Abstract
Background:
This study examined the clinical significance of NAC1 and the expression level of its potential downstream target fatty acid synthase (FASN) in ovarian clear cell carcinomas (OCCCs), and evaluated the NAC1/FASN pathway as a potential therapeutic target.
Methods:
NAC1 and FASN expression and NACC1 gene amplification were assessed in ovarian cancers by immunohistochemistry, fluorescence in situ hybridisation, and clinical data collected by a retrospective chart review. C75, a FASN inhibitor, was used to assess whether this pathway represented a therapeutic target in OCCC.
Results:
High NAC1 expression was most frequent in clear cell tumours (40.0%:24/60). NACC1 gene amplification was identified in none of the 58 OCCCs. The frequency of NACC1 gene amplification was significantly higher in the high-grade serous histology than in the clear cell histology (P<0.01). NAC1 expression was significantly correlated with FASN expression in both OCCC samples and OCCC cell lines. Either high NAC1 expression or high FASN expression significantly correlated with shorter progression-free and overall survival (P=0.002 and 0.0048). NAC1 overexpression stimulated FASN expression, and NAC1 silencing using siRNA decreased FASN expression in OCCC cell lines. Profound growth inhibition was observed in C75-treated carcinoma cells with FASN overexpression when compared with the response in carcinoma cells without FASN expression.
Conclusion:
These findings indicate that NAC1/FASN overexpression is critical to the growth and survival of a subset of OCCC. The FASN silencing by the C75-induced phenotypes depends on the expression status of the targeted cell line. Therefore, NAC1/FASN pathway-targeted therapy may benefit selected OCCC patients.
Similar content being viewed by others
Main
Ovarian carcinoma is the most lethal gynaecological malignancy worldwide (Wingo et al, 1995). Despite a dramatic improvement in survival following the introduction of platinum–taxane combination chemotherapy, the overall 5-year survival remains around 45%. Ovarian carcinoma is classified into four major histological types (serous, mucinous, endometrioid, and clear cell), which are distinct on a molecular level (Cho and Shih, 2009). Ovarian clear cell carcinoma (OCCC), which constitutes around 25% of ovarian carcinoma in Japan, carries a poor prognosis despite 60% being diagnosed at an early stage (Sugiyama et al, 2000). Unlike its serous counterpart, OCCC is more frequently platinum resistant (Winter et al, 2007). This may be a reflection of the fundamental molecular differences between OCCC and serous carcinomas. Therefore, chemotherapeutics targeting genes and pathways unique to OCCC may represent one option for improving survival. One such gene, which has recently been shown to have a role in taxane resistance, is NAC1 (Ishibashi et al, 2008; Jinawath et al, 2009).
NAC1 is a member of the BTB/POZ family of proteins that participate in several cellular functions including proliferation, apoptosis, transcription control, and cell morphology maintenance (Stogios et al, 2005). By serial analysis of gene expression levels in all 130 deduced human BTB/POZ genes, we identified NACC1 as a carcinoma-associated BTB/POZ gene (Nakayama et al, 2006). NAC1 is a transcription repressor that is involved in the self-renewal and maintenance of pluripotency in embryonic stem cells (Wang et al, 2006). NAC1 is significantly overexpressed in several types of human carcinomas (Nakayama et al, 2006; Ishibashi et al, 2008; Yeasmin et al, 2008). The levels of NAC1 expression correlate with tumour recurrence in ovarian serous carcinomas, and intense NAC1 immunoreactivity in primary ovarian tumours predicts early recurrence (Nakayama et al, 2006; Ishibashi et al, 2008). Using a quantitative proteomic method employing tandem mass spectrometry (MS/MS) and spectral counting, Ueda et al (2010) demonstrated that NAC1 regulated fatty acid synthase (FASN) expression. Fatty acid synthase (FAS), the enzyme responsible for the de novo synthesis of fatty acids, has emerged as a potential therapeutic target in human cancers. Fatty acid synthase catalyses the condensation of malonyl-CoA and acetyl-CoA to produce long-chain fatty acids (Wakil, 1989). High levels of FAS expression have been found in ovarian cancer (Gansler et al, 1997; Alo et al, 2000; Ueda et al, 2010) and in most human solid tumours (Kuhajda, 2006). The upregulation of FAS expression in cancer cells has been linked to both mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways through the sterol regulatory binding element binding protein 1c (Van de Sande et al, 2002; Yang et al, 2002; Yang et al, 2003). Fatty acid synthase protein expression denotes poor prognosis in breast and prostate cancers (Alo et al, 1996; Pizer et al, 2001).
NAC1 and FASN expression has not been investigated with respect to ovarian carcinoma in histological subtypes other than serous (Pizer et al, 2001; Ueda et al, 2010). In this study, we further investigated the clinicopathological role of NAC1-regulated FASN expression in OCCC, as well as the possibility of FASN-based therapeutics in OCCC.
Materials and methods
Tissue samples
Formalin-fixed, paraffin-embedded tissue samples of 144 ovarian cancers including 44 serous carcinomas, 10 mucinous carcinomas, 60 clear cell carcinomas, and 20 endometrioid carcinomas were used in this study. Samples were obtained from the Department of Obstetrics and Gynaecology at the Shimane University Hospital and the Department of Obstetrics and Gynaecology at Seirei Hamamatsu General Hospital. Diagnosis was based on conventional morphological examination of sections stained with hematoxylin and eosin (H&E), and tumours were classified according to the WHO classification. Tumour staging was performed according to the International Federation of Gynaecology and Obstetrics (FIGO) classification. All patients were primarily treated with cytoreductive surgery and adjuvant platinum and taxane or CPT-11 chemotherapy (CBDCA AUC 5 with paclitaxel 175 mg m−2 or docetaxel 70 mg m−2 or CDDP 60 mg m−2 with CPT-11 180 mg m−2). All patients received 6–12 courses of this combination regimen. Acquisition of tissue specimens and clinical information was approved by an institutional review board (Shimane University and Serrei Hamamatsu General Hospital). The paraffin tissue blocks were organised into tissue microarrays, each was made by removing 3-mm diameter cores of tumour from the block. Selection of the area to core was made by a gynaecological oncologist (KN) and pathology technician (KI), and was based on review of the H&E slides.
Immunohistochemistry
The method for NAC1 immunohistochemistry and evaluation criteria has been detailed in a previous report (Ishibashi et al, 2008). For immunohistochemistry, paraffin sections after deparaffinisation were incubated with a primary mouse FASN antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1 : 100 in a 4 °C moist chamber overnight. Two independent observers scored the FASN immunoreactivity using a categorical scoring system from 0 (not detectable) to 3 (intense) with the mean score recorded from triplicates.
Fluorescence in situ hybridisation
BAC clones (RP11-356L15 and CTD-2508D10) containing the genomic sequences of the 19p13.2 amplicon were purchased from Bacpac Resources (Childrens’ Hospital, Oakland, CA, USA) and Invitrogen (Carlsbad, CA, USA). Bac clones located at Ch19P13.11 (CTD-2518O18) were used to generate reference probes. The method for fluorescence in situ hybridisation (FISH) has been described in detail in a previous report (Nakayama et al, 2007).
Cell culture and cell lines
ES2 (clear cell carcinoma) human ovarian cancer cell line was obtained from the American Tissue Culture Centre (Rockville, MD, USA). JHOC9 (clear cell carcinoma) and JHOC5 (clear cell carcinoma) human ovarian cancer cell lines were also obtained from Riken Bioresource Centre (Ibaragi, Japan). The OCCC cell lines OVISE, OVTOKO, and RMG1 were obtained from the Japanese Health Science Research Resources Bank. The OCCC cell line OV207 was a kind gift from Dr Vijayalakshmi Shridhar, Mayo Clinic (Rochester, MN, USA).
Western blot analysis
Cell lysates were prepared by dissolving cell pellets in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA) supplemented with 5% beta-mercaptoethanol (Sigma, St Louis, MO, USA). Western blot analysis was performed on ovarian cancer cell lines ES2, JHOC9, JHOC5, OVISE, OVTOKO, RMG1, and OV207. Similar amounts of total protein from each lysate were loaded and separated on 10% Tris-Glycine-SDS polyacrylamide gels (Novex, San Diego, CA, USA) and electroblotted to Millipore Immobilon-P polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were probed with NAC1 antibody (1 : 100) (Novus Biologicals, Littleton, CO, USA) or FASN (1 : 1000) (Santa Cruz Biotechnology) followed by a peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin (1 : 20 000). Western blots were developed by chemiluminescence (Pierce, Rockford, IL, USA). The same membrane was probed with an antibody that reacted with GAPDH (1 : 10 000) (Cell Signaling Technology, Beverly MA, USA) for loading controls and was developed by chemiluminescence (Pierce).
Silencing RNA knockdown of NAC1 gene expression
Two silencing RNAs (siRNAs) that targeted NAC1 were designed with the following sense sequences: 5′-UGAUGUACACGUUGGUGCCUGUCACCA-3′ and 5′-GAGGAAGAACUCGGUGCCCUUCUCCAU-3′. Control siRNA (luciferase siRNA) was purchased from IDT (Coralville, IA, USA). Cells were seeded into 96-well plates and transfected with siRNAs using Oligofectamine (Invitrogen). Following siRNA transfection, cells were collected at 48 h for quantitative real-time PCR analysis of NAC1 and FASN mRNA expression levels.
Quantitative real-time PCR analysis
Relative transcript expression levels were measured by quantitative real-time PCR using a method described previously (Nakayama et al, 2006). The primers for NAC1 and FASN were 5′-CCAGACACTGCAGATGGAGA-3′ (forward), 5′-AAGCTGAGGATCTGCTGGAA-3′ (reverse) and 5′-CATCCAGATAGGCCTCATGAC-3′ (forward), 5′-CTCCATGAAGTAGGAGTGGAAG-3′ (reverse). PCR reactions were performed in triplicate using an iCycler (Bio-Rad Laboratories). The amplified products were quantified by the fluorescence intensity of SYBR Green I (Molecular Probes, Eugene, OR, USA). Average fold changes were calculated by differences in threshold cycles (Ct) between pairs of samples to be compared. The amyloid beta (A4) precursor protein was used for normalising the cDNA concentration of each sample.
Cell proliferation assay
Cells were seeded in 96-well plates at a density of 3000 cells per well. Cell number was determined indirectly by an MTT assay (Nakayama et al, 2001). Data were expressed as the mean ±1 s.d. of triplicate determinations. An MTT cell growth assay was performed 96 h after treating the cells with C75 (Calbiochem, La Jolla, CA, USA) at 10 μM or with DMSO (control). The data were expressed as a percentage of the DMSO control. The mean and s.d. were obtained from three experiments.
Plasmid constructs and the generation of stable clones
To assess whether NAC1 overexpression induced FASN overexpression in OCCC, we used a mammalian expression vector, pCMV/NAC1, with a V5 tag at the COOH terminus as previously described (Nakayama et al, 2006).
The pCMV/NAC1 vector was stably transfected into the ES2 cell line using the Nucleofector II electroporator (Amaxa, Köln, Germany). Selection of pCMV/NAC1 stable transfected cells was performed in selection medium containing 3∼6 μg ml−1 blasticidine (Invitrogen).
Statistical methods for clinical correlation
Progression-free and overall survivals were calculated from the date of diagnosis to the date of first relapse or last follow-up. Age and performance status distributions were similar between patients expressing NAC1 or FASN and those not expressing it. The data were plotted as Kaplan–Meier curves, and the statistical significance was determined by the log-rank test. Data were censored when patients were lost to follow-up. The Student's t-test (for comparison of two groups) or one-way analysis of variance (for comparison of more than two groups) were used to evaluate numeric data.
Results
Relationship between NAC1 protein expression and histological subtype in ovarian carcinoma
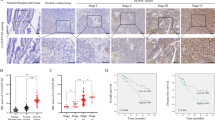
NAC1 immunoreactivity was detected in tumour cell nuclei (Figure 1A). High expression of NAC1 (NAC1 immunointensity 2+ and 3+) was observed in 33.3% (44/132) of all analysed tumours. High expression of NAC1 was identified in 15/43 (35%) serous, 1/11 (9%) mucinous, 4/18 (22%) endometrioid, and 24/60 (40%) of clear cell carcinomas. The relationships between NAC1 protein expression and histological subtypes are summarised in Table 1A. Interestingly, the frequency of high NAC1 protein expression was highest in clear cell type in ovarian carcinomas. As OCCC most frequently had high expression, we focused on OCCCs for the following experiments.
Immunoreactivity of NAC1 and FASN in OCCC tissues. (A, D) Showing H&E staining of corresponding tissues in upper and lower panels, respectively. (B) Intense NAC1 immunoreactivity is present in the nuclei of OCCC cells (upper centre panel). (C) Intense FASN immunoreactivity is present in the cytoplasm of OCCC cells (upper centre panel). (E, F) An OCCC case with negative staining of both NAC1 and FASN respectively.
NACC1 gene amplification was a rare event in OCCCs
Previously, we reported that NACC1 was a potential oncogene in ovarian cancer and that NACC1 was amplified in 20% of high-grade serous carcinomas (Nakayama et al, 2006; Shih Ie et al, 2011). Of the 60 OCCCs, 58 had informative cases. None of the clear cell carcinomas, however (0%: 0/58), had detectable NACC1 gene amplification. A total of 9 out of 43 (20.9%) serous high-grade carcinomas showed significant amplification of NACC1 (P<0.05, χ2 test) (Table 1B).
Relationship between NAC1 protein expression and clinicopathological factors in OCCCs
High expression of NAC1 (NAC1 immunointensity 2+ and 3+) was observed in 40% (24/60) of the analysed tumours. Patients were stratified into one of the two groups depending on the status of NAC1 immunostaining. The relationships between NAC1 protein expression and clinicopathological factors are shown in Table 2. There were no significant correlations between high NAC1 expression and FIGO stage, CA125 levels, patient age, status of endometriosis, Ki-67 labelling index, chemotherapy regimen, or status of residual tumour (P=0.077). The results in Table 2 demonstrate that high NAC1 expression is significantly correlated with high FASN expression status (P=0.0185).
Effect of NAC1 protein expression on progression-free/overall survival in OCCC
Kaplan–Meier estimates of disease-free/overall survival are plotted in Figure 2. The 60 patients of OCCCs studied included cancers diagnosed at stages I–IV. Among them, the 24 patients with high NAC1 expression tended to have a shorter progression-free and overall survival than those without low NAC1 expression (P=0.119 and 0.318 respectively; log-rank test) (Figure 2A and B).
(A, B) NAC1 overexpression tended to correlate with shorter progression-free/overall survival in patients with OCCC who received cytoreductive surgery, followed by a standard platinum-based chemotherapy regimen (P=0.119 and 0.318 respectively, log-rank test). (C) Kaplan–Meier survival analysis showing that high expression of FASN was associated with a shorter progression-free survival in comparison to low FASN expression in OCCCs (P=0.021, log-rank test). (D) Kaplan–Meier survival analysis showing that high FASN expression tended to correlate with a shorter overall survival than low FASN expression in OCCC (P=0.075, log-rank test). (E, F) Either high NAC1 or high FASN expression correlated with shorter progression-free/overall survival in OCCC (P=0.004 and 0.037 respectively, log-rank test).
Effect of FASN protein expression on overall/progression-free survival in OCCC
Recently, FASN was identified as a potential downstream target of NAC1 (Ueda et al, 2010). In the immunohistochemical analysis, NAC1 and FASN were significantly positively correlated. We then assessed for any prognostic effects of FASN protein expression in OCCC. Kaplan–Meier estimates of overall survival are plotted in Figure 2C and D. High expression of FASN correlated with shorter progression-free survival in patients with ovarian carcinomas treated with platinum-based chemotherapy (P=0.021; log-rank test) (Figure 2C). High expression of FASN tended to be associated with shorter overall survival in patients with ovarian carcinomas treated with platinum-based chemotherapy but was not statistically significant (P=0.075; log-rank test) (Figure 2D).
Interestingly, when patients with OCCC treated with platinum-based chemotherapy were classified using a two-tier system of expression levels (low or high), patients with high NAC1 expression or high FASN expression had significantly shorter progression-free and overall survival compared with patients with low expression of these genes (P=0.004 and 0.037), respectively, log-rank test (Figure 2E and F).
Correlation of NAC1 protein expression and FASN protein expression in OCCC cell lines
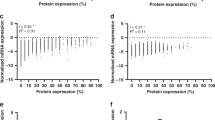
NAC1 and FASN expression levels measured with immunohistochemistry were then correlated with protein expression levels by western blot in several OCCC cell lines (Figure 3A). NAC1 protein expression levels significantly correlated with FASN protein expression level in the OCCC cell lines (r=0.877, P<0.01) (Figure 3B).
(A) Western blot analysis showing higher expression levels of NAC1 protein in OV207, OVTOKO, and JHOC9 cells than in OVISE and ES2 cells. Western blot analysis showing a higher expression level of FASN protein in OV207, OVTOKO, and JHOC9 cells than in OVISE and ES2 cells in which FASN protein is not detectable. (B) NAC1 protein expression is significantly correlated with FASN protein expression in OCCC cell lines (r=0.877, P<0.01). (C) Gene expression analysis showing a significant reduction of FASN gene expression in NAC1 siRNA-treated cells compared with control siRNA-treated cells in OV207 and JHOC9 cell lines. *P<0.05. (D) Gene expression analysis showing a significant induction of FASN gene expression in NAC1-transfected cells compared with control vector-treated cells in ES2 cell line. *P<0.05. (E) Western blot analysis showing a reduction of FASN in C75-treated cells compared with control DMSO-treated cells in JHOC9 cell line. (F) C75 suppresses cell growth depending on FASN expression levels in ovarian clear cell carcinomas. *P<0.05.
NAC1 knockdown leads to decreased FASN expression in OCCC cell lines
We previously showed that siRNA knockdown of NAC1 significantly reduced NAC1 expression compared with control siRNA treatment (Nakayama et al, 2010). Reduction of NACC1 gene expression significantly inhibited FASN gene expression in OCCC lines OV207 and JHOC9 (Figure 3C).
Constitutive expression of NAC1 leads to increased FASN expression in OCCC cell lines
Next, to confirm the results of NAC1-knockdown experiments, we generated stable NAC1-expressing cells from ES2 cells, which have low endogenous NAC1 expression. This cell line was stably transfected with a NAC1 pCMV vector. When compared with vector-transfected controls, the ES2 cell line that expressed NAC1 had higher FASN gene expression levels as measured by real-time PCR (Figure 3D).
C75 suppresses growth in OCCC cells
The above findings suggest that FASN is one of the downstream targets of NAC1, a gene overexpressed in a subset of OCCC OCCCs. This raises the possibility that FASN expression contributes to the OCCC phenotype. To assess the contribution of FASN expression to OCCC cell growth and survival, OCCC cell lines were treated with the FASN inhibitor C75, a FASN inhibitor, and FASN levels measured and cell growth assessed. The FASN protein expression status tended to be correlated with growth inhibition by the C75 FASN inhibitors (Figure 3E and F).
Discussion
Ovarian clear cell carcinomas, which comprise ∼25% of all ovarian carcinomas in Japan, display a distinct gene expression profile relative to other histological types (Schwartz et al, 2002; Yamaguchi et al, 2010). They are more aggressive and carry a worse prognosis than stage-matched serous adenocarcinomas (Goff et al, 1996; Sugiyama et al, 2000). This is likely because OCCC is frequently refractory to platinum-based chemotherapy (Goff et al, 1996; Sugiyama et al, 2000). The current immunohistochemical analysis of a panel of ovarian tumours demonstrated strong expression of NAC1 in 40% of clear cell carcinomas, in comparison with high expression in 9–35% of serous, endometrioid, and mucinous tumours. Given this, NAC1 may represent a potential molecular target in patients with OCCCs. We have demonstrated that high NAC1 expression correlates with shorter survival in patients with ovarian carcinoma treated with platinum-based chemotherapy regardless of histological type (Ishibashi et al, 2008; Nakayama et al, 2010). Recently, Zhang et al (2012) reported that NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. It is plausible that while high NAC1 expression in OCCC is one possible explanation for why some of these tumours have a worse prognosis, clearly this applies to only a subset of tumours; therefore other mechanisms likely exist.
NACC1 gene amplification accounts for the increased expression in many high-grade ovarian serous carcinomas; however, some serous carcinomas did have increased NAC1 expression in the absence of gene amplification (Nakayama et al, 2010; Shih Ie et al, 2011). In contrast, NACC1 gene amplification was undetectable in all clear cell carcinoma specimens tested, which suggests that NAC1 in this histology may be regulated at the transcriptional level.
Recently, Ueda et al (2010) reported that FASN is a potential downstream target of NAC1 in serous high-grade ovarian carcinoma; however, it is unclear if this is the case in other histological subtypes. Therefore, to assess the relationship between NAC1 and FASN in clear cell histology, we used both knockdown and overexpression systems. We first knocked down NAC1 in OCCC lines, JHOC9, and OV207, using a previously designed siRNA (Yeasmin et al, 2008). Reduction of NAC1 expression resulted in decreased FASN expression in the NAC1-knockdown cell line, indicating that FASN was a likely downstream target of NAC1. Conversely, an ES2 cell line overexpressing NAC1 had significantly increased FASN gene expression. These reciprocal findings suggest that FASN is a potential downstream target of NAC1 in OCCCs. Our observations augment the growing body of evidence suggesting that the transcriptional factor NAC1 regulates FASN in multiple histological types of ovarian carcinomas.
In the present study, we demonstrated that OCCC cell lines with FASN overexpression were more sensitive to a potent FASN inhibitor, C75, suggesting that FASN-targeted therapy may have activity in this subset of OCCC. The mechanism underlying the upregulation of FASN in OCCC is not clear and likely involves multiple pathways. In several types of carcinoma, including ovarian carcinoma, FASN overexpression robustly induces de novo lipogenesis. The generated lipids are integrated into membrane lipid rafts and modulate membrane receptor tyrosine kinases (for example, the EGFR family). This, in turn, results in the initiation of oncogenic signalling pathways involving cell survival, proliferation, migration, and invasion (Jackowski et al, 2000; Menendez et al, 2005). The FASN overexpression increases EGFR and HER2 protein expression and tyrosine phosphorylation, and thereby amplifies oncogenic signalling pathways that contribute to tumourigenic transformation (Vazquez-Martin et al, 2008). The effects of the FASN pathway are likely mediated by both the FASN kinase activity itself as well as factors along the pathway, for example, in prostate cancer in which caveolin-1 and KLF5/SREBP-1 function upstream of FASN (Di Vizio et al, 2007; Zhou et al, 2007). Thus, at least in OCCCs, the NAC1 pathway represents another mechanism for controlling FASN expression and pathway activity. Unlike other members of the BTB/POZ family, NAC1 has a BEN domain instead of the zinc-finger DNA-binding domain (Abhiman et al, 2008). The BEN theoretically may function as a DNA-binding domain (Abhiman et al, 2008); however, this has not been directly demonstrated. It does act as a transcription co-repressor with other BTB/POZ proteins (Korutla et al, 2009). Moreover, it has also been shown to interact with nuclear proteins potentially involved in tumourigenesis, including Nanog (Ma et al, 2009), CoREST (Korutla et al, 2007), and HDAC3 and HDAC4 (Nakayama et al, 2010). Thus, it is possible that FASN expression is indirectly controlled by NAC1 through binding with its specific partner(s). Identification of the NAC1/FASN pathway sheds new light on the molecular mechanism by which NAC1 promotes tumour progression in OCCC. Further studies are required to elucidate the transcriptional regulation of FASN by NAC1.
As with other carcinomas of the colon, prostate, and breast (Alo et al, 1996; Rashid et al, 1997; Rossi et al, 2003), FASN overexpression appears to correlate with worse prognosis, and overexpression is found more frequently in the aggressive OCCC and to a lesser-degree serous carcinomas. More importantly, in patients with OCCC, the high level of FASN significantly correlated with poor prognosis, suggesting that FASN contributed to the aggressive phenotype. A similar observation has been reported for NAC1 (Ishibashi et al, 2008; Nakayama et al, 2010). It is therefore plausible that the NAC1/FASN pathway represents one factor driving OCCC progression. How FASN contributes to disease aggressiveness in OCCC remains to be elucidated. Besides endowing drug resistance, FASN may enhance oncogenesis via cellular mechanisms such as enhancing the Wnt (Fiorentino et al, 2008), c-Met (Coleman et al, 2009), and proteosome pathways (Little et al, 2008). Moreover, upregulation of FASN confers a growth and survival advantage by blocking apoptosis under hypoxia, a common condition in solid tumours and malignant effusions (Harris, 2002; Furuta et al, 2008).
In conclusion, we have demonstrated that NAC1, a member of the BTB/POZ family, and its potential downstream target FASN are overexpressed in a subset of OCCC. Of special interest is the finding that NAC1 expression is most common in the clear cell subtype of ovarian carcinomas, which are frequently chemoresistant (Goff et al, 1996; Sugiyama et al, 2000). Furthermore, NAC1/FASN expression is a biomarker of poor outcome for patients treated with conventional platinum-based chemotherapy in OCCCs. New-generation FASN inhibitors deserve consideration in future clinical trials involving OCCC, particularly for patients who are refractory to platinum-based chemotherapy.
Change history
03 July 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abhiman S, Iyer LM, Aravind L (2008) BEN: a novel domain in chromatin factors and DNA viral proteins. Bioinformatics 24: 458–461
Alo PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, Serpieri DE, Di Tondo U (2000) Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol Rep 7: 1383–1388
Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U (1996) Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer 77: 474–482
Cho KR, Shih Ie M (2009) Ovarian cancer. Annu Rev Pathol 4: 287–313
Coleman DT, Bigelow R, Cardelli JA (2009) Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol Cancer Ther 8: 214–224
Di Vizio D, Sotgia F, Williams TM, Hassan GS, Capozza F, Frank PG, Pestell RG, Loda M, Freeman MR, Lisanti MP (2007) Caveolin-1 is required for the upregulation of fatty acid synthase (FASN), a tumor promoter, during prostate cancer progression. Cancer Biol Ther 6: 1263–1268
Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, Nguyen PL, Migita T, Zamponi R, Di Vizio D, Priolo C, Sharma C, Xie W, Hemler ME, Mucci L, Giovannucci E, Finn S, Loda M (2008) Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest 88: 1340–1348
Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K (2008) Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68: 1003–1011
Gansler TS, Hardman W, Hunt DA, Schaffel S, Hennigar RA (1997) Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28: 686–692
Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, Nikrui N, Tamimi HK, Cain JM, Greer BE, Fuller AF (1996) Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol 60: 412–417
Harris AL (2002) Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38–47
Ishibashi M, Nakayama K, Yeasmin S, Katagiri A, Iida K, Nakayama N, Fukumoto M, Miyazaki K (2008) A BTB/POZ gene, NAC-1, a tumor recurrence-associated gene, as a potential target for Taxol resistance in ovarian cancer. Clin Cancer Res 14: 3149–3155
Jackowski S, Wang J, Baburina I (2000) Activity of the phosphatidylcholine biosynthetic pathway modulates the distribution of fatty acids into glycerolipids in proliferating cells. Biochim Biophys Acta 1483: 301–315
Jinawath N, Vasoontara C, Yap KL, Thiaville MM, Nakayama K, Wang TL, Shih IM (2009) NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene 28: 1941–1948
Korutla L, Degnan R, Wang P, Mackler SA (2007) NAC1, a cocaine-regulated POZ/BTB protein interacts with CoREST. J Neurochem 101: 611–618
Korutla L, Wang P, Jackson TG, Mackler SA (2009) NAC1, a POZ/BTB protein that functions as a corepressor. Neurochem Int 54: 245–252
Kuhajda FP (2006) Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 66: 5977–5980
Little JL, Wheeler FB, Koumenis C, Kridel SJ (2008) Disruption of crosstalk between the fatty acid synthesis and proteasome pathways enhances unfolded protein response signaling and cell death. Mol Cancer Ther 7: 3816–3824
Ma T, Wang Z, Guo Y, Pei D (2009) The C-terminal pentapeptide of Nanog tryptophan repeat domain interacts with Nac1 and regulates stem cell proliferation but not pluripotency. J Biol Chem 284: 16071–16081
Menendez JA, Vellon L, Lupu R (2005) Targeting fatty acid synthase-driven lipid rafts: a novel strategy to overcome trastuzumab resistance in breast cancer cells. Med Hypotheses 64: 997–1001
Nakayama K, Miyazaki K, Kanzaki A, Fukumoto M, Takebayashi Y (2001) Expression and cisplatin sensitivity of copper-transporting P-type adenosine triphosphatase (ATP7B) in human solid carcinoma cell lines. Oncol Rep 8: 1285–1287
Nakayama K, Nakayama N, Davidson B, Sheu JJ, Jinawath N, Santillan A, Salani R, Bristow RE, Morin PJ, Kurman RJ, Wang TL, Shih Ie M (2006) A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci USA 103: 18739–18744
Nakayama K, Nakayama N, Jinawath N, Salani R, Kurman RJ, Shih Ie M, Wang TL (2007) Amplicon profiles in ovarian serous carcinomas. Int J Cancer 120: 2613–2617
Nakayama K, Rahman MT, Rahman M, Yeasmin S, Ishikawa M, Katagiri A, Iida K, Nakayama N, Miyazaki K (2010) Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol Oncol 119: 469–478
Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB (2001) Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate 47: 102–110
Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR (1997) Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol 150: 201–208
Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M (2003) Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res 1: 707–715
Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Fearon ER, Hanash SM, Cho KR (2002) Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res 62: 4722–4729
Shih Ie M, Nakayama K, Wu G, Nakayama N, Zhang J, Wang TL (2011) Amplification of the ch19p13.2 NACC1 locus in ovarian high-grade serous carcinoma. Mod Pathol 24: 638–645
Stogios PJ, Downs GS, Jauhal JJ, Nandra SK, Prive GG (2005) Sequence and structural analysis of BTB domain proteins. Genome Biol 6: R82
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88: 2584–2589
Ueda SM, Yap KL, Davidson B, Tian Y, Murthy V, Wang TL, Visvanathan K, Kuhajda FP, Bristow RE, Zhang H, Shih Ie M (2010) Expression of fatty acid synthase depends on NAC1 and is associated with recurrent ovarian serous carcinomas. J Oncol 2010: 285191
Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV (2002) Role of the phosphatidylinositol 3'-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res 62: 642–646
Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA (2008) Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif 41: 59–85
Wakil SJ (1989) Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28: 4523–4530
Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH (2006) A protein interaction network for pluripotency of embryonic stem cells. Nature 444: 364–368
Wingo PA, Tong T, Bolden S (1995) Cancer statistics, 1995. CA Cancer J Clin 45: 8–30
Winter WE, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP (2007) Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25: 3621–3627
Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, Matsui S, Murphy SK, Konishi I (2010) Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene 29: 1741–1752
Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES (2002) Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res 279: 80–90
Yang YA, Morin PJ, Han WF, Chen T, Bornman DM, Gabrielson EW, Pizer ES (2003) Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res 282: 132–137
Yeasmin S, Nakayama K, Ishibashi M, Katagiri A, Iida K, Purwana IN, Nakayama N, Miyazaki K (2008) Expression of the bric-a-brac tramtrack broad complex protein NAC-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin Cancer Res 14: 1686–1691
Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM (2012) NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene 31: 1055–1064
Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP (2007) Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res 67: 2964–2971
Acknowledgements
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology in Japan, Suzuken Memorial Foundation, and Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rahman, M., Nakayama, K., Rahman, M. et al. Fatty acid synthase expression associated with NAC1 is a potential therapeutic target in ovarian clear cell carcinomas. Br J Cancer 107, 300–307 (2012). https://doi.org/10.1038/bjc.2012.246
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.246
Keywords
This article is cited by
-
SIK2 enhances synthesis of fatty acid and cholesterol in ovarian cancer cells and tumor growth through PI3K/Akt signaling pathway
Cell Death & Disease (2020)
-
Nucleus accumbens-associated protein-1 promotes glycolysis and survival of hypoxic tumor cells via the HDAC4-HIF-1α axis
Oncogene (2017)
-
Genetic variants in de novo lipogenic pathway genes predict the prognosis of surgically-treated hepatocellular carcinoma
Scientific Reports (2015)
-
Fatty acid synthase inhibition engages a novel caspase-2 regulatory mechanism to induce ovarian cancer cell death
Oncogene (2015)
-
Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer
Medical Oncology (2015)