Abstract
Background:
To evaluate the safety, pharmacokinetics (PKs), and pharmacodynamics of aflibercept, and to identify the recommended phase II dose (RP2D) of aflibercept in combination with pemetrexed and cisplatin.
Methods:
Aflibercept was administered at escalating doses of 2, 4, or 6 mg kg−1 in combination with fixed doses of pemetrexed (500 mg m−2) plus cisplatin (75 mg m−2) every 3 weeks. Blood samples were collected for PK analyses. Serum antiaflibercept antibodies were quantified to assess their impact on systemic aflibercept concentrations.
Results:
Eighteen patients were enrolled. One patient dosed at 4 mg kg−1 experienced grade 3 hypophosphatemia (dose-limiting toxicity; DLT), which prompted a cohort expansion. No further DLTs were observed in the 4 mg kg−1 cohort or the 6 mg kg−1 dose cohort. Most common adverse events (AEs) of all grades included (%): fatigue (89), anaemia (89), nausea (83), hyponatremia (78), and neutropenia (72). Grade ⩾3 AEs consistent with anti-vascular endothelial growth factor therapy included (%): hypertension (22), pulmonary embolism (11), and deep vein thrombosis (6). Five patients (28%) experienced mild neurocognitive disturbance. No episodes of reversible posterior leukoencephalopathy syndrome (RPLS) were noted.
Conclusion:
The results of this phase I study allowed further evaluation of the combination of aflibercept with pemetrexed and cisplatin in a phase II study. The RP2D of aflibercept was 6 mg kg−1, to be administered intravenously every 3 weeks in combination with pemetrexed and cisplatin.
Similar content being viewed by others
Main
Vascular endothelial growth factor (VEGF) is a promoter of tumour angiogenesis (Kowanetz and Ferrara, 2006). Vascular endothelial growth factor signals through its receptors (VEGFR) including VEGFR-1 (FLT-1) and VEGFR-2 (FLK 1/KDR), which are expressed in normal and tumour vasculature endothelia. Vascular endothelial growth factor-mediated signalling is thought to be important in the development and progression of multiple solid tumours, and VEGF mRNA and protein overexpression are prognostic of poor outcome (Bonnesen et al, 2009; Delli Carpini et al, 2010). Thus, the use of VEGF- and VEGFR-targeted agents as cancer therapy has increased dramatically in recent years (Cook and Figg, 2010).
Aflibercept (VEGF Trap; Regeneron Pharmaceuticals, Tarrytown, NY, USA, and Sanofi Oncology, Cambridge, MA, USA) is a recombinant protein consisting of domain 2 from VEGFR-1 fused to domain 3 from VEGFR-2, attached to the hinge region of the Fc(a) domain of human immunoglobulin IgG1. Aflibercept binds all isoforms of VEGF-A, VEGF-B, and placental growth factor. Aflibercept exerts its antiangiogenic effects through regression in the normalisation and remodelling of surviving tumour vessels, and inhibition of neovascularisation (Holash et al, 2002).
Previously reported clinical trials have shown that aflibercept has antitumour activity both as a single agent and in combination with chemotherapy (Holash et al, 2002; Tang et al, 2008; Lockhart et al, 2010; Coleman et al, 2011; Tabernero et al, 2011). The recommended phase II dose (RP2D) is 4 mg kg−1 intravenously (i.v.) every 2 weeks when given as a single agent and either 4 mg kg−1 administered every 2 weeks or 6 mg kg−1 administered every 3 weeks in combination with chemotherapy (Lockhart et al, 2010). The most common treatment-related toxicities were consistent with prior studies of anti-VEGF agents, and included proteinuria, hypertension, fatigue, and hoarseness. Combination studies with cytotoxic chemotherapy have shown some increase in chemotherapy-related toxicities (Freyer et al, 2008; Limentani et al, 2008; Rixe et al, 2008; Kuhnowski et al, 2010; Novello et al, 2011; Tabernero et al, 2011).
Pemetrexed in combination with cisplatin is used first-line in the treatment of patients with locally advanced or metastatic non-squamous non-small-cell lung cancer (NSCLC) (Scagliotti et al, 2008), and in patients with advanced malignant pleural mesothelioma (MPM) (Vogelzang et al, 2003). The addition of a VEGF inhibitor, such as bevacizumab, to chemotherapy has proven to be effective in non-squamous NSCLC, with an acceptable toxicity profile (Sandler et al, 2006; Spigel et al, 2012).
The primary objective of this phase I combination trial was to determine the dose-limiting toxicities (DLTs) and RP2D of aflibercept administered i.v. every 3 weeks in combination with pemetrexed and cisplatin. Secondary objectives were to assess the safety profile of the combination, to characterise the pharmacokinetics (PKs) of aflibercept and pemetrexed, and to evaluate the immunogenicity of aflibercept.
Materials and methods
Patient eligibility
Patients were required to have a histologically confirmed advanced, incurable malignancy that was refractory to conventional therapy, or for which treatment with pemetrexed and/or cisplatin was considered appropriate. Patients had to have measurable disease by Response Evaluation Criteria in Solid Tumours (RECIST) (version 1.1), (Eisenhauer et al, 2009) an Eastern Cooperative Oncology Group (ECOG) performance status (PS) ⩽1, and adequate haematological, hepatic, and renal function. Prior anti-VEGF therapy ⩾4 weeks from initial administration of aflibercept was allowed. Key exclusion criteria included: (a) prior treatment with aflibercept or pemetrexed; (b) patients whose disease had progressed during cisplatin administration or relapsed within 6 months of completion of cisplatin-based therapy; (c) surgery within the last 28 days; (d) uncontrolled hypertension defined as systolic blood pressure (BP) ⩾150 mm Hg and/or diastolic pressure ⩾100 mm Hg (prior antihypertensive medication was allowed); (e) bleeding diathesis or coagulopathy; (f) brain or leptomeningeal metastases (brain imaging was mandatory for study participation).
The study was conducted at two sites and the institutional review board of both participating centres approved the study.
Study design
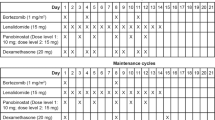
This was an open-label, dose-escalation phase I trial; three dose levels were planned for aflibercept: (a) 2 mg kg−1, (b) 4 mg kg, and (c) 6 mg kg−1. No intra-patient dose escalation was permitted. In the event patients experienced a DLT at the first dose level, a dose level −1 (aflibercept given at 1 mg kg−1) was planned. Aflibercept, pemetrexed, and cisplatin were administered i.v. on day 1 of each 3-week cycle. Aflibercept was administered over 1 h, followed in sequence by fixed doses of pemetrexed (500 mg m2 i.v. over 10 min) and cisplatin (75 mg m2 i.v. administered as per institutional practice; typically 2 h). Patients were supplemented with vitamin B12 (1000 mcg intramuscularly) 1 week before the first pemetrexed dose and every three cycles thereafter. A low-dose folic acid preparation or multivitamin with folic acid was administered by mouth daily at doses ranging from 350 to 1000 mcg. This supplementation started at least 5 days before the first dose of pemetrexed, continued throughout the treatment period, and for 30 days after the last dose of pemetrexed. Oral or i.v. dexamethasone (4 mg) was given twice daily the day before, the day of, and the day after pemetrexed administration unless medically contraindicated. Three patients were enrolled in the first-dose level; dose escalation proceeded, following the standard 3+3 rule, until>one patient experienced a DLT during the first cycle of therapy. The RP2D was then selected as the dose level at which ⩽1 of 6 patients encountered a DLT during the first cycle of therapy.
Toxicity was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 3.0 (NCI CTCAE v.3.0). Dose-limiting toxicities were defined as adverse events (AEs) attributed as being possibly, probably, or definitely related to the study agents, and fulfilling one of the following criteria: (a) grade 3 or 4 neutropenia complicated by fever ⩾38.5 °C or infection, or grade 4 neutropenia of at least 7 days duration, (b) grade 3 thrombocytopenia complicated by haemorrhage or grade 4 thrombocytopenia, or (c) any grade 3 or higher non-haematologic toxicity (except fatigue, anorexia, nausea, vomiting, or diarrhoea that was not optimally controlled with appropriate medical intervention). Grade 3 hypertension (BP ⩾150/100 mm Hg), or BP ⩾180/100 mm Hg (if the patient had a history of isolated systolic hypertension) that could be controlled within 3 weeks of initiation of oral antihypertensive therapy was not considered a DLT. Likewise, grade 3 proteinuria (>3.5 grams per 24 h) that recovered to <2 grams per 24 h within 3 weeks of onset and/or grade 3 laboratory abnormalities that could reflect tumour burden were not considered as DLTs.
Patient evaluation
Pre-treatment evaluations were performed within 2 weeks of treatment initiation and included history and physical examination, ECOG PS, haematology, serum chemistry, prothrombin time/INR, PTT, and urinalysis. Physical examinations were repeated on day 1 of each subsequent cycle; haematology, chemistry, and urinalysis were measured weekly for the entire study duration.
Baseline radiological investigations were performed within 28 days of treatment initiation. Objective tumour response was assessed by RECIST (version 1.1) every two cycles (Eisenhauer et al, 2009). Complete and partial responses (PR) had to be confirmed at least 4 weeks after the initial observation.
Dose modifications
Patients were required to meet the following criteria to receive study drugs on day 1 of a treatment cycle: absolute neutrophil count ⩾1.5 × 109 l−1, platelets ⩾100 × 109 l−1, creatinine clearance (CrCl) ⩾60 ml/min, and non-haematologic toxicity recovered to grade ⩽1.
In the event a DLT occurred during cycle 1 or later, treatment with the triplet regimen was interrupted temporarily. A recovery period of up to 6 weeks was allowed. Patients could resume dosing at a lower dose level upon recovery to grade 2 or better. Recurrence of a drug-related DLT after one dose reduction led to withdrawal of the patient from the study. Patients who required permanent discontinuation from aflibercept were withdrawn from the study. If either cisplatin or pemetrexed was permanently discontinued, the administration of aflibercept and the remaining chemotherapeutic agent was allowed to continue at the same (or lower) dose.
Duration of therapy
Study treatment continued until disease progression, an unacceptable AE, patient’s decision to withdraw from the study, or changes in the patient’s condition rendering further treatment unacceptable.
Pharmacokinetic analysis
Blood samples were collected to characterise the plasma PK profiles of aflibercept and pemetrexed. For aflibercept, samples were collected on cycle 1 day 1 before aflibercept infusion, at the end of aflibercept infusion, and at 1, 2, 4 and 8 h after completion of pemetrexed infusion; day 2 (24 h), day 8, and day 15. For cycle 2 and beyond, trough samples for aflibercept were taken before aflibercept infusion. For pemetrexed, samples were collected on cycle 1 day 1 before aflibercept infusion, at the end of pemetrexed infusion, and at 0.25, 0.5, 1, 2, and 4 h after completion of pemetrexed infusion, and on day 2 (24 h).
Aflibercept (bound to VEGF or free) in plasma samples was quantified using a validated, direct enzyme-linked immunosorbent assay, with a lower limit of quantification of 15.6 ng ml−1 in human plasma for free aflibercept and 43.9 ng ml−1 in human plasma for bound aflibercept (Tew et al, 2010). To calculate the total aflibercept concentration, the amount of aflibercept present in the bound complex needed to be determined. As 1 ng of complex contains 0.717 ng of aflibercept and 0.283 ng of human VEGF, the concentration of the complex was multiplied by 0.717 to give the adjusted-bound aflibercept concentration (Equation 1).

Total aflibercept concentrations were calculated by adding the adjusted-bound aflibercept concentrations to the free aflibercept concentrations at the corresponding time points (Equation 2). Total aflibercept concentrations were only calculated for samples for which both free and bound aflibercept concentrations were available.

Pemetrexed plasma concentration determination was performed by LC-MS/MS. Briefly, pemetrexed and its internal standard was extracted from a 0.050 ml aliquot of human K2-EDTA plasma using an automated protein precipitation procedure. The lower limit of quantitation of the assay is 1.00 mcg ml−1. Detection of antiaflibercept antibodies was performed in acid-treated serum samples using an electrochemiluminescence bridging immunoassay (Tew et al, 2010).
Observed PK parameters were calculated using non-compartmental analysis (WinNonLin v. 5.3, Pharsight Corporation, Mountain View, CA, USA). Observed half-life (t½), clearance (CL), volume of distribution (Vss), maximum plasma concentration (Cmax), dose-adjusted Cmax (Cmax/D), last observed concentration (Clast), time of maximum plasma concentration (tmax), time of last observed concentration (tlast), area under the concentration-time curve (AUCinf), and dose-adjusted AUCinf (AUCinf/D) were calculated for free aflibercept and for pemetrexed. Cmax, Cmax/D, Clast, tmax, and tlast were calculated for adjusted-bound aflibercept–VEGF complex. AUCinf was calculated using the log-linear trapezoidal rule.
Results
Patient demographics
Between October 2008 and November 2010, 18 patients were enrolled at the two participating institutions. A median of four cycles of aflibercept (range 1–12) were administered for the entire group. Treatment duration of the 18 patients ranged from 21 days to 315 days (0.7–10 months). At the time of this report, all patients are off study treatment. Table 1 shows their baseline demographics.
Dose escalation and maximum tolerated dose
Four, seven, and seven patients were enrolled in dose levels 1, 2, and 3, respectively. Treatment is summarised in Table 2. Two patients (1 in the 4 mg kg−1 cohort and 1 in the 6 mg kg−1 cohort) were replaced owing to disease progression before cycle 1 completion (without experiencing a DLT). No cycle 1 DLT was observed at the first dose level (2 mg kg−1). One patient at the 4 mg kg−1 dose level experienced treatment-related grade 3 hypophosphatemia (DLT), thus prompting a cohort expansion with an additional three patients. No additional DLT was observed in the expansion cohort allowing for further dose escalation. When aflibercept was dosed at 6 mg kg−1 in the next cohort, no DLTs were observed. Thus, aflibercept 6 mg kg−1 i.v. every 3 weeks was selected as the RP2D in combination with pemetrexed and cisplatin.
Safety and compliance
All 18 treated patients were evaluable for toxicity and experienced at least one AE during the course of the study. The most frequently reported treatment-related AEs are listed in Table 3.
Fatigue (89%) and nausea (83%) were the most frequently reported non-haematologic all grade AEs. Three patients (17%) treated at higher doses of aflibercept had grade 3 fatigue. Nausea (83%), constipation (61%), anorexia (61%), and vomiting (56%) were the most relevant gastrointestinal toxicities. These were grade 1 and 2 in all cases, and were managed with supportive care measures. Other toxicities, commonly associated with antiangiogenic therapy, were hypertension (56%), dysphonia (39%), thromboembolic-related events (TREs, 17%), and proteinuria (6%). Hypertension seemed to be more frequent with increasing doses of aflibercept. Four patients (22%) experienced grade 3 hypertension (one patient treated at 2 mg kg−1 and three patients treated at 6 mg kg−1), which was managed with oral antihypertensive medication. No patient discontinued study treatment owing to this AE. Dysphonia was mild, and did not lead to dose modification, delay, or treatment discontinuation. Two patients, both treated in the 4 mg kg−1 cohort, had grade 4 pulmonary embolism (PE). One patient had a bilateral PE as well as deep vein thrombosis and was withdrawn from the study; the other was asymptomatic and had an incidental unilateral PE documented on a restaging computed tomography scan showing progressive disease (PD). No major haemorrhagic events were observed. One episode of reversible grade 2 proteinuria was observed, in the 6 mg kg−1 cohort. It did not require modification of the treatment dose.
Five patients (28%) experienced mild neurocognitive disturbance. These symptoms mostly consisted of a vague sensation of dizziness and mild headache. All episodes were grade 1 in intensity, and mostly observed in female patients. Four patients were treated at dose level 1 (2 mg kg−1 of aflibercept) and one patient was at dose level 2 (4 mg kg−1 of aflibercept). There was no apparent association with hypertension, or with the cumulative dose of aflibercept. Magnetic resonance imaging (MRI) of the brain was performed in two of these patients and results were normal. The development of these symptoms did not correlate with a specific number of cycles and, after its resolution, patients who were rechallenged did not experience a recurrence of their symptoms. There were no grade 3 or 4 events. These symptoms did not lead to treatment cessation and were reversible in three of the five patients (60%). One patient reported mild cognitive impairment at the end of the study but did not have a formal neurocognitive assessment, and the other patient was lost to follow-up.
A summary of the most common laboratory abnormalities observed throughout the study can be found in Table 3. Grade 3 neutropenia was observed in six patients (33%), leading to dose delay and/or dose reduction of the chemotherapy part of the regimen in the majority of cases. No episodes of febrile neutropenia were documented. Three patients died during the study: two of disease progression and one patient of a pleural effusion, assessed as unrelated to the study treatment.
Dose delays and dose modifications for pemetrexed and cisplatin are provided in Table 4. There were no dose delays or reductions for aflibercept.
Activity
Two patients (11%) achieved a confirmed PR, both treated at the 6 mg kg−1 cohort. One patient, a 56-year-old male, with a previously untreated NSCLC achieved PR at cycle 6, and discontinued treatment owing to an inflammatory cholangitis (unrelated to study treatment). A 65-year-old male with a chemo-naïve mesothelioma achieved a PR at cycle 8, and received 12 cycles of therapy, until PD was documented. Eleven (61%) patients had SD as their best response.
Pharmacokinetic analysis
The PK parameters for unbound (free) and adjusted-bound aflibercept are shown in Table 5. The mean Cmax of free aflibercept increased in a dose-proportional manner when comparing the 2, 4, and 6 mg kg−1 cohorts. The concentration–time profiles of free aflibercept are characterised by a consistent t½ over the dosing interval at all three dose levels (Figure 1). The mean CL and Vss of free aflibercept did not change over the 2–6 mg kg−1 dose range, suggesting target (endogenous VEGF) saturation. The mean dose-adjusted AUC during the first dosing interval (AUC0-21 day/dose) of free aflibercept was dose-proportional at the two higher dose levels of 4 and 6 mg kg−1, indicating near-target saturation in the systemic circulation after one dose. Following i.v. administration, free aflibercept binds endogenous VEGF to form a monomeric aflibercept–VEGF complex. The complex reaches a plateau, and the concentration of adjusted-bound complex remains constant with repeat dosing of aflibercept at 2, 4, and 6 mg kg−1 over the 21-day dosing interval. Bound aflibercept concentrations were comparable at all three dose levels, suggesting saturation of endogenous VEGF binding (Figure 1). The cohorts differed only in their time-to-plateau, which was longest for the 2 mg kg−1 cohort and shorter for the 4 and 6 mg kg−1 cohorts.
Pemetrexed PK parameters were calculated to assess the effect, if any, of aflibercept administration on systemic pemetrexed concentrations (Table 5). The systemic concentration of and exposure to pemetrexed were not altered by concomitant administration of aflibercept. In particular, the kinetics of pemetrexed were linear over the first dosing interval, consistent with literature values of pemetrexed PK parameters when pemetrexed is administered with cisplatin in the same study population composition as this study (Dickgreber et al, 2009). The pemetrexed Cmax, AUC, tmax, and t½ were not statistically different (P<0.05) between the 2, 4, and 6 mg kg−1 aflibercept cohorts. In addition, the mean plasma concentration of pemetrexed at any given time point was the same regardless of aflibercept dose. Further, free and bound aflibercept drug concentrations in combination with pemetrexed are comparable to those observed with aflibercept monotherapy (Tew et al, 2010), suggesting that aflibercept PK are not affected by concomitant pemetrexed administration.
No antiaflibercept antibodies were detected in analysed samples.
Conclusion
This study has evaluated the feasibility of the combination of i.v. aflibercept in conjunction with cisplatin and pemetrexed in patients with advanced solid tumours. The RP2D of aflibercept was determined to be 6 mg kg−1 i.v. every 3 weeks. The most common treatment-related clinical AEs were fatigue and nausea. Fatigue was observed more frequently than in prior studies with cisplatin and pemetrexed combinations both in NSCLC and MPM patients (Shepherd et al, 2001; Vogelzang et al, 2003; Scagliotti et al, 2008). The most frequently reported haematologic toxicities (i.e., neutropenia and thrombocytopenia) do not appear to differ significantly from previous studies (Vogelzang et al, 2003; Kim et al, 2008; Scagliotti et al, 2008).
Anti-VEGF therapy has been associated with several side effects common to this class of agents. The incidence of vascular-related toxicities (i.e., hypertension, proteinuria, thrombosis, and haemorrhage) in this study was generally similar to that previously reported in prior studies of anti-VEGF therapy (Kuenen et al, 2002), including aflibercept with chemotherapy (Freyer et al, 2008; Limentani et al, 2008; Rixe et al, 2008; Kuhnowski et al, 2010). Prior reports have indicated a potential negative interaction between VEGF modulators and cisplatin (Kuenen et al, 2002; Marx et al, 2002). As VEGF has an important role in endothelial cell homeostasis and modulates the production of nitric oxide, deprivation of VEGF can potentially lead to a shift in endothelial cells to a prothrombotic state (Li and Keller, 2000). Chemotherapeutic agents, especially cisplatin, can induce activation of platelets, monocytes, and endothelial cells (Togna et al, 2000); as such, further exacerbation of hypercoagulability could potentially occur, resulting in a high rate of thromboembolic events. Two patients (11%) experienced three TREs in this study (two episodes of PE and one episode of DVT) deemed to be at least possibly related to the study treatment. The reported incidence of TREs in prior studies of aflibercept and chemotherapy ranges from 3 to 22% (Tabernero et al, 2012). In addition, a meta-analysis of anti-VEGF class AEs in three placebo-controlled phase III trials with aflibercept demonstrated that venous thrombotic events were not increased with aflibercept (Tabernero et al, 2012). The incidence of TREs in unselected patient populations with NSCLC and/or mesothelioma is 3–31% (Chew et al, 2008). It is therefore difficult to ascertain whether the addition of aflibercept to the cisplatin–pemetrexed combination actually poses a higher risk of developing TREs.
Vascular endothelial growth factor has an important role in regulating glomerular permeability (Kanellis et al, 2004). Thus, the inhibition of VEGF in the kidney glomeruli can result in proteinuria. Several clinical trials of aflibercept have confirmed that proteinuria is a potential toxicity, with an overall incidence of grade 3/4 up to 8% (Tabernero et al, 2012). Nephrotoxicity is one of the major side effects of cisplatin, which occurs in a dose-dependent manner in 25–30% of patients receiving a single dose of cisplatin. Renal toxicity is also accumulative. Glomerular injury is one of the potential mechanisms of nephrotoxicity when cisplatin is used (Sanchez-Gonzalez et al, 2011). Although glomerular injury occurs less frequently (because it is associated with high drug exposures) than tubular toxicity, vascular or interstitial damage, it may lead to proteinuria. Cisplatin-induced glomerular injury is characterised by a marked fall in the glomerular filtration rate. In this study, the frequencies of decreased CrCl and of proteinuria were both low (n=1 each, 6%) and no grade ⩾3 of such events were observed (Table 3).
In this study, we observed an increased rate of neurological symptoms, particularly in the form of a poorly defined, mild neurocognitive disturbance, often described by patients as dizziness or lightheadedness. There was no apparent dose dependence, as four episodes of this neurocognitive disturbance were documented when aflibercept was administered at 2 mg kg−1, and only one patient reported it in the 4 mg kg−1 cohort. Evaluation of these vague neurological symptoms steered towards the consideration of RPLS, which has been associated with the administration of anti-VEGF agents and/or cisplatin (Ito et al, 1998; Marinella and Markert, 2009; Leighl et al, 2010). However, differences between the symptoms observed in this patient subgroup and the most commonly reported characteristics of RPLS were noted. First, most cases of RPLS reported in the literature have been associated with severe and often acute hypertension; this was not the case in our study. All patients had their BP monitored on a weekly basis. For those who developed these mild neurocognitive symptoms, no significant changes were observed between BP reading before and after the development of symptoms (data not shown). Endothelial dysfunction has been implicated in the pathophysiology of RPLS, and has been observed in other, nontumoral, clinical settings, such as chronic renal failure, nephritis, haemolytic uraemic syndrome, and/or metabolic disturbances (e.g., hypomagnesemia) (Hinchey et al, 1996). Therefore, we further investigated if the development of this cognitive disorder was associated with decreased CrCl, the onset of proteinuria, or the presence of electrolyte abnormalities. All patients who developed neurocognitive symptoms had a normal CrCl at baseline, and no changes were observed in association with the onset of symptoms (data not shown). Second, other potentially serious neurological symptoms (e.g., alteration in mental status, hallucinations, and seizures) are usually present in the development of RPLS. These symptoms were not observed in patients in our study though reports of mild headache and memory impairment were noted in several patients. Finally, abnormalities in MRI, including vasogenic oedema, have been described consistently and are key to diagnosis in patients affected by RPLS (Hinchey et al, 1996). In this phase I study, in those patients who had MRI scans (n=2) after developing neurocognitive symptoms, no MRI abnormalities were noted.
Pharmacokinetic analysis showed that trough concentrations of free (unbound) aflibercept were higher than adjusted-bound aflibercept trough concentrations in patients receiving either 4 or 6 mg kg−1 aflibercept. Maintenance of systemic free aflibercept concentration above the adjusted-bound aflibercept trough concentration ensures maintenance of target saturation. As target saturation is thought to parallel clinical efficacy, PK analysis suggests that a dose of 6 mg kg−1 i.v. every 3 weeks has the best chance of clinical efficacy via maintenance of target saturation over the entire dosing interval. With respect to the potential impact of aflibercept administration on pemetrexed concentrations, the systemic concentration of and exposure to pemetrexed were not altered by concomitant administration of aflibercept.
In summary, this study showed that the administration of aflibercept 6 mg kg−1 i.v. every 3 weeks in combination with cisplatin and pemetrexed led to a slightly higher than expected rate of the side effects (mainly increased fatigue) than that observed with the chemotherapy regimen alone. The mild neurocognitive disturbances seen in five patients were investigated and did not suggest RPLS at this stage, but led to a higher level of observation for this toxicity in the phase II study. The phase II study of this regimen was conducted in previously untreated nonsquamous NSCLC (clinicaltrials.gov identifier: NCT00794417) and results will be reported separately, but it is noted that the study was discontinued early owing to a higher than anticipated incidence of RPLS.
Change history
30 July 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bonnesen B, Pappot H, Holmstav J, Skov BG (2009) Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: relation to prognosis. Lung Cancer 66 (3): 314–318
Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH (2008) The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost 6 (4): 601–608
Coleman RL, Duska LR, Ramirez PT, Heymach JV, Kamat AA, Modesitt SC, Schmeler KM, Iyer RB, Garcia ME, Miller DL, Jackson EF, Ng CS, Kundra V, Jaffe R, Sood AK (2011) Phase 1-2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol 12 (12): 1109–1117
Cook KM, Figg WD (2010) Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin 60 (4): 222–243
Delli Carpini J, Karam AK, Montgomery L (2010) Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis 13 (1): 43–58
Dickgreber NJ, Fink TH, Latz JE, Hossain AM, Musib LC, Thomas M (2009) Phase I and pharmacokinetic study of pemetrexed plus cisplatin in chemonaive patients with locally advanced or metastatic malignant pleural mesothelioma or non-small cell lung cancer. Clin Cancer Res 15 (1): 382–389
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45 (2): 228–247
Freyer G, Fumoleau P, You B, Isambert N, Chevalier P, Trillet Lenoir V (2008) A phase I dose escalation and pharmacokinetic (PK) study of intravenous (iv) aflibercept (VEGF Trap) plus docetaxel (D) and cisplatin (C) in patients (pts) with advanced solid tumors: preliminary results. J Clin Oncol 26 (Suppl): abstr 14539
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR (1996) A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334 (8): 494–500
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99 (17): 11393–11398
Ito Y, Arahata Y, Goto Y, Hirayama M, Nagamutsu M, Yasuda T, Yanagi T, Sobue G (1998) Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 19 (3): 415–417
Kanellis J, Levidiotis V, Khong T, Cox AJ, Stacker SA, Gilbert RE, Cooper ME, Power DA (2004) A study of VEGF and its receptors in two rat models of proteinuria. Nephron Physiol 96 (1): P26–P36
Kim YH, Chung HC, Kang WK, Park SR, Kim CS, Kim TY, Shin SW, Park BJ, Cha SJ, Bang YJ (2008) Pemetrexed and cisplatin in patients with advanced gastric cancer: a Korean cancer study group multicenter phase II study. Cancer Chemother Pharmacol 62 (2): 263–270
Kowanetz M, Ferrara N (2006) Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 12 (17): 5018–5022
Kuenen BC, Rosen L, Smit EF, Parson MR, Levi M, Ruijter R, Huisman H, Kedde MA, Noordhuis P, van der Vijgh WJ, Peters GJ, Cropp GF, Scigalla P, Hoekman K, Pinedo HM, Giaccone G (2002) Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol 20 (6): 1657–1667
Kuhnowski F, Thieblemont C, Jardin F, Broussais-Guillemot F, Meignan M, Cabecadas J, Gaulard P, Tilly H, Oprea C, Haioun C (2010) A phase I study of IV aflibercept (Afl) in combination with R-CHOP in untreated patients (pts) with B-cell lymphoma. J Clin Oncol 28 (Suppl 15s): abstr 8010
Leighl NB, Raez LE, Besse B, Rosen PJ, Barlesi F, Massarelli E, Gabrail N, Hart LL, Albain KS, Berkowitz L, Melnyk O, Shepherd FA, Sternas L, Ackerman J, Shun Z, Miller VA, Herbst RS (2010) A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol 5 (7): 1054–1059
Li W, Keller G (2000) VEGF nuclear accumulation correlates with phenotypical changes in endothelial cells. J Cell Sci 113 (Part 9): 1525–1534
Limentani S, Just R, Purdham A, Mulay M, Bair A, Tamby JF, Chap LI, Rosen LS (2008) A phase I dose escalation and pharmacokinetic (PK) study of intravenous (iv) aflibercept (VEGF Trap) plus FOLFOX4 in patients (pts) with advanced solid tumors: preliminary results. J Clin Oncol 26 (Suppl): abstr 3556
Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, Buzenet G, Koehler E, Sosman JA, Schwartz LH, Gultekin DH, Koutcher JA, Donnelly EF, Andal R, Dancy I, Spriggs DR, Tew WP (2010) Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol 28 (2): 207–214
Marinella MA, Markert RJ (2009) Reversible posterior leucoencephalopathy syndrome associated with anticancer drugs. Intern Med J 39 (12): 826–834
Marx GM, Steer CB, Harper P, Pavlakis N, Rixe O, Khayat D (2002) Unexpected serious toxicity with chemotherapy and antiangiogenic combinations: time to take stock!. J Clin Oncol 20 (6): 1446–1448
Novello S, Ramlau R, Gorbunova VA, Ciuleanu TE, Ozgurolu M, Goksel T, Baldotto C, Bennouna J, Shepherd FA, Scagliotti G (2011) Aflibercept in combination with docetaxel for second-line treatment of locally advanced or metastatic non-small-cell lung cancer (NSCLC): final results of a multinational placebo-controlled phase III trial (EFC10261-VITAL). 14th World Conference on Lung Cancer. Abstract O4302. 3–7 July 2011 Amsterdam, The Netherlands
Rixe O, Verslype C, Khayat D, Tejpar S, Billemont B, Crabbe M, Meric JB, Assadourian S, Van Cutsem E (2008) A phase I dose escalation (DE) and pharmacokinetics (PK) study of intravenous aflibercept (VEGF Trap) plus irinotecan, 5-fluorouracil, and leucovorin (I-LV5FU2) in patients with advanced solid tumors (STs). J Clin Oncol 26 (Suppl): abstr 3557
Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI (2011) An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol 41 (10): 803–821
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355 (24): 2542–2550
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26 (21): 3543–3551
Shepherd FA, Dancey J, Arnold A, Neville A, Rusthoven J, Johnson RD, Fisher B, Eisenhauer E (2001) Phase II study of pemetrexed disodium, a multitargeted antifolate, and cisplatin as first-line therapy in patients with advanced nonsmall cell lung carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group. Cancer 92 (3): 595–600
Spigel DR, Hainsworth JD, Shipley DL, Ervin TJ, Kohler PC, Lubiner ET, Peyton JD, Waerhouse DM, Burris HA, Greco FA (2012) A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer. J Thor Oncol 7 (1): 196–202
Tabernero J, Allegra CJ, Rougier PR, Scagliotti G, Philip PA, Lakomy R, Ramlau R, Assadourian S, Chevalier S, Van Cutsem E (2012) Meta-analysis of anti-VEGF class adverse events from three double-blind (db), placebo (pbo)-controlled phase III trials with IV aflibercept (Afl). J Clin Oncol 30 (Suppl): abstr 3579
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Van Hazel G, Moisevenko V, Ferry D, Mckendrick J, Soussan-Lazard K, Boelle E, Allegra C (2011) Results from VELOUR, a phase 3 study of aflibercept versus placebo in combination with FOLFIRI for the treatment of patients with previously treated metastatic colorectal cancer. European Multidisciplinary Congress. Abstract 6LBA, Stockholm, 23–27 September 2011
Tang P, Cohen SJ, Bjarnason GA, Kollmannsberger C, Virik K, MacKenzie MJ, Brown J, Wang L, Chen AP, Moore MJ (2008) Phase II trial of aflibercept (VEFG Trap) in previously treated patients with metastatic colorectal cancer (MCRC): a PMH phase II consortium trial. J Clin Oncol 26 (Suppl): abstr 4027
Tew WP, Gordon M, Murren J, Dupont J, Pezzulli S, Aghajanian C, Sabbatini P, Mendelson D, Schwartz L, Gettinger S, Psyrri A, Cedarbaum JM, Spriggs DR (2010) Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin Cancer Res 16 (1): 358–366
Togna GI, Togna AR, Franconi M, Caprino L (2000) Cisplatin triggers platelet activation. Thrombosis Res 99 (5): 503–509
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21 (14): 2636–2644
Acknowledgements
The present study has been supported by Regeneron Pharmaceuticals and SanofiOncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LLS and HAW receive research funding from Regeneron Pharmaceuticals. FAS has received honoraria from Regeneron Pharmaceuticals. AT, LL, JL, BG, and EBL are employees of Regeneron Pharmaceuticals. All remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Diaz-Padilla, I., Siu, L., San Pedro-Salcedo, M. et al. A phase I dose-escalation study of aflibercept administered in combination with pemetrexed and cisplatin in patients with advanced solid tumours. Br J Cancer 107, 604–611 (2012). https://doi.org/10.1038/bjc.2012.319
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.319
Keywords
This article is cited by
-
A phase II multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non-small cell lung cancer
British Journal of Cancer (2014)
-
Aflibercept—a Decoy VEGF Receptor
Current Oncology Reports (2014)
-
Efficacy and Safety of Aflibercept and Its Role in the Treatment of Metastatic Colorectal Cancer
Rare Cancers and Therapy (2013)