Abstract
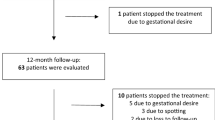
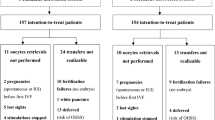
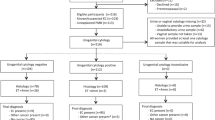
Tamoxifen (tam) is used extensively for treatment of patients with breast cancer and is being evaluated for chemoprevention in healthy women. It has, however, been reported to increase the risk of endometrial cancer in post-menopausal women, probably by an oestrogenic effect on the endometrium. It also causes endometrial cysts and polyps. The aims of this study were to identify the incidence of endometrial thickening, polyps and cysts by transvaginal ultrasound (TVUS) screening of a population of post-menopausal healthy women in the Royal Marsden tamoxifen chemoprevention trial and to evaluate the possible benefit from the use of intermittent norethisterone (NE) in women with persistent changes. Since 1990, we have undertaken regular TVUS, using an endovaginal B mode probe, of the 463 post-menopausal women in the trial randomized to tam (20 mg day(-1)) or placebo (plac), without breaking the randomization code. Endometrial thickening (ET) was defined as > or = 8 mm at the widest point across the myometrial cavity in the longitudinal plane, including any stromal changes. Cystic changes were defined as more than one hypoechogenic area > 1 mm. Polyps were identified using saline hydrosonography. Oral NE (2.5 mg day(-1)) was used for 21 days out of 28 for three consecutive cycles by women with persistent endometrium > or = 8 mm, including cystic and polypoid changes. TVUS was repeated after the three courses to evaluate any change caused by NE and endometrial biopsies, including hysteroscopy, was performed on those women with persistent abnormalities. A persistent ET > or = 8 mm was identified in 56 (24%) of the 235 women on tamoxifen compared with only 5 (2%) of 228 women on placebo (P <0.0005). Stromal changes, including cysts, were detected in 36 (15%) and polyps in 26 (11%) of the women on tamoxifen compared with only two (< 1%) of the women on placebo (P < < 0.0005). After 3 months of cyclical norethisterone, 39 of 47 women (83%) on tamoxifen had persistent ultrasound changes. However, 45 (96%) had a progesterone withdrawal bleed. Hysteroscopy was performed in 39 women on tamoxifen (28 endometrial biopsy, 15 polypectomy), five of whom had histological evidence of a proliferative endometrium and a further three had an atypical hyperplastic endometrium (one of whom had a focus of invasive carcinoma). The cysts and polyps which were detected in women on tam could not be reversed by NE and were presumably stromal and not of malignant risk. However, 96% of the women had withdrawal NE bleeding, indicating an oestrogenically primed endometrium which could be a mechanism for an increased risk of endometrial cancer. Further studies are required to ascertain whether a progestin would protect against this risk. As in other studies, these results indicate that any increased risk of endometrial cancer caused by tamoxifen is low, and that TVUS screening is probably not justified for asymptomatic women on tamoxifen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Powles, T., Bourne, T., Athanasiou, S. et al. The effects of norethisterone on endometrial abnormalities identified by transvaginal ultrasound screening of healthy post-menopausal women on tamoxifen or placebo. Br J Cancer 78, 272–275 (1998). https://doi.org/10.1038/bjc.1998.477
Issue Date:
DOI: https://doi.org/10.1038/bjc.1998.477
This article is cited by
-
Progestins and the endometrium in patients receiving tamoxifen
British Journal of Cancer (1999)