Abstract
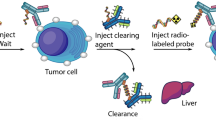
Radioimmunotherapy (RIT) is currently limited by toxicity to normal tissues as a result of prolonged circulating radioantibody in the blood. In this study, the use of a clearing antibody was investigated (second antibody) in an attempt to reduce blood background levels of [90Y]A5B7 immunoglobulin G (IgG) activity, and, therefore, improve the therapeutic tumour-blood ratio in nude mice bearing human colorectal tumour xenografts. The second antibody was raised against the 12N4 macrocycle group used for chelation of 90Y, and is, thus, applicable to any anti-tumour antibody labelled with this methodology. Second antibody was administered 18, 24 or 48 h after radiolabelled antibody injection and produced up to a tenfold reduction in blood levels and a tenfold improvement in tumour-blood ratios. This has the advantage of reducing the risk of myelotoxicity caused by prolonged retention of activity in the blood. For all normal tissues, there was a similar or slightly lower uptake of [90Y]IgG with second antibody clearance, apart from a transient rise in liver activity due to complexes of primary and secondary antibody clearing via the liver. As a result of clearance of [90Y]IgG from the blood pool, there was an associated fall in the amount of antibody at the tumour site (up to 3.3-fold) at later time points for mice injected with second antibody. However, despite this, tumour-blood ratios remained superior to the control group at these later time points. Estimated dosimetry evaluation revealed that total dose to normal tissues, blood and tumour was lower than for the non-clearance group. Surprisingly, however, there was little improvement in total estimated tumour-blood dose ratio over the time period studied. This was probably because the majority of the dose was delivered to both the blood and tumour within the first 24 h after administration of [90Y]IgG, so that giving the clearing agent after this time did not produce a large difference in total estimated dose. The anti-macrocycle second antibody proved to be a successful clearing agent and could potentially be applied to any anti-tumour antibody coupled with the 12N4 macrocycle. In the light of the estimated dosimetry results described here, it would probably be most useful given at earlier time points (i.e. before 18 h after injection of primary antibody) to produce an improved tumour-blood ratio of total dose. Development of this strategy may allow higher levels of activity to be administered for RIT, and repeated dosing regimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casey, J., King, D., Pedley, R. et al. Clearance of yttrium-90-labelled anti-tumour antibodies with antibodies raised against the 12N4 DOTA macrocycle. Br J Cancer 78, 1307–1312 (1998). https://doi.org/10.1038/bjc.1998.676
Issue Date:
DOI: https://doi.org/10.1038/bjc.1998.676