Abstract
Imatinib mesylate (IM) therapy has been shown to induce lower T cell counts in chronic myelogenous leukemia (CML) patients and an interference of IM with T cell receptor (TCR) signaling has been invoked to explain this observation. However, IL-7 and TCR signaling are both essential for lymphocyte survival. This study was undertaken to determine whether IM interferes with IL-7 or TCR signaling to explain lower T cell counts in patients. At diagnosis, CML patients have typically lower CD4+ counts in their blood, yet CD8+ counts are normal or even increased in some. Following the initiation of IM treatment, CD4+ counts were further diminished and CD8+ T lymphocytes were dramatically decreased. In vitro studies confirmed IM interference with TCR signaling through the inhibition of ERK phosphorylation and we showed a similar effect on IL-7 signaling and STAT5 phosphorylation (STAT5-p). Importantly however, using an in vivo mouse model, we demonstrated that IM impaired T cell survival through the inhibition of IL-7 and STAT5-p but not TCR signaling which remained unaffected during IM therapy. Thus, off-target inhibitory effects of IM on IL-7 and STAT5-p explain how T cell lymphopenia occurs in patients treated with IM.
Similar content being viewed by others
Key points
-
Imatinib disrupts T cell homeostasis through the inhibition of IL-7 and STAT5 phosphorylation.
-
Imatinib attenuates cytokine signaling in different clinical settings of immune dysfunctions.
Introduction
Imatinib mesylate (IM) is currently the drug of choice for first line therapy in patients with Philadelphia chromosome-positive chronic myelogenous leukemia (CML). Despite the relatively high specificity of IM treatment towards the BCR-ABL fusion protein, off-target multikinase inhibitory effects occur and can interfere with normal hematopoiesis.1, 2 For instance, non-specific inhibition of Flt3L has been associated with disruption of dendritic cell (DC) homeostasis and functions in both mice and humans.3 In addition, studies have reported an interference of IM with T cell counts and activation.4 T lymphocytes require T cell receptor (TCR) stimulation by MHC-I or MHC-II and IL-7 signaling in order to survive and persist in the periphery. While TCR signaling induces the phosphorylation and activation of AKT by the lipid kinase phosphatidylinositol 3-kinase (PI3K), IL-7 signaling induces the phosphorylation of STAT5 (STAT5-p) by Jak1-3 protein kinases; these pathways constitute potential targets for IM.5, 6 Despite ample evidence that IM can inhibit TCR signaling in vitro, the precise mechanism of action of IM on T cell homeostasis has remained inconclusive in vivo. The present studies were undertaken to precise the impact of IM on TCR or IL-7 inhibition in order to explain T cell lymphopenia in CML patients.
Materials and methods
Clinical samples
Healthy donors (n=25), CML patients at diagnosis (n=22) and patients treated with IM (n=10) were recruited. Patients treated with IM received between 200 and 600 mg/day. The median time of IM treatment was 2.9 years (range: 0.5–10.9) and the median time of remission post-IM was 1.1 years (range: 0.3–3). Blood samples were obtained under protocols approved by the HMR Ethics Committee, and written informed consent was obtained from all patients and healthy donors.
Flow cytometry analyses
The percentage and absolute counts of naive CD4+ or CD8+ T lymphocytes; (CD3+CD45RA+CCR7+), central memory; (CD3+CD45RA+CCR7neg) and effector memory (TEM); (CD3+CD45RAnegCCR7neg) were determined by flow cytometry. FITC-CD3, Pacific Blue-CD4, APCcy7-CD8, APC-CCR7, PEcy7-CD45RA were used to evaluate naive and memory T cells (BD Bioscience, San Diego, CA, USA). PE-CD56 was used to evaluate NK cells (BD Biosciences). Non-specific binding was determined using isotypic controls. Flow cytometry acquisition was performed on LSRII and analysis with Flowjo software (Treestar, Ashland, OR, USA).7 The percentages and absolute T-cell counts were calculated based on lymphocytes and monocytes gating.
IL-7 and TCR signaling
PBMCs from normal subjects were cultured at 2 × 106 cells/ml in RPMI+10% FCS and incubated with IM (3 μmol/ml) at 37 °C for 24 h.8 For STAT5 phosphorylation, cells were washed and incubated in serum-free medium for 1 h before stimulation with rhIL-7 (Cytheris) for 30 min at 37 °C. After fixation and permeabilization in Perm-Buffer III (BD Biosciences),9 cells were stained for STAT-5p (PE-STAT5, clone pY694, BD Biosciences) and cell surface receptors. For ERK phosphorylation (PE-ERK1-2, T202/pY204, BD Biosciences) following anti-CD3 stimulation (OKT3; BioXCell, West Lebanon, NH, USA), cells were serum starved for 1 h, incubated with 1 mg/ml of anti-CD3 for 15 min and then cross-linked with anti-mouse immunoglobulin-G (Sigma, Oakville, ON, Canada) at 5 mg/ml for 15 min. Intracellular staining was performed as described above.
Mouse studies
All experiments were approved by the Animal Ethic Committee of the HMR. For adoptive transfer of T cells into Rag−/− mice (CD45.2+), 1 × 106 of enriched lymph node T cells (CD45.1+) were labeled with cell trace violet (Invitrogen, Burlington, ON, Canada) and adoptively transferred through the tail vein.7 Mice were treated by gavage with IM 100 mg/kg twice a day for 7 days. Cell trace violet content was analyzed in CD45.1+ T cells by flow cytometry.
Statistical analysis
Prism 5.0 (GraphPad, La Jolla, CA, USA) was used for statistical analysis. The Mann Whitney U test was used to compare paired data, whereas the Kruskal–Wallis test followed by Dunn's post-test was used to compare three or more groups.
Results and discussion
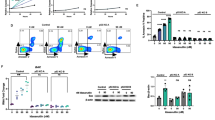
Lymphopenia has been reported in CML patients under IM therapy,10 and T cells were evaluated before and after IM treatment. At diagnosis, CML patients have lower naive and memory CD4+ T cells, while CD8+ counts were not diminished. Following IM treatment, CD4+ counts remained diminished and naive and memory CD8+ T cells were much lower (Figures 1a–c). We confirmed an effect of IM with TCR signaling4 by showing the inhibition of ERK-1/2 phosphorylation in response to suboptimal TCR simulation in CD4+ and CD8+ T cells exposed to 3 μM of IM (Figure 1d). As IL-7 is essential for naive and memory T cell survival,9, 11 we evaluated IM effect on STAT5-p in response to IL-7 stimulation in T cells. While 0.5 and 1 ng/ml of IL-7 are sufficient for inducing STAT5-p in T cells, much higher concentrations of IL-7 are required when these cells are exposed to 3 μM of IM (Figure 1e). Thus, in addition to TCR stimulation, our in vitro studies confirm that IM can interfere with IL-7 signaling and STAT5-p in T cells.
(a) Graphical summary of the absolute number (cells/μl) of CD4+ T cells and CD8+ T cells enumerated in the blood of age match controls (n=12), CML at diagnosis (n=11) and CML patients treated by TKI (n=6). (a–c) Absolute counts of naive (CCR7+CD45+ cells) and memory (CCR7+CD45RAneg, CCR7negCD45RAneg and CCR7negCD45RA+) CD4+ and CD8+ T cells. Differences between groups were assessed by Kruskal-Wallis U test (Dunns post-test). *P<0.05; ** P<0.01; ***P<0.001. (d) Evaluation of ERK phosphorylation in CD4+ and CD8+ T cells incubated with 3 μM IM and then exposed to anti-CD3 stimulation. Results are representative of two independent experiments. (e) Evaluation of STAT5 phosphorylation in T cells incubated overnight with IM 3 μM and then exposed to varying concentrations of rhIL-7 (ng/ml). Results are representative of three independent experiments. Differences between groups were assessed by Kruskal-Wallis U test (Dunns post-test). *P<0.05; **P<0.01; ***P<0.001.
While lower systemic IL-7 concentrations induce survival through Stat5-p and BCL-2 synthesis,12, 13 higher concentrations of IL-7 signal through PI3K and synergize with TCR signaling to induce homeostatic proliferation of T cells.14, 15 We therefore used lymphopenic Rag−/− mice to evaluate the in vivo effect of IM on survival and homeostatic proliferation of adoptively transferred T lymphocytes (Figure 2a). After 7 days of IM treatment, lymphocyte counts were much lower, indicating a potential defect in survival and/or homeostatic proliferation of transferred T cells (Figure 2b). Importantly, IM treatment did not reduce homeostatic proliferation of T cells, thus confirming that TCR signaling remains functional during IM treatment and supporting a model wherein the loss of T cells is predominantly mediated through the inhibition of IL-7 signaling and STAT5-p (Figure 2d). Previous studies have invoked a potential role for TCR inhibition by IM to explain diminished delayed type hypersensitivity in mice.16 However, we showed herein that IM induces DC depletion in humans and mice and this could contribute to limit delayed type hypersensitivity development (Figures 2c and e).17, 18 Furthermore, IL-7 can act as an adjuvant to facilitate T cell activation and the inhibition of IL-7 signaling could probably impair delayed type hypersensitivity response.19, 20 Finally, while the inhibition of TCR signaling by IM could perhaps explain lower naive CD4+, CD8+ and memory CD4+ T cell counts, it does not explain lower memory CD8+ counts, as these cells do not require TCR stimulation for their peripheral maintenance.21 Thus, despite conclusive in vitro evidences showing an interference of IM with TCR signaling,4, 16, 22 our in vivo data are more consistent with an effect of IM on STAT5 to explain lower T cell counts. STAT5 is required for the signaling of other cytokines and it is possible that the effect of IM on T cells is not entirely restricted to IL-7 signaling.23 Additional studies are needed in order to understand the full spectrum of cytokines signaling inhibited by IM.
(a) Schematic representation of the in vivo mouse model to evaluate IM effect on T cells. (b) Absolute numbers of congenic CD4+ and CD8+ T cells recovered in IM treated mice. (c) Absolute numbers of CD11c+ DCs after 7 days of IM treatment. (d) CD4+ and CD8+ T cell proliferation 7 days after transfer into lymphopenic recipients treated or not with IM. Data are representative of two independent experiments, four mice per group. (e) Graphical summary of the median percentage (left) and absolute counts (right) of pDC (HLADR+CD14negCD303+CD123+), mDC1 (HLADR+CD14negCD11chiCD1c+), mDC2 (HLADR+CD14negCD11c+CD141chi), mDC3 (HLADR+CD14negCD11chiCD16+) in the blood of healthy subjects (n=25), CML at diagnosis (n=22), and CML patients treated by IM (n=10).
In conclusion, this study has several potential clinical implications. First, lymphopenia occurs in most CML patients following IM treatment. The use of IL-7 therapy could perhaps improve T cell counts in patients at higher risk of infections such as those receiving IM for persistent disease after allogeneic stem cell transplant.24 Second, although promising results were initially obtained for the treatment of steroid refractory chronic graft-versus-host disease, <50% of patients respond to IM therapy and its use has not been widely accepted in the transplant community.25, 26 IL-7 can facilitate T cell activation but once activated, IL-7 receptor is down-modulated; this could limit the IM effect on T cells involved in chronic graft-versus-host disease and explain the disappointing results observed clinically. Third, in acute lymphoblastic leukemia, IL-7 can contribute to the survival of acute lymphoblastic leukemia cells and it is not excluded that part of IM effectiveness in Philadelphia-positive acute lymphoblastic leukemia cells may be related to the inhibition of IL-7 signaling and stat5p.27, 28 However, the benefit of IM in Philadelphia-positive acute lymphoblastic leukemia patients who do not undergo allogeneic transplant is often of a short duration, and rises in systemic IL-7 that typically occurs during lymphopenia may reduce the effectiveness of IM at blocking stat5p (Figure 1e).29 In contrast, decrease in systemic IL-7 occurs following allogeneic stem cell transplantation despite profound lymphopenia, and IM treatment can still successfully prevent relapse in most patients.7, 30, 31 Whether IL-7 can induce IM resistance after allogeneic transplant remains unknown. Nonetheless, the finding that IL-7-stat5p can be inhibited by IM provides a novel perspective on IM treatment that should be further studied in future clinical trials.
Change history
04 November 2020
A correction to this paper has been published and can be accessed via a link at the top of the paper.
References
Ruchatz H, Puttini M, Cleris L, Pilotti S, Gambacorti-Passerini C, Formelli F . Effect of imatinib on haematopoietic recovery following idarubicin exposure. Leukemia 2003; 17: 298–304.
Chand M, Thakuri M, Keung YK . Imatinib mesylate associated with delayed hematopoietic recovery after concomitant chemotherapy. Leukemia 2004; 18: 886–888.
Taieb J, Maruyama K, Borg C, Terme M, Zitvogel L . Imatinib mesylate impairs Flt3L-mediated dendritic cell expansion and antitumor effects in vivo. Blood 2004; 103: 1966–1967.
Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005; 105: 2473–2479.
Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science 1993; 262: 1877–1880.
Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I . Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: comparative analysis of gamma(c), Jak3, and gamma(c) and Jak3 double-deficient mice. Int Immunol 2000; 12: 123–132.
Gauthier SD, Leboeuf D, Manuguerra-Gagne R, Gaboury L, Guimond M . Stromal-derived factor-1alpha and interleukin-7 treatment improves homeostatic proliferation of naive CD4(+) T cells after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2015; 21: 1721–1731.
Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 2008; 111: 4022–4028.
Hennion-Tscheltzoff O, Leboeuf D, Gauthier SD, Dupuis M, Assouline B, Gregoire A et al. TCR triggering modulates the responsiveness and homeostatic proliferation of CD4+ thymic emigrants to IL-7 therapy. Blood 2013; 121: 4684–4693.
Legros L, Ebran N, Stebe E, Rousselot P, Rea D, Cassuto JP et al. Imatinib sensitizes T-cell lymphocytes from chronic myeloid leukemia patients to FasL-induced cell death: a brief communication. J Immunother 2012; 35: 154–158.
Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA 2001; 98: 8732–8737.
Jiang Q, Benbernou N, Chertov O, Khaled AR, Wooters J, Durum SK . IL-7 induces tyrosine phosphorylation of clathrin heavy chain. Cell Signal 2004; 16: 281–286.
Kittipatarin C, Li WQ, Bulavin DV, Durum SK, Khaled AR . Cell cycling through Cdc25A: transducer of cytokine proliferative signals. Cell Cycle 2006; 5: 907–912.
Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA . Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med 2004; 200: 659–669.
Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N . IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 2007; 109: 1034–1042.
Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S . Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood 2004; 104: 1094–1099.
Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 2002; 17: 211–220.
Kim HJ, Barajas B, Chan RC, Nel AE . Glutathione depletion inhibits dendritic cell maturation and delayed-type hypersensitivity: implications for systemic disease and immunosenescence. J Allergy Clin Immunol 2007; 119: 1225–1233.
Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL . Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood 2001; 97: 1525–1533.
Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL . Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest 2005; 115: 1177–1187.
Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R . Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 1999; 286: 1377–1381.
Cwynarski K, Laylor R, Macchiarulo E, Goldman J, Lombardi G, Melo JV et al. Imatinib inhibits the activation and proliferation of normal T lymphocytes in vitro. Leukemia 2004; 18: 1332–1339.
Leonard WJ, Shores EW, Love PE . Role of the common cytokine receptor gamma chain in cytokine signaling and lymphoid development. Immunol Rev 1995; 148: 97–114.
Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006; 29: 313–319.
Olivieri A, Cimminiello M, Corradini P, Mordini N, Fedele R, Selleri C et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 2013; 122: 4111–4118.
de Masson A, Bouaziz JD, Peffault de Latour R, Wittnebel S, Ribaud P, Rubio MT et al. Limited efficacy and tolerance of imatinib mesylate in steroid-refractory sclerodermatous chronic GVHD. Blood 2012; 120: 5089–5090.
Williams RT, den Besten W, Sherr CJ . Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev 2007; 21: 2283–2287.
Chalandon Y, Thomas X, Hayette S, Cayuela JM, Abbal C, Huguet F et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood 2015; 125: 3711–3719.
Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J et al. A potential role for interleukin-7 in T-cell homeostasis. Blood 2001; 97: 2983–2990.
Thiant S, Yakoub-Agha I, Magro L, Trauet J, Coiteux V, Jouet JP et al. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant 2010; 45: 1546–1552.
Thiant S, Moutuou MM, Leboeuf D, Guimond M . Homeostatic cytokines in immune reconstitution and graft-versus-host disease. Cytokine 2016; 82: 24–32.
Acknowledgements
We would like to thank Dr Denis-Claude Roy for providing clinical samples, Mrs Chantal Baron and Marylène Corriveau for their help with patient samples processing. We also thank Dr Janos Filep for review of the manuscript.
Author contributions
ST designed experiments, analyzed data, performed flow cytometric analysis of DCs and T cells, performed statistical analysis, wrote the manuscript. MMM performed IL-7 and TCR signaling assays. Developed the in vivo mouse model. PL performed flow cytometric analysis of DCs. RSB study DC in CML patients. DL provided scientific expertise in IL-7 and TCR signaling, edited the manuscript. LB recruited patients and provided scientific expertise in CML. JR provided scientific expertise in CML, stem cell transplant and edited the manuscript. MG designed the study, analyzed the data, and wrote the paper; all authors reviewed the paper and agree with its content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Thiant, S., Moutuou, M., Laflamme, P. et al. Imatinib mesylate inhibits STAT5 phosphorylation in response to IL-7 and promotes T cell lymphopenia in chronic myelogenous leukemia patients. Blood Cancer Journal 7, e551 (2017). https://doi.org/10.1038/bcj.2017.29
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2017.29
This article is cited by
-
Improvement of immune thrombocytopenia with imatinib therapy following chronic myeloid leukemia
International Journal of Hematology (2023)
-
COVID-19 in persons with chronic myeloid leukaemia
Leukemia (2020)
-
Disruption of an antimycobacterial circuit between dendritic and helper T cells in human SPPL2a deficiency
Nature Immunology (2018)