Abstract
Background:
Resistance to cisplatin-based chemotherapy is associated with poor prognosis in testicular germ cell cancer, emphasising the need for new therapeutic approaches. In this respect, the therapeutic concept of anti-angiogenesis is of particular interest. In a previous study, we presented two novel anti-angiogenic compounds, HP-2 and HP-14, blocking the tyrosine kinase activity of angiogenic growth factor receptors, such as vascular endothelial growth factor receptor-2 (VEGFR-2), and related signalling pathways in testicular cancer. In this study, we investigated the efficacy of these new compounds in platinum-resistant testicular germ cell tumours (TGCTs), in vitro and in vivo.
Methods and results:
Drug-induced changes in cell proliferation of the cisplatin-sensitive TGCT cell line 2102EP and its cisplatin-resistant counterpart 2102EP-R, both expressing the VEGFR-2, were evaluated by crystal violet staining. Both compounds inhibited the growth of cisplatin-resistant TGCT cells in a dose-dependent manner. In combination experiments with cisplatin, HP-14 revealed additive growth-inhibitory effects in TGCT cells, irrespective of the level of cisplatin resistance. Anti-angiogenic effects of HP compounds were confirmed by tube formation assays with freshly isolated human umbilical vein endothelial cells. Using TGCT cells inoculated onto the chorioallantoic membrane of fertilised chicken eggs (chicken chorioallantoic membrane assay), the anti-angiogenic and anti-proliferative potency of the novel compounds was also demonstrated in vivo. Gene expression profiling revealed changes in the expression pattern of genes related to DNA damage detection and repair, as well as in chaperone function after treatment with both cisplatin and HP-14, alone or in combination. This suggests that HP-14 can revert the lost effectiveness of cisplatin in the resistant cells by altering the expression of critical genes.
Conclusion:
The novel compound HP-14 effectively inhibits the growth of cisplatin-resistant TGCT cells and suppresses tumour angiogenesis. Thus, HP-14 may be an interesting new agent that should be further explored for TGCT treatment, especially in TGCTs that are resistant to cisplatin.
Similar content being viewed by others
Main
Today, more than 80% of patients with metastatic testicular germ cell tumours (TGCTs) can be cured using cisplatin-based combination chemotherapy (Bokemeyer et al, 2008; Feldman et al, 2008). Therefore, testicular cancer is considered the paradigm for curative cancer. However, patients with cisplatin-resistant or -refractory disease have an unfavourable prognosis, and long-term survival can be achieved in only 10–15% of these patients (Einhorn et al, 2007; Oechsle et al, 2011b).
New treatment options for refractory TGCTs are rare. In the last two decades, only a few chemotherapeutic agents, such as paclitaxel, gemcitabine, oxaliplatin and etoposide have been introduced (Einhorn et al, 2007; De Wit et al, 2011; Oechsle et al, 2011b).
Different combinations of these agents have improved the therapeutic efficacy, resulting in response rates of 51% (Oechsle et al, 2011b). However, these agents are associated with extensive side effects and long-term survival, as well as remission duration are still poor. Therefore, it is mandatory to identify new chemotherapeutic agents for more effective treatment of patients with refractory TGCTs, ideally with reduced side effects, to improve survival and quality of life of mostly very young patients (Schrader et al, 2009).
The process of cisplatin resistance is determined by multiple factors on different cellular levels, such as changes in cellular drug uptake and efflux, leading to decreased drug accumulation. Furthermore, increased drug detoxification in the cytoplasm by the glutathione system and changes in DNA repair leading to an increased removal of cisplatin-induced lesions from the DNA have been described in drug resistance. Another important factor is the modification of apoptotic cell death pathways. Here, altered apoptosis induction has been described because of a decreased expression or loss of function of pro-apoptotic factors on the one hand, or an overexpression of anti-apototic factors on the other hand (Köberle et al, 2010; Galluzzi et al, 2012). The molecular basis of TGCT chemoresistance is still poorly understood. Mismatch repair deficiency and microsatellite instability seem to have a pivotal role (Honecker et al, 2009). Furthermore, the relevance of the mutation status of P53 and MDM2 has been discussed (Houldsworth et al, 1998; Koster et al, 2011). Further studies revealed a correlation between the glutathione levels and cisplatin resistance, as well as between a number of export pumps, such as the ABC transporters and cisplatin resistance (Sark et al, 1995; Masters et al, 1996; Kollmannsberger et al, 2006). Besides factors putatively involved in chemoresistance of TGCTs, other proteins, such as the cell cycle regulatory proteins p21 and Cyclin D1 (Koster et al, 2010; Noel et al, 2010), or proteins involved in the regulation of apoptosis, such as BAX and BCL-2 (Mayer et al, 2003), were proposed.
Preliminary studies suggest that the vascular endothelial growth factor (VEGF) and its receptor (VEGFR) have an important role in the development and metastatic process of TGCTs (Viglietto et al, 1996; Fukuda et al, 1999; Jones et al, 2000; Bentas et al, 2003; Devouassoux-Shisheboran et al, 2003). Increased expression of VEGF and VEGFR-2 has been found in patients with TGCTs, suggesting that the therapeutic concept of anti-angiogenesis may be of particular interest in innovative TGCT treatment (Adam et al, 2003). Angiogenesis is necessary to support the growth of many tumour types and is considered to be a key factor in tumour growth and progression (Folkman, 1971). On the basis of these findings, a number of anti-neoplastic drugs has been designed to target the VEGF pathway in urologic cancers such as the renal cell carcinoma. Anti-angiogenic agents, such as sorafenib, sunitinib, pazopanib or bevacizumab, have expanded the current treatment options (Motzer et al, 2006; Rini et al, 2008; Escudier et al, 2009). In cisplatin-resistant TGCTs, anti-angiogenic agents such as sunitinib showed good activity in vitro and also showed some clinical activity, indicating that anti-angiogenic approaches may also be promising for the treatment of TGCTs, which are resistant to conventional chemotherapy (Castillo-Ávila et al, 2009; Oechsle et al, 2011a).
In a recent study, we identified two novel compounds (HP-2 and HP-14) with anti-tumour activity in VEGFR-expressing TGCTs. The compounds inhibited the growth and migration of TGCT cells and slowed down microvessel network development of fertilised chicken eggs (Nitzsche et al, 2010).
In the present study, we investigated the suitability of HP-2 and HP-14 for growth inhibition of platinum-resistant TGCT cells, and examined the anti-neoplastic and anti-angiogenic effects in vivo by using a modified chicken chorioallantoic membrane (CAM) model (Gloesenkamp et al, 2012b). Moreover, we investigated possible additive anti-neoplastic and/or cisplatin-resensitising effects of a combination treatment of the HP compounds together with cisplatin.
Materials and methods
Cell lines
The human embryonal carcinoma cell line 2102EP, its cis-diamminedichloroplatinum (II; cisplatin)-resistant subline 2102EP-R and the renal cell carcinoma cell line Caki-1 were used (Oechsle et al, 2011a; Port et al, 2011). 2102EP is a cell line derived from a primary tumour classified histologically as teratocarcinoma with yolk sac tumour. 2102EP-R was derived via long-term culture under increasing concentrations of cisplatin from the established TGCT line 2102EP. To induce resistance, cells were incubated with a concentration of cisplatin that causes 10% growth inhibition after 2 days. At reaching 50% lethality upon long-time exposure, the addition of cisplatin was paused and cells were allowed to recover over three passages until the next dose escalation step (Wang et al, 1980; Port et al, 2011). Both cell lines were cultured in DMEM/HAM’s F12 medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin. Caki-1 cells were maintained in RPMI 1640 medium (Biochrom AG) supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin.
Tube formation assays were performed with freshly prepared human umbilical vein endothelial cells (HUVECs) of the passages 1–2. Isolation and preparation was performed as described (Chlench et al, 2007). Cells were cultured in endothelial cell basal medium supplemented with the SupplementPack MV (PromoCell, Heidelberg, Germany). The cell cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. Culture medium was changed every second day, and once a week the cells were passaged using 1% Trypsin/EDTA.
Drugs
Cisplatin was obtained from TEVA (Radebeul, Germany). HP-2 and HP-14 were purchased from Ambinter (Paris, France). Stock solutions were prepared in DMSO, stored at −20 °C and diluted to the final concentration in fresh media before each experiment. In all experiments, the final DMSO concentration was<0.2%.
Measurement of growth inhibition
Drug-induced changes in cell numbers were evaluated by crystal violet staining, as described (Gillies et al, 1986). In brief, cells in 96-well plates were fixed with 1% glutaraldehyde and stained with 0.1% crystal violet. The unbound dye was removed by washing with water. Bound crystal violet was solubilised with 0.2% Triton-X-100 (Sigma-Aldrich, Steinheim, Germany). Light extinction, which increases linearly with the cell number, was analysed at 570 nm using an ELISA-Reader (Dynex Technologies, Denkendorf, Germany).
Flow cytometric analysis
Cell cycle distribution and apoptosis were analysed by flow cytometry after staining the cells with propidium iodide (Fried et al, 1976). 2102EP and 2102EP-R cells were treated with 10 μ M HP-14 or 1 μ M cisplatin for 48 h. Cells were trypsinised, washed with PBS/formaldehyde 2% (vol/vol), fixed with ice-cold ethanol/PBS (2 : 1 vol/vol) overnight at −20 °C and pelleted. Resuspension in PBS containing 40 μg ml−1 RNase A followed. After incubation for 30 min at 37 °C, cells were pelleted again and resuspended in PBS containing 50 μg ml−1 propidium iodide. Stained cells were analysed using a FACSCalibur flow cytometer (BD Biosciences, Heidelberg, Germany) and FlowJo Software (Tree Star, Ashland, OR, USA). The proportion of cells in G0/G1, S and G2/M phase was examined. Identification of apoptotic cells achieved by determination of hypoploid cell populations commonly referred to as ‘sub-G1 peak’. For each experiment, a minimum of 10 000 events per sample were analysed.
Tube formation assay
The HUVEC cells were incubated in serum-free medium overnight. Matrigel (BD Biosciences) was placed onto the bottom of a 96-well microtitre plate and hardened for 30 min at 37 °C. A total of 50 000 serum-starved HUVEC cells per well were seeded on the Matrigel and treated for 6 h with 30 ng ml−1 VEGF and 10 μ M HP-14 (controls with VEGF only). Images were taken with an Axiovert 135 microscope (Carl Zeiss, Jena, Germany) with the Kappa digital camera system (Gleichen, Germany). The images were analysed with the tube formation module of Wimasis (Wimasis, Munich, Germany).
DNA analysis
DNA microarray analysis and RT-PCR (including primer and incubation times) were performed as described previously (Nitzsche et al, 2010). For microarray analysis in brief, total cellular RNA was extracted from cells using ArrayGrade Total RNA Isolation Kit (SABiosciences, Frederick, MD, USA). RNA concentration was measured by absorption spectrophotometry (GeneQuant, Biochrom, Cambridge, UK). Using the True-Labeling AMP 2.0 amplification kit (SABiosciences), the mRNA was reversely transcribed into cDNA and converted to biotin-labelled cRNA using biotin-16-UTP (Roche, Mannheim, Germany) by in vitro transcription. The cRNA samples were purified with an ArrayGrade cRNA cleanup kit (SABiosciences). Thereafter, the probes were hybridised to the pretreated Oligo GEArray Human Toxicology and Drug Resistance Microarray (OHS-401, SABiosciences). After several washing steps, array spots binding cRNA were detected by chemiluminescence staining. Image acquisition was performed using X-ray films and a digital scanner. Spots were analysed and converted to numerical data by using the GEArray Expression Analysis Suite software (SABiosciences). Data evaluation included background correction (subtraction of minimum value) and normalisation to reference genes. The cut-off value for upregulation was set at a 1.5-fold increase of the ratio of genes in the treated samples, whereas downregulation was set at a 0.5-fold expression of genes in the treated samples.
Gene ontology (GO) analysis of differentially expressed genes with a focus on biological processes was performed by using DAVID software tool (Huang et al, 2009a, 2009b). Venn diagrams were created using Venny software tool (Oliveros, 2007; http://bioinfogp.cnb.csic.es/tools/venny/index.html).
Chicken chorioallantoic membrane assay
The CAM assay was performed as described previously (Gloesenkamp et al, 2012a). Briefly, 2 × 106 cells were resuspended in 10 μl growth medium and mixed with 10 μl growth factor-reduced Matrigel (BD Biosciences). The cell suspensions were implanted on fertilised chicken eggs on day 8 of incubation, using a silicone ring of 5 mm in diameter. After 24 h, tumours were topically treated for 72 h with 20 μl PBS containing cisplatin (1 μ M), HP-2 (20 μ M) or HP-14 (10 μ M). Application of PBS alone served as a negative control. Tumour growth and viability of the developing embryo were controlled daily by stereo microscopy. At the end of the experiment, tumours were recovered, pictures were taken using a stereomicroscope equipped with a Kappa digital camera system and the tumour area (mm2) was calculated. Tumour sizes were measured using a ruler. After that, the tissues were fixed in 4% freshly prepared paraformaldehyde, paraffin-embedded, cut in 5-μm thin sections and stained with haematoxylin and eosin for histopathology.
Immunhistochemistry of CAM tumours
Immunohistochemistry was performed as described (Vogler et al, 2008). In brief, paraffin sections (5 μm) were mounted on poly-L-lysine-coated slides. After deparaffinising and rehydration, antigen retrieval of sections was done by heating in 10 mol l−1 citrate buffer, pH 6.0. Non-specific binding sites were blocked with goat serum. Antibodies directed against Ki-67 (1 : 100; Dako, Hamburg, Germany), pan-cytokeratin (1 : 100 Dako) and desmin (1 : 100; Dako) were applied according to the manufacturer’s protocol. The Histostain-Plus streptavidin peroxidase staining procedure (Zymed Laboratories, Inc., San Francisco, CA, USA) was used with AEC chromogen staining for detection. At optimal colour development, sections were immersed in sterile water, counterstained with Mayer’s haematoxylin and covered using cover slips. Quantification of positive cells was determined by counting Ki-67 and desmin-positive cells in 16 distinct randomly chosen fields per sample.
Results
HP compounds inhibit the proliferation of VEGFR-2-expressing TGCT cells
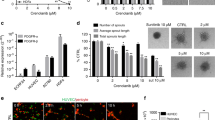
The VEGFR-2 expression of all cell lines used in this study was examined by RT-PCR (Figure 1A). Growth inhibition of cisplatin-sensitive and -resistant TGCT cells, both expressing the VEGFR-2, was determined after 48 h of incubation with increasing concentrations of HP compounds (0–20 μ M). HP-2 and HP-14 led to a dose-dependent decrease in the proliferation of both cisplatin-sensitive 2102EP cells and cisplatin-resistant 2102EP-R cells by up to >95% (Figure 1B and C). The IC50 values of HP-2 were determined as 10.6 μ M (2102EP) and 11.5 μ M (2102EP-R), respectively. For HP-14, the IC50 values were 4.2 μ M (2102EP) and 5.9 μ M (2102EP-R), respectively. In contrast, VEGFR-2 lacking Caki-1 cells did not respond to HP treatment. After 48 h of incubation with HP-2 (0–20 μ M) or HP-14 (0–20 μ M), only a non-significant growth inhibition<5% was observed for both compounds (data not shown).
Effects of HP-2 and HP-14 on the proliferation of VEGFR-2-expressing, cisplatin-sensitive and cisplatin-resistant TGCT cells. The VEGFR-2 expression was determined by RT-PCR (A). TGCT cells were treated with increasing concentrations of HP-2 and HP-14 for 48 h, and drug-induced changes in cell numbers were evaluated by crystal violet staining. Both compounds inhibit the growth of the platinum-sensitive 2102EP cells (B) and cisplatin-resistant 2102EP-R cells (C) in a dose-dependent manner.
Anti-neoplastic potency of HP compounds in combination with cisplatin
To test additive anti-proliferative effects of the combination treatment of HP-2 or HP-14 together with cisplatin, 2102EP and 2102EP-R cells were treated for 48 h with HP compounds at increasing doses from 0 to 10 μ M, and cisplatin at a fixed dose of 1 μ M. When given as a single agent, cisplatin (1 μ M) reduced the growth of cisplatin-sensitive 2102EP cells by 42% (Figure 2A), whereas the growth of cisplatin-resistant 2102EP-R cells was inhibited by only 19% (Figure 2C). Combination treatment resulted in additive growth inhibition of cisplatin-sensitive TGCT cells (Figure 2A and B). In cisplatin-resistant TGCT cells, the combination of HP compounds and cisplatin also led to a dose-dependent and additive reduction of cell growth of >90% (Figure 2C and D).
Combination treatment with HP-2 or HP-14, and cisplatin. TGCT cells were treated with HP-2 or HP-14 and 1 μ M cisplatin for 48 h, and the proliferation was measured in 2102EP (A, B), 2102EP-R (C, D) and in Caki-1 (E, F). The combination of HP-2 and HP-14 with cisplatin led to additive growth-inhibitory effects in TGCT cells 2102EP and 2102EP-R, whereas no marked growth inhibition was observed in the VEGFR-2-negative renal cell carcinoma cell line Caki-1.
Again, the VEGFR-2 lacking Caki-1 cells did not respond to the HP-based combination treatments. Treatment with rising concentrations of HP-2 or HP-14 alone, or in combination with cisplatin (1 μ M) resulted in a minor growth inhibition of up to 15%, which mainly reflected the single effect of cisplatin on Caki-1 cells (Figure 2E and F).
Effect of combination treatment on cell cycle regulation
To determine whether HP-induced growth inhibition of TGCT cells was connected with cell cycle-arresting effects, flow cytometry was performed. After 48 h of treatment, HP-14 induced only a marginal increase of cells in the G0/G1 phase of the cell cycle, in both the cisplatin-sensitive and -resistant cell lines (Figure 3A and B). Cisplatin monotherapy induced a strong shift of the cisplatin-sensitive cells into the S- and G2/M phases of the cell cycle, accompanied by a respective decrease in the proportion of cells in the G0/G1 phase, whereas the cisplatin-resistant cells showed only minimal changes in the distribution of the cell cycle phases compared with untreated cells. The combination treatment of HP-14 and cisplatin led to an arrest of the cisplatin-sensitive cells in the S- and G2/M phase of the cell cycle, whereas the cisplatin-resistant cells again showed almost no changes in the cell cycle regulation.
Tables show cell cycle phase distribution of cisplatin-sensitive (A) and cisplatin-resistant (B) TGCT cells treated with HP-14 alone or in combination with cisplatin. After treatment, cells were collected, fixed with ethanol and stained with propidium iodide. Changes in cell cycle phase distribution were measured by DNA flow cytometric analysis.
No significant increase in apoptotic cells in the sub-G1 population was detected in both cell lines (Figure 3).
Combination treatment differentially alters the gene expression profile in cisplatin-sensitive vs cisplatin-resistant TGCT cells
The effects of cisplatin, HP-14, or a combination of both, on the expression of a set of 263 genes related to the metabolic processes of cell stress, cell toxicity, drug resistance and drug metabolism was analysed using cDNA microarrays. Cisplatin-sensitive (2102EP) and -resistant (2102EP-R) cells were treated either with cisplatin (1 μ M), HP-14 (1 μ M) or the combination of HP-14 and cisplatin (1 μ M each) for 48 h. Of note, treatment with 1 μ M HP-14 represents a sublethal dose, as it did not significantly reduce cell growth in neither 2102EP nor 2012EP-R. Interestingly, the combination treatment reduced the growth of the cell lines 2102EP and 2102EP-R by 60% and 45%, respectively, suggesting a cisplatin-sensitising effect of even low doses of HP-14 in the resistant cells (Figure 2B and D). Treatment-induced changes are summarised in Figure 4A (for further details see Supplementary Tables 1 and 2 in the Supplementary Files). In 2102EP cells, cisplatin, HP-14 and the combination of both affected the expression of 18, 26 and 33 genes, respectively. Gene expression analyses of 2102EP-R cells detected a set of 22, 27 or 29 altered genes after treatment with cisplatin, HP-14 or the combination of both agents. Strikingly, a number of genes known to be directly or indirectly involved in DNA damage detection and repair, such as topoisomerase I, GADD45a/b and BRCA1, were altered by cisplatin in 2102EP, but not in 2102EP-R when cells were treated with cisplatin.
(A) Venn diagram showing the overlap of differentially expressed genes in cisplatin-sensitive 2102EP and cisplatin-resistant 2102EP-R cells after treatment with cisplatin, HP-14 and the combination of both. (B, C) Venn diagram showing the overlap of differentially expressed genes in 2102EP and 2102EP-R cells, and the corresponding GO terms for biological processes showing enrichment after treatment with cisplatin or HP-14.
The GO analysis of genes exclusively affected by cisplatin in 2102EP cells revealed enrichment for genes involved in DNA replication. In contrast, genes regulated by cisplatin in 2102EP-R cells were not involved in DNA repair or DNA synthesis, but were assigned to biological processes regulating gene expression (Figure 4B). Moreover, cisplatin treatment affected the expression of seven genes in both cell lines. Interestingly, the GO analysis of these genes illustrated a significant overrepresentation of genes regulating cell cycle, probably through different pathways in both cell lines (Figure 4B). Furthermore, cisplatin induced expression of chaperones, such as CCT4 and DNAJB11, in 2102EP, but not in 2102EP-R. Interestingly, an opposite effect on gene expression was seen on treatment with HP-14 in 2102EP-R cells, where, for example, expression of topoisomerase I, GADD45b and DNAJB11 decreased (Figure 4C). Alterations in all three samples, both treated by the single substances or the combination, were seen in 7 of 263 genes in 2102EP, and 14 out of 263 genes in 2102EP-R, again suggesting that in 2102EP-R cells the response to the combination treatment was mainly determined by HP-14 and not by cisplatin. In only two cases, namely BAG1 and FGF2, disconcordant gene regulation by single substance and combination treatment was seen, both occurring in the 2102EP-R cells, and expression of both genes was predominantly determined by HP-14 in the combination treatment (Figure 4C and Supplementary Table 2).
Examination of genes regulated by HP-14 in 2102EP and 2102EP-R revealed that HP-14 affects proliferation of both cell lines through activation of different pathways. In 2102EP cells, HP-14 regulated the expression of 15 genes with a function in the regulation of apoptosis and response to endogenous stimuli and substances (Figure 4C). In contrast, in 2012EP-R cells, HP-14 affected the expression of 17 genes directly involved in cell death (Figure 4C). Taken together, the gene expression analysis suggests that the effects of cisplatin and HP-14 on both cell lines are based on the activation of different sets of genes and their corresponding pathways. Notably, the gene expression pattern shows further that the additive effects of both drugs on the proliferation of sensitive and resistant cells are reflected by a co-operative effect on gene expression.
HP treatment attenuates tumour growth in vivo and leads to impaired tumour angiogenesis
Using a modified CAM assay, the effects of HP compounds on tumour formation and growth of TGCTs were evaluated in vivo. Cisplatin-sensitive (2102EP) and -resistant (2102EP-R) TGCT cells were inoculated onto the CAM of 8-day-old chicken embryos and quantitatively examined for HP-induced growth reduction after 72 h of treatment. Both 2102EP and 2102EP-R cells formed substantial tumours (Figure 5B and C) with mean tumour areas of 7.66 (2102EP) and 8.01 mm2 (2102EP-R) after 3 days. Cisplatin reduced tumour formation of cisplatin-sensitive cells by 47% and by 8% in tumours of cisplatin-resistant cells.
Suppression of TGCT growth in vivo. Tumour plaques were inoculated onto the CAM. After 24 h, solid tumours had developed, which were then treated with either HP-2, HP-14 or cisplatin for 72 h, and the tumour area (mm2) was quantified. (A) Quantification of tumour area of tumour plaques treated with HP-2, HP-14 or cisplatin. The lower micrographs show representative pictures of cisplatin-sensitive 2102EP (B) and cisplatin-resistant 2102EP-R (C) tumours after 3 days of treatment. Results represent the mean±s.e.m. of at least five tumours. Data were analysed by Student’s t-test. *P<0.05 compared with untreated controls.
Treatment of cisplatin-sensitive TGCT tumours with HP-2 at the relatively high concentration of 20 μ M did not lead to an appreciable reduction of tumour growth (growth reduction by 8%). In cisplatin-resistant tumours, HP-2 induced a growth reduction of 16%, which was still less pronounced than that seen after treatment with HP-14.
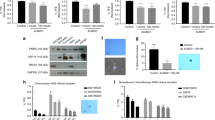
HP-14 treatment at 10 μ M led to a significant reduction in tumour growth of cisplatin-sensitive tumours of 57% and to a comparable reduction of 64% in tumours of cisplatin-resistant cells (Figure 5A). Tumour reduction by HP-14 corresponded with a reduced proliferation index, as evidenced by Ki-67 staining, which dropped by 20% in cisplatin-sensitive tumours and by 45% in cisplatin-resistant tumours (Figure 6C and E). Tumour cell origin was determined by anti-human cytokeratin antibody staining (Figure 6A).
Immunohistochemical analysis of CAM tumours. (A) Paraffin-embedded cisplatin-sensitive (upper panel) and cisplatin-resistant (lower panel) tumours were subjected to immunohistochemistry with specific antibodies directed against human cytokeratin, Ki-67 and desmin. (B, D) Quantification of HP-induced inhibition of blood vessel formation (desmin immunostaining) of cisplatin-sensitive (B) and -resistant (D) tumours. Data are given as the percentage of desmin-positive cells after treatment with HP-2 or HP-14, as compared with untreated controls. (C, E) Quantification of HP-induced changes in Ki-67 staining in cisplatin-sensitive (C) and -resistant tumours (E). Immunoreactivity was quantified by counting positive cells. For each slide, 16 fields were randomly chosen and, using a defined field area (1.72 mm2), all positive cells per field were counted. Results represent the mean±s.e.m. of three tumours. Data were analysed by Student’s t-test. *P<0.05 compared with controls.
The anti-angiogenic potency of the treatment was determined by immunohistochemical analysis of tumour feeding chicken blood vessel formation, using anti-desmin as a specific antibody for chicken endothelial cells (Azoitei et al, 2010). HP-14 treatment significantly reduced the percentage of desmin-positive blood vessel cells by 58% in cisplatin-sensitive and 55% in cisplatin-resistant tumours. Again, compared with HP-14, the effect of HP-2 was less pronounced (Figure 6B and D).
The anti-angiogenic potency of HP-14 was confirmed by performing a tube formation assay with freshly isolated HUVEC cells. HP-14 (10 μ M for 6 h) significantly reduced the length of the tubes, the number of branching points and the number of newly formed loops (Figure 7A and B).
Inhibition of tube formation by HP-14. (A) The HUVEC cells were treated with 10 μ M HP-14 for 6 h. The formation of tubes was analysed by examining different tube structures: total tube length (pixels, px), branching points and number of loops. Results represent the mean±s.e.m. of at three different experiments. *P<0.05 compared with controls. (B) Representative images of tube formation of untreated (left side) and HP-14-treated HUVEC cells (right side).
Discussion
In a recent study, we introduced two novel compounds, HP-2 and HP-14, as promising agents for treatment approaches in TGCTs (Nitzsche et al, 2010). However, their potential for the treatment of platinum-resistant TGCTs, the therapeutically most difficult subgroup of this tumour entity, has not been elucidated so far. Therefore, we explored the anti-proliferative and anti-angiogenic effects of the two novel small molecule inhibitors in platinum-sensitive and -resistant TGCT cell lines, and we could clearly demonstrate that at least one of the two novel HP compounds, HP-14, is able to potently inhibit cell growth by up to 95%, irrespective of the level of cisplatin resistance in the examined TGCT cells. This makes HP-14 an interesting candidate for urgently needed novel approaches beyond the standard cisplatin-based chemotherapy of TGCTs.
Increasingly, targeted therapies are applied in combination with standard chemotherapy. The rationale for this approach lies in the different modes of action of the co-applied agents, attacking the tumour at different sites or pathways, and is thus believed to exert (supra-) additive anti-neoplastic potency. Recent studies by Ramasubbaiah et al (2010) and Suddek (2011) demonstrated that the combination of anti-angiogenic compounds, such as sunitinib and bevacizumab, with standard chemotherapy can induce additive or even synergistic anti-neoplastic effects in TGCTs. In our study, we analysed the effect of the anti-angiogenic and anti-proliferative compounds, HP-2 and HP-14, in combination with cisplatin. In cisplatin-sensitive TGCT cells, a pronounced supra-additive effect was observed when HP-14 was combined with cisplatin, suggesting that the enhanced anti-neoplastic effect may occur due to the different modes of action of both agents. Although cisplatin acts as a cytotoxic, DNA-damaging agent, HP-14 inhibits the growth of TGCTs by interfering with the VEGFR-2-related pathways, eventually leading to cell cycle arrest (Nitzsche et al, 2010). Interestingly, in this analysis, the cisplatin-sensitive cell line 2102EP showed marked alterations in the cell cycle upon treatment with cisplatin, both alone and in combination with HP-14, whereas the cisplatin-resistant cell line 2102EP-R showed only minimal alterations in the cell cycle. This suggests that the increased toxicity of the combination of HP-14 and cisplatin in this cell line is independent of the cell cycle phase or the functionality of cell cycle checkpoints.
Using a cisplatin-resistant TGCT cell model, we analysed the anti-neoplastic potency of HP-14 in cisplatin-resistant TGCT cells. Upon treatment, we observed a pronounced effect on TGCT cell proliferation, indicating that HP-14 exerts its anti-tumour effect independently of the cisplatin-resistant phenotype. Interestingly, combined application of HP-14 together with cisplatin led to a supra-additive anti-neoplastic effect, especially in cisplatin-resistant 2102EP-R cells. This finding is in line with the results of Castillo-Ávila et al (2009), who investigated the combination of anti-angiogenic sunitinib and cisplatin in a mouse xenograft model for cisplatin-resistant TGCTs, and discovered an enhanced growth reduction as compared with the effects of either sunitinib or cisplatin alone. Suddek (2011) also reported improved response rates by combining sunitinib with cisplatin in non-urologic refractory solid tumours. As could be expected from a targeted agent, both agents, HP-2 and HP-14, showed only minimal effects, either as single agents or in combination with cisplatin in Caki-1 cells lacking VEGFR-2, underlining the importance of this pathway in HP-mediated cytotoxicity.
Clearly, the molecular basis for the observed marked resensitising effect of HP-14 on cisplatin remains to be elucidated in more detail. However, our screening approach using a gene microarray analysis suggests a dual effect of HP-14, both compensating for lost pro-apoptotic pathways and abrogating the consequences of upregulated protective factors in our cisplatin-resistant TGCT cells.
In our study, a number of differentially expressed proteins involved in cell growth, cellular response to stress, as well as changes in the expression levels of transcription factors and regulators after treatment with HP-14 and cisplatin were identified. For example, the growth arrest and DNA damage inducible proteins GADD45a and GADD45b are upregulated in the cisplatin-sensitive 2102EP cells upon treatment with cisplatin. These nuclear proteins are involved in stress signalling in response to DNA-damaging agents, which results in either cell cycle arrest, DNA repair, cell survival and senescence, or apoptosis (Wang et al, 1999; Zhan, 2005). Furthermore, cisplatin and HP-14 treatment in combination also altered the expression of other genes, such as BRCA1, ERCC3 or DNAJB11, each of them being implicated in the regulation of DNA repair and stress response, suggesting that cisplatin and HP-14 treatment leads to a more pronounced DNA-damaging effect in the sensitive cell line.
Interestingly, an opposite effect on gene expression of several genes was seen upon treatment with cisplatin and HP-14 in 2102EP-R cells, where, for example, expression of topoisomerase I, GADD45b and DNAJB11 decreased. Interestingly, more genes implicated in the regulation of cell growth and proliferation, and transcription factors and regulators were predominantly altered in 2102EP-R cells. For example, the expression of AKT-1 (protein kinase B), a downstream signal transduction molecule of VEGFR-2 (Takano et al, 2008), was decreased. Furthermore, the expression of the cell growth regulator dihydrofolate reductase was much more enhanced in 2102EP-R cells than in 2102EP cells. The dihydrofolate reductase catalyses the NADPH-dependent reduction of dihydrofolate to tetrahydrofolate needed in purine and pyrimidine synthesis, and has been implicated in the regulation of the cell cycle (Chen et al, 1984; Jensen et al, 1997). Interestingly, a cell line-specific downregulation of TP53, the tumour suppressor gene p53, was seen in 2102EP-R, both on treatment with HP-14 and cisplatin (and the combination of both). It is tempting to speculate that lack of upregulation of p53, which normally activates cell cycle checkpoints and leads to cell cycle arrest upon cell damage, might be underlying the observed absence of cell cycle regulation in 2102EP-R cells.
As also shown in the Venn diagrams, cisplatin and HP-14 regulate genes of different pathways. Therefore, we suggest that HP-14 compensates for the missing effects of cisplatin treatment in the resistant cell type. However, the exact mechanism underlying growth inhibition of 2102EP-R cells by combination treatment remains to be examined in forthcoming studies.
To extend our observations of in vivo efficiency of the two HP compounds, we employed a modified CAM assay to investigate the interaction between TGCT growth and angiogenesis. Both the cisplatin-sensitive and -resistant TGCT models were strongly tumorigenic. Inoculated tumour plaques grew fast, and formed solid and vascularised tumours on the CAM membrane, irrespective of the cisplatin-resistant or -sensitive phenotype. HP-14 treatment significantly reduced tumour growth by up to 64%, and was at least not less effective in tumours formed by 2102EP-R cells compared with tumours formed by 2102EP. Concomitantly, the vascularisation of implanted TGCTs was markedly reduced.
In summary, we could show that HP-14 has strong growth-inhibitory and anti-angiogenic activity in platinum-sensitive and -resistant TGCT cells, both in vitro and in vivo. Administration of HP-14 in combination with cisplatin additively enhanced the anti-neoplastic effects, as compared with those achieved by either single agent alone. Therefore, we conclude that HP-14 may be an interesting new drug for targeted therapy of testicular cancers, particularly those showing resistance to the conventionally applied platinum-based chemotherapies.
Change history
09 November 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adam M, Schmidt D, Wardelmann E, Wernert N, Albers P (2003) Angiogenetic protooncogene ets-1 induced neovascularization is involved in the metastatic process of testicular germ cell tumors. Eur Urol 44: 329–336
Azoitei N, Pusapati GV, Kleger A, Möller P, Küfer R, Genze F, Wagner M, van Lint J, Carmeliet P, Adler G, Seufferlein T (2010) Protein kinase D2 is a crucial regulator of tumour cell-endothelial cell communication in gastrointestinal tumours. Gut 59 (10): 1316–1330
Bentas W, Beecken W-D, Glienke W, Binder J, Schuldes H (2003) Serum levels of basic fibroblast growth factor reflect disseminated disease in patients with testicular germ cell tumors. Urol Res 30: 390–393
Bokemeyer C, Oechsle K, Honecker F, Mayer F, Hartmann JT, Waller CF, Böhlke I, Kollmannsberger C (2008) Combination chemotherapy with gemcitabine, oxaliplatin, and paclitaxel in patients with cisplatin-refractory or multiply relapsed germ-cell tumors: a study of the German Testicular Cancer Study Group. Ann Oncol 19: 448–453
Castillo-Ávila W, Piulats JM, Garcia del Muro X, Vidal A, Condom E, Casanovas O, Mora J, Germà JR, Capellà G, Villanueva A, Viñals F (2009) Sunitinib inhibits tumor growth and synergizes with cisplatin in orthotopic models of cisplatin-sensitive and cisplatin-resistant human testicular germ cell tumors. Clin Cancer Res 15: 3384–3395
Chen MJ, Shimada T, Moulton AD, Cline A, Humphries RK, Maizel J, Nienhuis AW (1984) The functional human dihydrofolate reductase gene. J Biol Chem 259: 3933–3943
Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A (2007) Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett 581: 673–680
De Wit R, Skoneczna IA, Gedske Daugaard K, de Santis M, Garin A, Aass N, Witjes JA, Albers P, White J, Germa-Lluch JR, Osanto S, Marreaud S, Collette L (2011) A randomized phase III study comparing paclitaxel-BEP (T-BEP) to standard BEP in patients with in intermediate prognosis germ cell cancer (GCC): an intergroup study of EORTC, German TCSG/AUO, MRC, and Spanish GCC group (EORTC 30983). ASCO 29: 4509
Devouassoux-Shisheboran M, Mauduit C, Tabone E, Droz JP, Benahmed M (2003) Growth regulatory factors and signalling proteins in testicular germ cell tumours. APMIS 111: 212–224, discussion 224
Einhorn LH, Brames MJ, Juliar B, Williams SD (2007) Phase II study of paclitaxel plus gemcitabine salvage chemotherapy for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. J Clin Oncol 25: 513–516
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM (2009) Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27: 3312–3318
Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ (2008) Medical treatment of advanced testicular cancer. J Am Med Assoc 299: 672–684
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186
Fried J, Perez AG, Clarkson BD (1976) Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J Cell Biol 71: 172–181
Fukuda S, Shirahama T, Imazono Y, Tsushima T, Ohmori H, Kayajima T, Take S, Nishiyama K, Yonezawa S, Akiba S, Akiyama S-i, Ohi Y (1999) Expression of vascular endothelial growth factor in patients with testicular germ cell tumors as an indicator of metastatic disease. Cancer 85: 1323–1330
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31: 1869–1883
Gillies RJ, Didier N, Denton M (1986) Determination of cell number in monolayer cultures. Anal Biochem 159: 109–113
Gloesenkamp C, Nitzsche B, Lim AR, Normant E, Vosburgh E, Schrader M, Ocker M, Scherübl H, Höpfner M (2012a) Heat shock protein 90 is a promising target for effective growth inhibition of gastrointestinal neuroendocrine tumors. Int J Oncol 40 (5): 1659–1667
Gloesenkamp CR, Nitzsche B, Ocker M, Di Fazio P, Quint K, Hoffmann B, Scherübl H, Höpfner M (2012b) AKT inhibition by triciribine alone or as combination therapy for growth control of gastroenteropancreatic neuroendocrine tumors. Int J Oncol 40 (3): 876–888
Honecker F, Wermann H, Mayer F, Gillis AJM, Stoop H, van Gurp RJLM, Oechsle K, Steyerberg E, Hartmann JT, Dinjens WNM, Oosterhuis JW, Bokemeyer C, Looijenga LHJ (2009) Microsatellite instability, mismatch repair deficiency, and Braf mutation in treatment-resistant germ cell tumors. J Clin Oncol 27: 2129–2136
Houldsworth J, Xiao H, Murty VV, Chen W, Ray B, Reuter VE, Bosl GJ, Chaganti RS (1998) Human male germ cell tumor resistance to cisplatin is linked to TP53 gene mutation. Oncogene 16: 2345–2349
Huang DW, Sherman BT, Lempicki RA (2009a) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57
Huang DW, Sherman BT, Lempicki RA (2009b) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13
Jensen DE, Black AR, Swick AG, Azizkhan JC (1997) Distinct roles for Sp1 and E2F sites in the growth/cell cycle regulation of the DHFR promoter. J Cell Biochem 67: 24–31
Jones A, Fujiyama C, Turner K, Fuggle S, Cranston D, Turley H, Valtola R, Bicknell R, Harris AL (2000) Angiogenesis and lymphangiogenesis in stage 1 germ cell tumours of the testis. BJU Int 86: 80–86
Köberle B, Tomicic MT, Usanova S, Kaina B (2010) Cisplatin resistance: preclinical findings and clinical implications. Biochim Biophys Acta 1806: 172–182
Kollmannsberger C, Nichols C, Bokemeyer C (2006) Recent advances in management of patients with platinum-refractory testicular germ cell tumors. Cancer 106: 1217–1226
Koster R, di Pietro A, Timmer-Bosscha H, Gibcus JH, van den Berg A, Suurmeijer AJ, Bischoff R, Gietema JA, de Jong S (2010) Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest 120: 3594–3605
Koster R, Timmer-Bosscha H, Bischoff R, Gietema JA, de Jong S (2011) Disruption of the MDM2-p53 interaction strongly potentiates p53-dependent apoptosis in cisplatin-resistant human testicular carcinoma cells via the Fas/FasL pathway. Cell Death Dis 2: e148
Masters JR, Thomas R, Hall AG, Hogarth L, Matheson EC, Cattan AR, Lohrer H (1996) Sensitivity of testis tumour cells to chemotherapeutic drugs: role of detoxifying pathways. Eur J Cancer 32A: 1248–1253
Mayer F, Stoop H, Scheffer GL, Scheper R, Oosterhuis JW, Looijenga LHJ, Bokemeyer C (2003) Molecular determinants of treatment response in human germ cell tumors. Clin Cancer Res 9: 767–773
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 16–24
Nitzsche B, Glösenkamp C, Schrader M, Ocker M, Preissner R, Lein M, Zakrzewicz A, Hoffmann B, Höpfner M (2010) Novel compounds with antiangiogenic and antiproliferative potency for growth control of testicular germ cell tumours. Br J Cancer 103: 18–28
Noel EE, Yeste-Velasco M, Mao X, Perry J, Kudahetti SC, Li NF, Sharp S, Chaplin T, Xue L, McIntyre A, Shan L, Powles T, Oliver RTD, Young BD, Shipley J, Berney DM, Joel SP, Lu Y-J (2010) The Association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am J Pathol 176: 2607–2615
Oechsle K, Honecker F, Cheng T, Mayer F, Czaykowski P, Winquist E, Wood L, Fenner M, Glaesener S, Hartmann JT, Chi K, Bokemeyer C, Kollmannsberger C (2011a) Preclinical and clinical activity of sunitinib in patients with cisplatin-refractory or multiply relapsed germ cell tumors: a Canadian Urologic Oncology Group/German Testicular Cancer Study Group cooperative study. Ann OncolE 22: 2654–2660
Oechsle K, Kollmannsberger C, Honecker F, Mayer F, Waller CF, Hartmann JT, Boehlke I, Bokemeyer C (2011b) Long-Term survival after treatment with gemcitabine and oxaliplatin with and without paclitaxel plus secondary surgery in patients with cisplatin-refractory and/or multiply relapsed germ cell tumors. Eur Urol 60: 850–855
Oliveros JC (2007) VENNY. An interactive tool for comparing lists with Venn Diagrams ( http://bioinfogp.cnb.csic.es/tools/venny/index.html )
Port M, Glaesener S, Ruf C, Riecke A, Bokemeyer C, Meineke V, Honecker F, Abend M (2011) Micro-RNA expression in cisplatin resistant germ cell tumor cell lines. Mol Cancer 10: 52
Ramasubbaiah R, Brames J, Johnston EL, Einhorn LH, Vaughn DJ, Perkins SM (2010) Phase II study of oxaliplatin (O) and bevacizumab (B) chemotherapy in refractory germ cell tumors (GCT). ASCO 28: e15054
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou S-S, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26: 5422–5428
Sark MW, Timmer-Bosscha H, Meijer C, Uges DR, Sluiter WJ, Peters WH, Mulder NH, de Vries EG (1995) Cellular basis for differential sensitivity to cisplatin in human germ cell tumour and colon carcinoma cell lines. Br J Cancer 71: 684–690
Schrader M, Kempkensteffen C, Christoph F, Hinz S, Weikert S, Lein M, Krause H, Stefan C, Jung K, Höpfner M, Albers P, Miller K, Schostak M (2009) Germ cell tumors of the gonads: a selective review emphasizing problems in drug resistance and current therapy options. Oncology 76: 77–84
Suddek GM (2011) Sunitinib improves chemotherapeutic efficacy and ameliorates cisplatin-induced nephrotoxicity in experimental animals. Cancer Chemother Pharmacol 67: 1035–1044
Takano H, Murasawa S, Asahara T (2008) Functional and gene expression analysis of hTERT overexpressed endothelial cells. Biol Targets Ther 2: 547–554
Viglietto G, Romano A, Maglione D, Rambaldi M, Paoletti I, Lago CT, Califano D, Monaco C, Mineo A, Santelli G, Manzo G, Botti G, Chiappetta G, Persico MG (1996) Neovascularization in human germ cell tumors correlates with a marked increase in the expression of the vascular endothelial growth factor but not the placenta-derived growth factor. Oncogene 13: 577–587
Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Gschwend JE, Simmet T, Debatin K-M, Fulda S (2008) Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res 68: 7956–7965
Wang N, Trend B, Bronson DL, Fraley EE (1980) Nonrandom abnormalities in chromosome 1 in human testicular cancers. Cancer Res 40: 796–802
Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O’Connor PM, Fornace AJ, Harris CC (1999) GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci 96: 3706–3711
Zhan Q (2005) Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res 569: 133–143
Acknowledgements
We are indebted to Felicitas Genze, Department of Urology, University of Ulm, Ulm, Germany, for expert technical support in the realisation of the CAM assay. We thank Dr Kerstin Kapp from the Institute of Molecular Biology and Bioinformatics, Free University Berlin, for her excellent support in FACS analysis. Bianca Nitzsche was funded by Urologic Research Foundation Berlin. Björn Hoffmann was supported by a grant (‘Therapeutic Systems Immunology’) of the Bundesministerium für Bildung und Forschung (BMBF). Friedemann Honecker was supported by a grant from the Werner Otto Stiftung, Hamburg.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Nitzsche, B., Gloesenkamp, C., Schrader, M. et al. Anti-tumour activity of two novel compounds in cisplatin-resistant testicular germ cell cancer. Br J Cancer 107, 1853–1863 (2012). https://doi.org/10.1038/bjc.2012.481
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.481
Keywords
This article is cited by
-
Testicular germ cell tumor: a comprehensive review
Cellular and Molecular Life Sciences (2019)
-
Molecular Mechanisms of Cisplatin Chemoresistance and Its Circumventing in Testicular Germ Cell Tumors
Current Oncology Reports (2018)
-
The genomic landscape of testicular germ cell tumours: from susceptibility to treatment
Nature Reviews Urology (2016)
-
Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours
Nature Communications (2015)
-
Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways
Scientific Reports (2015)