Abstract
In baseball parlance, a triple threat is a person who can run, hit and throw with aplomb. Leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) is a cell surface protein that antagonises ERBB receptor signalling by downregulating receptor levels. Over 10 years ago, Hedman et al postulated that LRIG1 might be a tumour suppressor. Recently, Powell et al provided in vivo evidence substantiating that claim by demonstrating that Lrig1 loss in mice leads to spontaneously arising, highly penetrant intestinal adenomas. Interestingly, Lrig1 also marks stem cells in the gut, suggesting a potential role for Lrig1 in maintaining intestinal epithelial homeostasis. In this review, we will discuss the ability of LRIG1 to act as a triple threat: pan-ERBB negative regulator, intestinal stem cell marker and tumour suppressor. We will summarise studies of LRIG1 expression in human cancers and discuss possible related roles for LRIG2 and LRIG3.
Similar content being viewed by others
Main
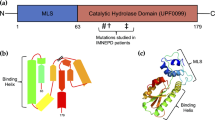
Leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) is a type 1 transmembrane protein whose extracellular domain contains 15 leucine-rich repeats (LRRs) and three immunoglobulin (Ig)-like domains (see Figure 1). Emerging data have underscored the importance of LRIG1 in growth suppression and cancer. Loss of Lrig1 in mice leads to heightened Egfr signalling in keratinocytes (Suzuki et al, 2002), and multiple groups have reported that LRIG1 regulates ERBB receptor degradation (Gur et al, 2004; Laederich et al, 2004; Shattuck et al, 2007; Ledda et al, 2008). Based on immunofluorescent analysis, Lrig1 was proposed to mark a population of quiescent stem cells in the mammalian epidermis (Jensen et al, 2009). This proposal was strengthened by a genetic study demonstrating that Lrig1 marks intestinal stem cells using lineage mapping (Powell et al, 2012). This study also showed that Lrig1 loss results in spontaneous tumour formation, supporting a tumour suppressor role for Lrig1. Interestingly, disrupting one allele of the tumour suppressor gene, adenomatous polyposis coli (Apc), in Lrig1+ cells results in highly dysplastic adenomas in the intestine, supporting the idea that creating an initiating event in Lrig1+ stem cells gives rise to intestinal tumours (Powell et al, 2012). In this review, we discuss the current understanding of the roles of LRIG1 in growth factor signalling modulation, and the evidence that Lrig1 may act as a tumour suppressor.
LRIG1 negatively regulates EGFR signalling. EGFR ligand binding results in phosphorylation of ERBBs and activation of downstream receptor tyrosine kinase signalling. In the current model, LRIG1 is postulated to associate with the EGFR ectodomain and accelerate recruitment of the E3 ligase c-CBL through a CBL-binding domain in the LRIG1 cytoplasmic tail. This effectively increases EGFR ubiquitylation and lysosomal degradation, resulting in decreased receptor levels at the cell surface. LRIG1 potentially downregulates other ERBBs through direct interaction or ERBB heterodimerisation with EGFR.
LRIG1 is a pan-ERBB negative regulator
Since the cloning of mouse Lrig1 (formerly called LIG-1) in 1996 (Suzuki et al, 1996), and human LRIG1 in 2001 (Hedman et al, 2002), studies have focused on deciphering its molecular function. In two independent studies, LRIG1 co-immunoprecipitated with and downregulated all four members of the ERBB receptor family by heterologous expression; in the specific case of EGFR, LRIG1 can reduce receptor levels by promoting receptor ubiquitylation and lysosomal degradation, independent of EGFR ligands (see Figure 1) (Gur et al, 2004; Laederich et al, 2004). The interaction between EGFR and LRIG1 is thought to occur via their ectodomains; disruption of both the LRRs and Ig-like domains of LRIG1 is required to abrogate co-immunoprecipitation with EGFR (Gur et al, 2004). EGFR degradation by LRIG1 appears to be dependent on the E3 ligase, Cbl, as recruitment of Cbl to either EGFR or LRIG1 is sufficient to induce EGFR ubiquitylation and degradation (Gur et al, 2004). There is a marked reduction of EGFR degradation in cells expressing a mutant EGFR that lacks the putative Cbl-binding site (Y1045F) and a mutant LRIG1 lacking the Cbl-binding site. Interestingly, LRIG1 also efficiently downregulates EGFRvIII, an EGFR mutant lacking most of the ectodomain, in the presence of a dominant-negative form of Cbl, suggesting a possible Cbl-independent mechanism for mutant receptor downregulation in this instance (Stutz et al, 2008).
Germline ablation of Lrig1 in two independent mouse models resulted in elevated levels of ErbB1-3 in the intestine (Powell et al, 2012; Wong et al, 2012), providing in vivo evidence to support its role in degrading ErbBs. LRIG1 can also associate with the receptor tyrosine kinase MET, independent of ligand stimulation and receptor activation, and is thought to enhance its degradation without affecting receptor ubiquitylation, independent of Cbl (Shattuck et al, 2007; Lee et al, 2012). Distinct from its mechanism of downregulating ERBB and MET, LRIG1 associates with the RET receptor tyrosine kinase and antagonises glial-derived neurotrophic factor ligand binding to and activating RET (Ledda et al, 2008). LRIG1 silencing in human glioma cells leads to increased proliferation and invasion (Mao et al, 2012). Thus, it appears that LRIG1 may control cell growth through antagonising multiple receptor tyrosine kinases. However, much remains to be learned about how endogenous LRIG1 is regulated, and the structural details through which it interacts with RTKs.
Lrig1 is a tumour suppressor in mouse
A tumour suppressor is a gene in which loss-of-function results in transformation. Classically, both alleles are affected; however, there are examples of haplo-insufficient tumour suppressors, such as p27 (Fero et al, 1998). It is also increasingly appreciated that a tumour suppressor may act at distinct steps in the neoplastic process, such as initiation, invasion or metastasis. Although LRIG1 has been proposed to be a tumour suppressor for over a decade, only recently has genetic evidence demonstrated that Lrig1 ablation in mice leads to spontaneous tumour formation (Powell et al, 2012). Powell et al (2012) engineered a mouse model in which a CreERT2 cassette was inserted into the translational start site of the endogenous Lrig1 locus; mice were generated on a 129S7/SvEv and C57BL/6 mixed background. Mice homozygous for Lrig1-CreERT2 are functionally null for Lrig1, which we refer to as Lrig1Cre/Cre (see Table 1). Of note, we have observed embryonic lethality in Lrig1Cre/Cre mice backcrossed into a pure C57BL/6 background (unpublished results), indicating that in this inbred background, Lrig1 is essential for development. Consistent with the known function of Lrig1 in negatively regulating ErbBs and downstream signalling, the intestines of Lrig1Cre/Cre mice exhibit significantly increased ErbB1-3 protein levels and phosphorylated Erk1/2 (p-Erk1/2), as measured by immunoblot and/or immunohistochemistry. Over 88% of Lrig1Cre/Cre mice develop low-grade duodenal tumours overlying significantly expanded Brunner’s glands; levels of ErbB1-3 and p-Erk1/2 in these tumours are higher than in matched grossly normal small intestinal tissue. Interestingly, these tumours do not exhibit nuclear β-catenin, suggesting that tumours are not due to increased canonical Wnt signalling, but more likely from enhanced ErbB signalling. This is consistent with the notion that proper calibration of ErbB signalling by Lrig1, especially in intestinal stem cells, is critical for intestinal cell and tissue homeostasis.
Genetic ablation of Lrig1 was first reported in 2002, when Suzuki et al (2002) engineered an Lrig1 null allele through insertion of a neomycin cassette after the first half of exon 1, resulting in a premature translational stop. These Lrig1 null mice, referred to as Lrig1neo/neo, also generated on a 129S7/SvEv and C57BL/6 mixed background, developed skin lesions resembling psoriasis on their tail, face and ears, but remained tumour free (see Table 1). Interestingly, as mentioned above, genetic background seems to affect phenotypes in Lrig1 mutant mice. Recently, when Wong et al (2012) crossed Lrig1neo/neo mice into an FVBN background, they observed increased intestinal size and crypt expansion throughout the small intestine, resulting from increased epithelial proliferation at postnatal day 6. The mice appeared to be extremely malnourished and had to be killed, eliminating the possibility of intestinal tumorigenesis studies. Of note, the neomycin cassette is retained in this mutant; constitutive expression of neomycin in the homozygous state has been reported to contribute to various phenotypes, such as embryonic lethality, depending on the gene that it affects (Scacheri et al, 2001). Thus, it cannot be ruled out that neomycin expression in Lrig1neo/neo mice potentially contributes to the phenotypes observed. Despite the different phenotypes in these two Lrig1-null mouse models, it is clear that ablation of Lrig1 in mice leads to enhanced ErbB activity and increased growth, supporting a role for Lrig1 in intestinal homeostasis.
Status of LRIG1 in human cancers
The LRIG1 locus, 3p14.3, is deleted in some human cancers, including nasopharyngeal (Sheu et al, 2009), renal (Willers et al, 1996) and breast cancers (Maitra et al, 2001). However, according to the TCGA cancer data sets (colorectal, lung, glioblastoma and ovarian), the LRIG1 locus is rarely lost or mutated. Here, we discuss multiple studies of LRIG1 expression status in human cancers (also summarised in Table 2).
In a recent bioinformatics gene expression analysis of five cancers (breast, lung, bladder, glioma and melanoma) from eight independent studies (Rouam et al, 2010), LRIG1 was one of four genes whose decreased expression best correlated with poor survival. Low LRIG1 expression also correlated with worse outcome in squamous epithelial uterine cervical cancer (Lindstrom et al, 2008), lymphocytic leukaemia (Hanlon et al, 2009) and colorectal cancer (Figure 2). In a genetic screen, using Villin-CreERT2 to drive an activating Kras mutation on a Cre-activatable Sleeping Beauty transposon background, Lrig1 was the second most frequent gene to be disrupted in the subset of adenomas that advanced to cancer (see discussion in Powell et al, 2012).
In other cancers, LRIG1 expression inversely correlates with tumour stage and can also differ by cancer subtype. For example, in squamous cell carcinoma (SCC) of the skin, LRIG1 was expressed at greater levels in well-differentiated SCC than in poorly differentiated SCC, and SCCs expressing lower LRIG1 correlated with metastasis and decreased survival (Tanemura et al, 2005). In addition, Thomasson et al (2012) reported that LRIG1 transcript and/or protein expression was decreased in clear cell renal cell carcinoma, but not in other histological subtypes.
LRIG1 expression in human cancer must be examined carefully, with attention to tissue context, cancer stage and cancer subtype. This is best exemplified in breast and prostate cancers, where oestrogen and androgen regulation of LRIG1 expression becomes a confounding factor (Miller et al, 2008; Thomasson et al, 2010; Krig et al, 2011). Miller et al (2008) reported decreased LRIG1 transcript and protein levels in 63% of breast cancers examined that inversely correlated with tumour grade, as determined by Oncomine database and immunoblot analyses, respectively. When these data were further scored based on ERBB2+ status, 76% of ERBB2+ breast cancer tumours displayed decreased LRIG1 transcript or protein expression, compared with patient-matched normal tissue. In contrast to ERBB2+ breast tumours, ERα+ breast tumours displayed increased levels of LRIG1 transcript by Oncomine database and immunoblot analysis, and intermediate-to-high LRIG1 gene expression correlated with longer relapse-free survival in ERα+ breast cancer patients (Krig et al, 2011).
These two studies provide in vitro mechanisms to reconcile the different LRIG1 expression patterns observed in ERBB2+ and ERα+ breast cancers. Miller et al (2008) showed that constitutively active ERBB2 had a negative effect on LRIG1 transcript and protein, suggesting that oncogenic ERBB2 may employ a mechanism to decrease the tumour suppressive benefits of LRIG1, thereby imparting an advantage to ERBB2+ breast cancers. In addition, Krig et al (2011) demonstrated that LRIG1 is a direct transcriptional target of ERα, thus suggesting higher LRIG1 expression in ERα+ tumours may be due to ERα activity. Further, they also showed that ERBB2 activation decreases ERα levels, indirectly antagonising LRIG1 expression. This provides a mechanism for disparate LRIG1 expression observed in these subtypes of breast cancer and illustrates the importance of context-specific analysis of LRIG1 expression in human cancer. In a separate analysis, based on intrinsic subtypes of breast cancer, low LRIG1 expression was confirmed in the ERBB2+ subset; LRIG1 expression was highest in the luminal A subtype, the subtype with the best clinical outcome, and high LRIG1 expression correlated with a greater probability of relapse-free survival (Figure 3, personal communication with Dr Charles M Perou).
LRIG1 expression in breast cancers. (A) Box plot of the LRIG1 gene signature in the UNC337 human breast tumour data set based on intrinsic subtypes. LRIG1 expression appears to be significantly upregulated only in the Luminal A intrinsic subtype compared with normal (P=5.83e–40). (B) When divided into high (50/128) and low (29/128) LRIG1-expression groups, patients with high LRIG1 expression exhibit longer relapse-free survival. P-values reflect statistical significance of analysis of variances (ANOVAs; P=0.00561).
Prostate cancer has emerged as an important example of how LRIG1 expression may not directly reflect a tumour suppressor effect. Thomasson et al (2010) examined two independent groups of prostate cancer patients; LRIG1 protein expression correlated with better survival of one group, in which patients had undergone prostatectomy, but worse survival of the second group, in which patients had not received treatment. To address this paradox, the authors showed that LRIG1 transcript and protein are upregulated by androgen signalling. In fact, LRIG1 expression may act as a surrogate marker for androgen activity, which is most often the main driver for prostate carcinogenesis. In this context, the authors suggest that although LRIG1 overexpression decreases PC3 prostate cancer cell growth, tumour-promoting androgen signalling prevails over the tumour suppressive impact of LRIG1 in patients with increased androgen activity and increased LRIG1 (Thomasson et al, 2010). Thus, in a high androgen environment, LRIG1 may reflect androgen signalling and may not exert enough tumour suppressive activity to overcome the threshold of oncogenic events, and thus expression should be carefully evaluated as its role may be masked by other factors.
Finally, a study by Hellberg et al (2009) exemplifies the significance of considering cancer biomarker expression in the context of other factors. This study demonstrated that the prognostic significance of LRIG1 protein expression in cervical cancer was dependent on how the data were correlated with other factors, such as cancer subtype, stage and other known tumour suppressors and oncogenes (Hellberg et al, 2009). Moving forward, it will be important to evaluate LRIG1 expression in human cancer with consideration of cancer stage, subtype and additional factors (such as estrogens) that may influence its expression.
Therapeutic potential of LRIG1
Given that LRIG1 is dysregulated in a number of human cancers, restoring its tumour suppressor function may attenuate growth factor signalling and reduce tumour growth. Interestingly, LRIG1 can associate with and destabilise EGFRvIII, a constitutively active mutant EGFR variant, suggesting there may be a possible therapeutic potential of LRIG1 in cancer, especially in glioblastoma where EGFRvIII is most commonly observed (Stutz et al, 2008). In addition, the soluble ectodomain of LRIG1, containing only the LRRs, associates with and inhibits EGFR activation, regardless of ligand stimulation, demonstrating a potentially novel mechanism of EGFR signalling modulation by LRIG1 (Goldoni et al, 2007). In this context, it has been shown recently that human glioblastomas expressing wild type or VIII mutant EGFR when placed within mouse brain are growth inhibited by nearby encapsulated cells secreting soluble LRIG1 ectodomain (Johansson et al, 2013). An in vitro study also showed that shedding of the LRIG1 ectodomain occurs endogenously and this ectodomain suppresses EGFR activation in a paracrine manner, without downregulating receptor levels (Yi et al, 2010).
Other LRIG family members: LRIG2 and LRIG3
The three LRIG protein family members have homologous functional domains. The extracellular regions of each contain 15 LRRs and three Ig-like extracellular domains and have 57–67% amino-acid sequence identity. Much less is known about the functions of LRIG2 and LRIG3 compared with LRIG1.
Overexpression of LRIG3 in HEK293T cells resulted in upregulation of ERBB receptors, in contrast to LRIG1, which is known to downregulate ERBB receptor levels (Abraira et al, 2010). However, when LRIG3 was knocked down in a human glioblastoma cell line, GL15, both total EGFR and phospho-EGFR were moderately upregulated, consistent with observed increases in proliferation, adhesion and invasion (Cai et al, 2009). Therefore, the precise function of LRIG3 in regard to ERBB receptor signalling modulation may be context-dependent.
The only genetic study regarding Lrig3 in mouse focused on inner ear morphogenesis. Abraira et al (2008) observed that Lrig3 expression was restricted to the lateral canal during embryogenesis; Lrig3 loss led to defects in inner ear morphogenesis and it was shown that during inner ear development, Lrig3 acts to repress Netrin transcription (see Table 1). Although LRIG3 can associate with EGFR, ERBB2 and ERBB4 in vivo, this inner ear phenotype is unlikely associated with ErbB signalling (Abraira et al, 2010). A separate study in Xenopus laevis reported Lrig3 expression in the neural plate and neural crest, and that loss-of-function prevented neural crest marker expression (Zhao et al, 2008). In contrast, Lrig3 gain-of-function induced neural crest marker expression and attenuated Fgf signalling in animal caps, similar to Wnt3a gain-of-function, suggesting that Lrig3 negatively modulates Fgf signalling during X. laevis development (Zhao et al, 2008).
Even less is known about the role of LRIG2 and LRIG3 in human cancers. Mutations in LRIG2 and LRIG3 are rare, according to the TCGA data set. High LRIG2 expression is a poor prognostic marker in early stages of cervical cancers by immunohistochemistry (Hedman et al, 2010). In brain tumours, by semi-quantitative immunohistochemistry, cytoplasmic expression of LRIG2 correlated with decreased survival in oligodendrogliomas (Holmlund et al, 2009) and higher tumour grade in meningiomas (Ghasimi et al, 2012). In addition, LRIG2 protein is highly expressed in invasive pituitary adenomas, but not expressed in non-invasive cases by qPCR (Zhang et al, 2011), suggesting LRIG2 may be differentially regulated during tumour progression. LRIG3 downregulation was identified as one of 12 promising serum biomarkers for early stage non-small-cell lung cancer (Ostroff et al, 2010). Although the three LRIG family members share structural similarities, it is unclear whether they exhibit functional redundancy. The Lrig1 and Lrig3 knockout mice display distinct phenotypes. However, the expression pattern of the Lrig family members in the affected tissues in these knockout mice is unknown. Future studies will need to address this issue of functional redundancy.
Conclusion
In a recent review by Hanahan and Weinberg, perturbation of negative-feedback mechanisms that attenuate proliferative signalling was noted as an emerging hallmark of cancer (Hanahan and weinberg, 2011). These negative-feedback programs are often regulated by tumour suppressor genes, directly or indirectly. LRIG1 qualifies as a negative-feedback inhibitor of ERBBs and other RTKs, and there is now in vivo evidence that it acts as a tumour suppressor. However, it remains to be demonstrated conclusively that its tumour suppressor activity is due directly to its ability to negatively regulate ErbBs and other RTKs. Going forward, it will be important to determine how LRIG1 physically interacts with EGFR and other RTKs, and how LRIG1 is regulated at the transcriptional and post-transcriptional level. It will also be of interest to determine by lineage labelling if Lrig1 marks stem cell populations in other organs than the intestine. Additional questions include whether Lrig1 maintains stem cell quiescence and whether this activity contributes to its tumour suppressor function. The triple threat features of LRIG1 – ERBB negative regulator, stem cell marker and tumour suppressor – clearly underscore the importance of understanding the function of LRIG1 in health and disease.
References
Abraira VE, Del Rio T, Tucker AF, Slonimsky J, Keirnes HL, Goodrich LV (2008) Cross-repressive interactions between Lrig3 and netrin 1 shape the architecture of the inner ear. Development 135: 4091–4099
Abraira VE, Satoh T, Fekete DM, Goodrich LV (2010) Vertebrate Lrig3-ErbB interactions occur in vitro but are unlikely to play a role in Lrig3-dependent inner ear morphogenesis. PLoS One 5: e8981
Cai M, Han L, Chen R, Ye F, Wang B, Han F, Lei T, Guo D (2009) Inhibition of LRIG3 gene expression via RNA interference modulates the proliferation, cell cycle, cell apoptosis, adhesion and invasion of glioblastoma cell (GL15). Cancer Lett 278: 104–112
Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ (1998) The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396: 177–180
Ghasimi S, Haapasalo H, Eray M, Korhonen K, Brannstrom T, Hedman H, Andersson U (2012) Immunohistochemical analysis of LRIG proteins in meningiomas: correlation between estrogen receptor status and LRIG expression. J Neurooncol 108: 435–441
Goldoni S, Iozzo RA, Kay P, Campbell S, McQuillan A, Agnew C, Zhu JX, Keene DR, Reed CC, Iozzo RV (2007) A soluble ectodomain of LRIG1 inhibits cancer cell growth by attenuating basal and ligand-dependent EGFR activity. Oncogene 26: 368–381
Gur G, Rubin C, Katz M, Amit I, Citri A, Nilsson J, Amariglio N, Henriksson R, Rechavi G, Hedman H, Wides R, Yarden Y (2004) LRIG1 restricts growth factor signalling by enhancing receptor ubiquitylation and degradation. Embo J 23: 3270–3281
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674
Hanlon K, Rudin CE, Harries LW (2009) Investigating the targets of MIR-15a and MIR-16-1 in patients with chronic lymphocytic leukaemia (CLL). PLoS One 4: e7169
Hedman H, Lindstrom AK, Tot T, Stendahl U, Henriksson R, Hellberg D (2010) LRIG2 in contrast to LRIG1 predicts poor survival in early-stage squamous cell carcinoma of the uterine cervix. Acta Oncol 49: 812–815
Hedman H, Nilsson J, Guo D, Henriksson R (2002) Is LRIG1 a tumour suppressor gene at chromosome 3p14.3? Acta Oncol 41: 352–354
Hellberg D, Tot T, Stendahl U (2009) Pitfalls in immunohistochemical validation of tumour marker expression--exemplified in invasive cancer of the uterine cervix. Gynecol Oncol 112: 235–240
Holmlund C, Haapasalo H, Yi W, Raheem O, Brannstrom T, Bragge H, Henriksson R, Hedman H (2009) Cytoplasmic LRIG2 expression is associated with poor oligodendroglioma patient survival. Neuropathology 29: 242–247
Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM (2009) Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4: 427–439
Johansson M, Oudin A, Tiemann K, Bernard A, Keunen O, Fack F, Golebiewska A, Stieber D, Wang B, Hedman H, Niclou SP (2013) The soluble form of the tumor suppressor Lrig1 potently inhibits in vivo glioma growth irrespective of EGF receptor status. Neuro-Oncology doi:10.1093/neuonc/not054
Krig SR, Frietze S, Simion C, Miller JK, Fry WH, Rafidi H, Kotelawala L, Qi L, Griffith OL, Gray JW, Carraway KL 3rd, Sweeney C (2011) Lrig1 is an estrogen-regulated growth suppressor and correlates with longer relapse-free survival in ERalpha-positive breast cancer. Mol Cancer Res 9: 1406–1417
Laederich MB, Funes-Duran M, Yen L, Ingalla E, Wu X, Carraway KL 3rd, Sweeney C (2004) The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. J Biol Chem 279: 47050–47056
Ledda F, Bieraugel O, Fard SS, Vilar M, Paratcha G (2008) Lrig1 is an endogenous inhibitor of Ret receptor tyrosine kinase activation, downstream signalling, and biological responses to GDNF. J Neurosci 28: 39–49
Lee JM, Kim B, Lee SB, Jeong Y, Oh YM, Song YJ, Jung S, Choi J, Lee S, Cheong KH, Kim DU, Park HW, Han YK, Kim GW, Choi H, Song PH, Kim KA (2012) Cbl-independent degradation of Met: ways to avoid agonism of bivalent Met-targeting antibody. Oncogene; e-pub ahead of print 3 December 2012; doi:10.1038/onc.2012.551
Lindstrom AK, Ekman K, Stendahl U, Tot T, Henriksson R, Hedman H, Hellberg D (2008) LRIG1 and squamous epithelial uterine cervical cancer: correlation to prognosis, other tumour markers, sex steroid hormones, and smoking. Int J Gynecol Cancer 18: 312–317
Ljuslinder I, Golovleva I, Palmqvist R, Oberg A, Stenling R, Jonsson Y, Hedman H, Henriksson R, Malmer B (2007) LRIG1 expression in colorectal cancer. Acta Oncol 46: 1118–1122
Maitra A, Wistuba II, Washington C, Virmani AK, Ashfaq R, Milchgrub S, Gazdar AF, Minna JD (2001) High-resolution chromosome 3p allelotyping of breast carcinomas and precursor lesions demonstrates frequent loss of heterozygosity and a discontinuous pattern of allele loss. Am J Pathol 159: 119–130
Mao F, Wang B, Xi G, Sun W, Zhang H, Ye F, Guo D, Lei T (2012) Effects of RNAi-mediated gene silencing of LRIG1 on proliferation and invasion of glioma cells. J Huazhong Univ Sci Technolog Med Sci 32: 227–232
Miller JK, Shattuck DL, Ingalla EQ, Yen L, Borowsky AD, Young LJ, Cardiff RD, Carraway KL 3rd, Sweeney C (2008) Suppression of the negative regulator LRIG1 contributes to ErbB2 overexpression in breast cancer. Cancer Res 68: 8286–8294
Ostroff RM, Bigbee WL, Franklin W, Gold L, Mehan M, Miller YE, Pass HI, Rom WN, Siegfried JM, Stewart A, Walker JJ, Weissfeld JL, Williams S, Zichi D, Brody EN (2010) Unlocking biomarker discovery: large scale application of aptamer proteomic technology for early detection of lung cancer. PLoS One 5: e15003
Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ (2012) The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158
Rouam S, Moreau T, Broet P (2010) Identifying common prognostic factors in genomic cancer studies: a novel index for censored outcomes. BMC Bioinformat 11: 150
Scacheri PC, Crabtree JS, Novotny EA, Garrett-Beal L, Chen A, Edgemon KA, Marx SJ, Spiegel AM, Chandrasekharappa SC, Collins FS (2001) Bidirectional transcriptional activity of PGK-neomycin and unexpected embryonic lethality in heterozygote chimeric knockout mice. Genesis 30: 259–263
Shattuck DL, Miller JK, Laederich M, Funes M, Petersen H, Carraway KL 3rd, Sweeney C (2007) LRIG1 is a novel negative regulator of the Met receptor and opposes Met and Her2 synergy. Mol Cell Biol 27: 1934–1946
Sheu JJ, Lee CH, Ko JY, Tsao GS, Wu CC, Fang CY, Tsai FJ, Hua CH, Chen CL, Chen JY (2009) Chromosome 3p12.3-p14.2 and 3q26.2-q26.32 are genomic markers for prognosis of advanced nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 18: 2709–2716
Stutz MA, Shattuck DL, Laederich MB, Carraway KL 3rd, Sweeney C (2008) LRIG1 negatively regulates the oncogenic EGF receptor mutant EGFRvIII. Oncogene 27: 5741–5752
Suzuki Y, Miura H, Tanemura A, Kobayashi K, Kondoh G, Sano S, Ozawa K, Inui S, Nakata A, Takagi T, Tohyama M, Yoshikawa K, Itami S (2002) Targeted disruption of LIG-1 gene results in psoriasiform epidermal hyperplasia. FEBS Lett 521: 67–71
Suzuki Y, Sato N, Tohyama M, Wanaka A, Takagi T (1996) cDNA cloning of a novel membrane glycoprotein that is expressed specifically in glial cells in the mouse brain. LIG-1, a protein with leucine-rich repeats and immunoglobulin-like domains. J Biol Chem 271: 22522–22527
Tanemura A, Nagasawa T, Inui S, Itami S (2005) LRIG-1 provides a novel prognostic predictor in squamous cell carcinoma of the skin: immunohistochemical analysis for 38 cases. Dermatol Surg 31: 423–430
Thomasson M, Hedman H, Ljungberg B, Henriksson R (2012) Gene expression pattern of the epidermal growth factor receptor family and LRIG1 in renal cell carcinoma. BMC Res Notes 5: 216
Thomasson M, Wang B, Hammarsten P, Dahlman A, Persson JL, Josefsson A, Stattin P, Granfors T, Egevad L, Henriksson R, Bergh A, Hedman H (2010) LRIG1 and the liar paradox in prostate cancer: a study of the expression and clinical significance of LRIG1 in prostate cancer. Int J Cancer 128: 2843–2852
Willers CP, Siebert R, Bardenheuer W, Lux A, Michaelis S, Seeber S, Luboldt HJ, Opalka B, Schutte J (1996) Genetic instability of 3p12-p21-specific microsatellite sequences in renal cell carcinoma. Br J Urol 77: 524–529
Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB (2012) Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14: 401–408
Yi W, Holmlund C, Nilsson J, Inui S, Lei T, Itami S, Henriksson R, Hedman H (2010) Paracrine regulation of growth factor signalling by shed leucine-rich repeats and immunoglobulin-like domains 1. Exp Cell Res 317: 504–512
Zhang H, Yan Q, Xu S, Ou Y, Ye F, Wang B, Lei T, Guo D (2011) Association of expression of Leucine-rich repeats and immunoglobulin-like domains 2 gene with invasiveness of pituitary adenoma. J Huazhong Univ Sci Technolog Med Sci 31: 520–523
Zhao H, Tanegashima K, Ro H, Dawid IB (2008) Lrig3 regulates neural crest formation in Xenopus by modulating Fgf and Wnt signaling pathways. Development 135: 1283–1293
Acknowledgements
We thank Charles M. Perou from University of North Carolina and Yan Guo from Vanderbilt University for performing the statistical analysis and providing figures for the UNC337 breast cancer data set and the TCGA colorectal cancer data set, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wang, Y., Poulin, E. & Coffey, R. LRIG1 is a triple threat: ERBB negative regulator, intestinal stem cell marker and tumour suppressor. Br J Cancer 108, 1765–1770 (2013). https://doi.org/10.1038/bjc.2013.138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.138
Keywords
This article is cited by
-
High-throughput functional screen identifies YWHAZ as a key regulator of pancreatic cancer metastasis
Cell Death & Disease (2023)
-
HYPOTHESIS: Do LRIG Proteins Regulate Stem Cell Quiescence by Promoting BMP Signaling?
Stem Cell Reviews and Reports (2023)
-
Lrig1-expression confers suppressive function to CD4+ cells and is essential for averting autoimmunity via the Smad2/3/Foxp3 axis
Nature Communications (2023)
-
LRIG1 is a conserved EGFR regulator involved in melanoma development, survival and treatment resistance
Oncogene (2021)
-
LRIG proteins regulate lipid metabolism via BMP signaling and affect the risk of type 2 diabetes
Communications Biology (2021)