after irradiation of young women with Hodgkin’s disease. Cancer 79 (6): 1203–1210
Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H (1979) Variations in the reporting of menstrual histories. Am J Epidemiol 109 (2): 181–185
Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows AT (1996) Breast cancer and other second neoplasms after childhood Hodgkin’s disease. New Engl J Med 334 (12): 745–751
Breslow NE, Day NE (1980) Statistical methods in cancer research. Volume I—The Analysis of Case-Control Studies International Agency for Research on Cancer: Lyon, France
Burrill W, Barber JB, Roberts SA, Bulman B, Scott D (2000) Heritability of chromosomal radiosensitivity in breast cancer patients: a pilot study with the lymphocyte micronucleus assay. Int J Radiat Biol 76 (12): 1617–1619
Butler L, Gold E, Greendale G, Crandall C, Modugno F, Oestreicher N, Quesenberry C, Habel L (2008) Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN). Breast Cancer Res Tr 112 (1): 165–174
Carel J-C, Léger J (2008) Precocious puberty. New Engl J Med 358 (22): 2366–2377
Chen J, Lee RJ, Tsodikov A, Smith L, Gaffney DK (2004) Does radiotherapy around the time of pregnancy for Hodgkin’s disease modify the risk of breast cancer? Int J Radiat Oncol Biol Phys 58 (5): 1474–1479
Colditz G, Baer H, Tamimi R (2006) Breast cancer. In Cancer Epidemiology and Prevention Scottenfeld D, Fraumeni J, (eds). 3rd edn, pp 995–1012. Oxford University Press: New York, NY, USA
De Bruin ML, Huisbrink J, Hauptmann M, Kuenen MA, Ouwens GM, van’t Veer MB, Aleman BMP, van Leeuwen FE (2008) Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood 111 (1): 101–108
De Bruin ML, Sparidans J, van’t Veer MB, Noordijk EM, Louwman MWJ, Zijlstra JM, van den Berg H, Russell NS, Broeks A, Baaijens MHA, Aleman BMP, van Leeuwen FE (2009) Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27 (26): 4239–4246
Diehl V, Thomas RK, Re D (2004) Part II: Hodgkin’s lymphoma—diagnosis and treatment. Lancet Oncol 5 (1): 19–26
Dvornyk V, Ul-Haq W (2012) Genetics of age at menarche: a systematic review. Hum Reprod Update 18 (2): 198–210
Gatti RA (2001) The inherited basis of human radiosensitivity. Acta Oncol 40 (6): 702–711
Gunnell D, Okasha M, Davey Smith G, Oliver SE, Sandhu J, Holly JMP (2001) Height, leg length, and cancer risk: a systematic review. Epidemiol Rev 23 (2): 313–342
Hancock SL, Tucker MA, Hoppe RT (1993) Breast cancer after treatment of Hodgkin’s disease. J Natl Cancer Inst 85 (1): 25–31
Hankinson SE, Colditz GA, Willett WC (2004) Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res 6 (5): 213–218
Hill DA, Gilbert E, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Wiklund T, Lynch CF, Van’t Veer M, Storm H, Pukkala E, Stovall M, Curtis RE, Allan JM, Boice JD, Travis LB (2005) Breast cancer risk following radiotherapy for Hodgkin lymphoma: modification by other risk factors. Blood 106 (10): 3358–3365
Inskip PD, Robison LL, Stovall M, Smith SA, Hammond S, Mertens AC, Whitton JA, Diller L, Kenney L, Donaldson SS, Meadows AT, Neglia JP (2009) Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol 27 (24): 3901–3907
Korenman SG (1980) The endocrinology of breast cancer. Cancer 46 (4 Suppl): 874–878
La Vecchia C, Negri E, Bruzzi P, Dardanoni G, Decarli A, Franceschi S, Palli D, Talamini R (1992) The role of age at menarche and at menopause on breast cancer risk: Combined evidence from four case-control studies. Ann Oncol 3 (8): 625–629
Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, Tokuoka S (2003) Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1990. Radiation Res 160 (6): 707–717
Largo RH, Prader A (1983) Pubertal development in Swiss girls. Helv Paediatr Acta 38 (3): 229–243
Ma YP, van Leeuwen FE, Cooke R, Broeks A, Enciso-Mora V, Olver B, Lloyd A, Broderick P, Russell NS, Janus C, Ashworth A, Houlston RS, Swerdlow AJ (2012) FGFR2 genotype and risk of radiation-associated breast cancer in Hodgkin lymphoma. Blood 119 (4): 1029–1031
Marshall WA, Tanner JM (1969) Variations in pattern of pubertal changes in girls. Arch Dis Child 44 (235): 291–303
Marti-Henneberg C, Vizmanos B (1997) The duration of puberty in girls is related to the timing of its onset. J Pediat 131 (4): 618–621
Maskarinec G, Pagano I, Lurie G, Kolonel LN (2006) A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidem Biomar 15 (4): 732–739
Mauch P, Kalish L, Marcus K, Coleman C, Shulman L, Krill E, Come S, Silver B, Canellos G, Tarbell N (1996) Second malignancies after treatment for laparotomy staged IA-IIIB Hodgkin’s disease: long-term analysis of risk factors and outcome. Blood 87 (9): 3625–3632
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidem Biomar 15 (6): 1159–1169
Metayer C, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, Joensuu T, van Leeuwen FE, van’t Veer MB, Curtis RE, Holowaty EJ, Andersson M, Wiklund T, Gospodarowicz M, Travis LB (2000) Second cancers among long-term survivors of hodgkin’s disease diagnosed in childhood and adolescence. J Clin Oncol 18 (12): 2435–2443
Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ (2011) Familial concordance for age at menarche: analyses from the Breakthrough Generations Study. Paediatr Perinat Epidemiol 25 (3): 306–311
Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM (2002) Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 155 (7): 672–679
Neinstein LS (1985) Menstrual dysfunction in pathophysiologic states. West J Med 143 (4): 476–484
Onland-Moret NC, Peeters PHM, van Gils CH, Clavel-Chapelon F, Key T, Tjønneland A, Trichopoulou A, Kaaks R, Manjer J, Panico S, Palli D, Tehard B, Stoikidou M, Bueno-De-Mesquita HB, Boeing H, Overvad K, Lenner P, Quirós JR, Chirlaque MD, Miller AB, Khaw KT, Riboli E (2005) Age at menarche in relation to adult height. Am J Epidemiol 162 (7): 623–632
Pantsiotou S, Papadimitriou A, Douros K, Priftis K, Nicolaidou P, Fretzayas A (2008) Maturational tempo differences in relation to the timing of the onset of puberty in girls. Acta Paediatr 97 (2): 217–220
Pierce MB, Leon DA (2005) Age at menarche and adult BMI in the Aberdeen Children of the 1950s Cohort Study. Am J Clin Nutr 82 (4): 733–739
Ronckers CM, Erdmann CA, Land CE (2005) Radiation and breast cancer: a review of current evidence. Breast Cancer Res 7 (1): 21–32
Russo J, Rivera R, Russo IH (1992) Influence of age and parity on the development of the human breast. Breast Cancer Res Tr 23 (3): 211–218
Russo J, Russo IH (1995) Hormonally induced differentiation: a novel approach to breast cancer prevention. J Cell Biochem Supplement 22: 58–64
Russo J, Russo IH (1997) Toward a unified concept of mammary carcinogenesis. Prog Clin Biol Res 396: 1–16
Sala E, Warren R, McCann J, Duffy S, Luben R, Day N (2000) High-risk mammographic parenchymal patterns, hormone replacement therapy and other risk factors: a case-control study. Int J Epidemiol 29 (4): 629–636
Sankila R, Garwicz S, Olsen JH, Döllner H, Hertz H, Kreuger A, Langmark F, Lanning M, Möller T, Tulinius H (1996) Risk of subsequent malignant neoplasms among 1,641 Hodgkin’s disease patients diagnosed in childhood and adolescence: a population-based cohort study in the five Nordic countries. Association of the Nordic Cancer Registries and the Nordic Society of Pediatric Hematology and Oncology. J Clin Oncol 14 (5): 1442–1446
StataCorp (2007) Stata Statistical Software : Release 10. College Station, TX: StataCorp LP, see http://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/
Swerdlow AJ, Cooke R, Bates A, Cunningham D, Falk SJ, Gilson D, Hancock BW, Harris SJ, Horwich A, Hoskin PJ, Linch DC, Lister TA, Lucraft HH, Radford JA, Stevens AM, Syndikus I, Williams MV (2012) Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: A National Cohort Study. J Clin Oncol 30 (22): 2745–2752
Titus-Ernstoff L, Tosteson AN, Kasales C, Weiss J, Goodrich M, Hatch EE, Carney PA (2006) Breast cancer risk factors in relation to breast density (United States). Cancer Cause Control 17 (10): 1281–1290
Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Wiklund T, Lynch CF, Van’t Veer MB, Glimelius I, Storm H, Pukkala E, Stovall M, Curtis R, Boice JD Jr., Gilbert E (2003) Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 290 (4): 465–475
Trichopoulos D, MacMahon B, Cole P (1972) Menopause and breast cancer risk. J Natl Cancer Inst 48 (3): 605–613
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA (2000) Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Cause Control 11 (7): 653–662
van den Brandt PA, Spiegelman D, Yaun S-S, Adami H-O, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ (2000) Pooled Analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 152 (6): 514–527
van Leeuwen FE, Klokman WJ, Stovall M, Dahler EC, esvan’t Veer MB, Noordijk EM, Crommelin MA, Aleman BM, Broeks A, Gospodarowicz M, Travis LB, Russell NS (2003) Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst 95 (13): 971–980
van Leeuwen FE, Swerdlow AJ, Travis LB (2007) Second cancers after treatment of hodgkin lymphoma. In Hodgkin LymphomaHoppe RT, Mauch PT, Armitage JO, Diehl V, Weiss LM, (eds). 2nd edn, pp 347–370. Lippincott Williams & Wilkins: Philadelphia, PA, USA
Acknowledgements
This work was supported by Breakthrough Breast Cancer (grant number BR 02/04); and the European Commission (grant number 223497). The Institute of Cancer Research and Royal Marsden Hospital acknowledge funding from the National Institute for Health Research to the Biomedical Research Centre. We thank the large number of health service staff who contributed to the National Notification process and to obtaining data and approaching patients, including doctors, secretaries, nurses, radiographers and data managers at hospitals across the country, staff of the 37 cancer networks, staff of the regional cancer registries and breast screening centres, and Justine Windsor, Cheryl Cavanagh and Michael Richards. We also thank the patients; Ha Nguyen, Deborah Hogben, Zongkai Qiao, Beverley Smith, Joanne Micic and Robert McCann for administrative help; Alison Butlin, Alison Hart, Margo Pelerin, Jill Wood and Sue Chell for help in data extraction; Ausra Kesminiene, Craig Higgins, Alan Ashworth, Peter Clayton, Nick Orr and Danielle Morris for advice; and Richard Houlston and his team for FGFR2 genotype data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
APPENDIX 1
APPENDIX 1
The Group includes those above plus: Gabriel Anghel, Lincoln Hospital; Brian Attock, North Devon District Hospital; Jane Barrett, Royal Berkshire Hospital; Andrew Bates, Southampton General Hospital; Andrew Bell, Poole Hospital; Kim Benstead, Cheltenham General Hospital; Eric M Bessell, Nottingham University Hospital; Ashoke Biswas, Royal Preston Hospital; Norbert Blesing, Great Western Hospital, Swindon; Caroline Brammer, New Cross Hospital, Wolverhampton; Jill Brock, Clatterbridge Centre for Oncology; Alison Brownell, Queens Hospital, Romford; A Murray Brunt, University Hospital of North Staffordshire; Peter B Coates, Queen Elizabeth Hospital, King’s Lynn; Matthew P Collinson, Royal Cornwall Hospital; Neville Davidson, Essex County Hospital; Sian Davies, North Middlesex University Hospital; Ian Fentiman, Guy’s Hospital; Eve Gallop-Evans, Velindre Hospital; Angel Garcia, Glan Clwyd Hospital; Andrew Goodman, Royal Devon & Exeter Hospital; Adrian N Harnett, Norfolk & Norwich University Hospital; Clive JR Irwin, University Hospital, Coventry; Stephen A Kelly, Derriford Hospital; Judith Kingston, Great Ormond Street Hospital; Christopher Knechtli, Royal United Hospital, Bath; Matthew Lyttelton, Kettering General Hospital; Zor Maung, University Hospital of North Tees; Christopher McNamara, Royal Free Hospital; Jamey S Morgan, Ipswich Hospital; Philip Murray, Colchester General Hospital; Hugh O’Brien, Cumberland Royal Infirmary; Ann O’Callaghan, St Mary’s Hospital, Portsmouth; Nigel O’Connor, Shrewsbury Hospital; Russell Patmore, Hull Royal Infirmary; Mojca Persic, Derby City General Hospital; Christopher Pocock, Kent & Canterbury Hospital; Saad MB Rassam, Kent & Canterbury Hospital; Hamish Ross, Northampton General Hospital; Amanda Salisbury, Churchill Hospital, Oxford; Philip M Savage, Charing Cross Hospital/Hammersmith Hospital; Joanna Simpson, Royal Sussex Hospital; Roger E Taylor, Singleton Hospital; Gill Thomas, Leicester Royal Infirmary; Colin Trask, Southend Hospital; Nicholas West, Whitehaven Hospital; Stephen J Whitaker, Royal Surrey County Hospital.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Cooke, R., Jones, M., Cunningham, D. et al. Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br J Cancer 108, 2399–2406 (2013). https://doi.org/10.1038/bjc.2013.219
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.219
Keywords
This article is cited by
-
Tailored to a Woman’s Heart: Gender Cardio-Oncology Across the Lifespan
Current Cardiology Reports (2023)
-
Second malignant neoplasms in lymphomas, secondary lymphomas and lymphomas in metabolic disorders/diseases
Cell & Bioscience (2022)
-
Pregnancy-associated cancer and the risk of second primary cancer
Cancer Causes & Control (2022)
-
Individual response of humans to ionising radiation: governing factors and importance for radiological protection
Radiation and Environmental Biophysics (2020)
-
Differential effect of parity on rat mammary carcinogenesis after pre- or post-pubertal exposure to radiation
Scientific Reports (2018)