Abstract
Peripheral T-cell lymphomas (PTCLs) are a heterogenous group of aggressive non-Hodgkin’s lymphomas that are incurable in the majority of patients with current therapies. Outcomes associated with anthracycline-based therapies are suboptimal, but remain the standard of care for most patients, even though the benefits of this approach remain uncertain. This study retrospectively examined outcomes in a cohort of North American PTCL patients treated with both anthracycline- and nonanthracycline-containing regimens. The incorporation of anthracycline-containing regimens was associated with improved progression-free survival (PFS) and overall survival (OS). Patients treated with nonanthracycline-containing regimens were more likely to have high-risk features and were less likely to undergo high-dose therapy and stem cell transplantation. However, anthracycline use remained an independent predictor of improved PFS and OS when adjusting for these confounding variables. Anthracycline-based regimens and consolidation with high-dose therapy and autologous stem cell transplantation in appropriately selected patients remains a viable option for patients unable to participate in a clinical trial. Long-term disease-free survival is not optimal, highlighting the need for an improved understanding of disease pathogenesis, and the development of novel therapeutic strategies.
Similar content being viewed by others
Introduction
Peripheral T-cell lymphomas (PTCLs) represent a heterogenous group of aggressive non-Hodgkin’s lymphomas that are derived from mature (post-thymic) T cells. PTCLs are associated with significantly inferior outcomes when compared with diffuse large B-cell lymphomas. Anthracycline-based regimens (usually CHOP (cyclophosphamide/doxorubicin/vincristine/prednisone)) are considered the standard of care in the front-line treatment of these patients. However, in contrast to diffuse large B-cell lymphomas, few patients will enjoy long-term disease-free survival with this approach. A survival advantage was not observed with anthracycline-based regimens by the International T-cell Lymphoma Project (ITCLP).1 Current strategies include both the incorporation of additional agents with CHOP and the development of nonanthracycline-containing regimens. A paucity of randomized controlled trials addresses the relative merits of these divergent approaches. Approximately 75% of patients comprising the ITCLP are from Europe and Asia. Given the significant geographic variation in the prevalence of PTCL subtypes, including those who are multidrug resistant, this study sought to report outcomes in North American PTCL patients treated with anthracycline- and nonanthracycline-containing regimens. In this multicenter study, to the best of our knowledge the largest North American PTCL study organized to date, superior survival rates were observed in patients receiving anthracycline-based regimens. Despite the apparent advantage associated with standard anthracycline-based regimens, long-term survival rates are poor, and improved therapeutic strategies are greatly needed.
Materials and methods
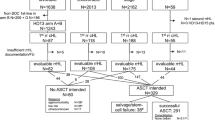
Consecutive patients ⩾18 years of age with biopsy-confirmed PTCLs who were evaluated at the Mayo Clinic (between 1994 and 2009; n=196) or the University of Michigan (between 1988 and 2011; n=246) were included in this study. Study approval was granted by the Institutional Review Board of each respective institution, in accordance with the US federal regulations and the Declaration of Helsinki Principles. Clinical characteristics, including Ann Arbor stage, lactate dehydrogenase, performance status, extent of extranodal disease and treatments received were retrospectively collected. Whenever possible, diagnostic biopsy specimens were reviewed (by ALF, NGB and MSL) and classified in accordance with the 2008 World Health Organization classification. Out of the 442 cases examined, tissue exhaustion precluded reclassification in 122 cases. Progression-free survival (PFS) was calculated from the time of initial diagnosis to the time of progression, relapse or death. Overall survival (OS) was calculated from the time of initial diagnosis to the time of death. Patients who did not experience any of these events were censored at the time of last contact. PFS and OS were estimated using the Kaplan–Meier method and two-tailed log-rank test.2 The Cox proportional hazards model was used to evaluate dichotomized variables and to adjust for the International Prognostic Index (IPI).3 Similar to previous studies, this study observed that high–intermediate-risk patients (IPI 3) experienced a PFS and OS closely resembling that observed in high-risk patients (IPI 4/5; data not shown). Therefore, a cutoff of ⩾3 was selected when the IPI was analyzed as a dichotomized variable.4, 5, 6, 7, 8, 9 All data were analyzed using JMP 8 software (SAS, Cary, NC, USA).
Results
The median age at diagnosis for this cohort of 442 PTCL patients was 57 years (range, 18–91 years). Median follow-up after diagnosis was 13 months for all patients (range, 0–206 months), and 28 months for censored patients. The median PFS and OS were 7 and 13 months, respectively, for the entire cohort. The estimated 5-year overall survival was 35%. As summarized in Table 1, peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) was the most common subtype (39%), followed by angioimmunoblastic T-cell lymphoma (AITL, 20%) and anaplastic large-cell lymphoma (24%). The clinical characteristics of these patients, including risk stratification by the IPI, are summarized in Table 1. The majority of patients (65%) were initially treated with anthracycline-based multiagent chemotherapy regimens, most commonly CHOP (93%), and 9% of patients received nonanthracycline-based regimens. Patients for whom treatment was unknown (8%), who did not receive any treatment (11%), who received palliative single-agent therapies or radiation (6%) were excluded from subsequent analyses.
For the remaining cohort of 326 patients, front-line treatment with an anthracycline-based regimen was associated with superior PFS and OS when compared with those patients treated with non-anthracycline-containing regimens (Figures 1a and b). A median PFS of 12 months and 2-year PFS of 39% were observed for anthracycline-treated patients. Median PFS (2 months) and 2-year PFS (23%) was poor for patients receiving non-anthracycline-containing regimens (Figure 1a). The improved PFS observed in anthracycline-treated patients was associated with a 30-month improvement in median OS, and a 37% absolute improvement in 2-year OS (Figure 1b). Tissue exhaustion precluded pathologic review in 28% of cases. When these cases were excluded, anthracycline use was associated with significant improvements in both PFS and OS (Supplementary Figures 1a and b). To evaluate whether or not the inclusion of anaplastic large-cell lymphoma patients (94% of whom received an anthracycline) in this analysis may have biased the apparent survival advantage associated with anthracyclines, PTCL-NOS and AITL cases (n=191) were analyzed in isolation. Among these patients, anthracycline use was also associated with improved PFS and OS (Figures 1c and d), with a median PFS and OS of 11 and 27 months, respectively. In contrast, median PFS and OS were each <6 months in patients receiving nonanthracycline-containing regimens. The improved PFS and OS associated with anthracycline use was also observed when cases for which pathologic re-review was not performed were excluded. Among these patients, anthracycline use was associated with a median PFS and OS of 12 and 29 months, respectively. Use of a nonanthracycline-containing regimen was associated with a median PFS of 7 months, and a median OS of 4 months (Supplementary Figures 1c and d).
In order to identify other confounding variables, characteristics of both anthracycline- and nonanthracycline-treated patients were compared for all histologies (Table 2) and for PTCL-NOS/AITL cases (Table 3). Nonanthracycline use was associated with higher risk disease (Table 2), although this difference was diminished among PTCL-NOS/AITL patients (Table 3). Therefore, a multivariate analysis was performed. When adjusting for the IPI (⩾3 risk factors), the association between nonanthracycline-based regimens and poorer survival remained significant, both for the entire cohort and for PTCL-NOS/AITL patients (Table 4). The IPI retained its prognostic utility on multivariate analysis (Table 4), and was able to risk-stratify patients receiving anthracycline-containing regimens (Figures 2a and b). Furthermore, when only high-risk (IPI ⩾3) patients were analyzed, anthracycline-containing regimens were associated with significantly improved PFS and OS (Figures 2c and d). The median PFS and OS were 10 and 18 months, respectively, for anthracycline-treated patients. In contrast, the median PFS and OS were each <2 months in nonanthracycline-treated patients. Consequently, only 6% of these patients were alive at 2 years, whereas the 2-year OS in high-risk patients receiving an anthracycline was 43%.
Anthracycline-containing regimens are associated with improved survival in high-risk PTCL. Progression-free survival (a) and overall survival (b) for all patients treated with anthracycline-based combination chemotherapy regimens stratified by the IPI. Progression-free survival (c) and overall survival (d) in high-risk (IPI ⩾3) patients treated with (—) and without (–-) an anthracycline.
Although the improved survival observed in anthracycline-treated patients remained statistically significant after adjusting for the IPI, the increased prevalence of high-risk features observed in nonanthracycline-treated patients may have led to treatment selection bias. To further examine this possibility, we analyzed the regimens that were employed among nonanthracycline-treated patients. Thirteen patients (34%) received palliative regimens (for example, methylprednisolone/nitrogen mustard, alkylating agent/prednisone). In contrast, 53% of patients received conventional nonanthracycline-containing regimens (for example, CVP, CEPP, COEP, GemOx, PEGS), whereas 13% received regimens often reserved for relapsed/refractory patients (for example, ICE, DHAP, ESHAP). The use of high-dose chemotherapy and autologous (or allogeneic) stem cell transplantation was examined in these patients. Among anthracycline-treated patients, 28% underwent transplant (64% autologous), either in first remission (56%), second remission (35%) or in the setting of primary refractory disease (8%). Remission status at the time of transplant was unknown in one patient. In comparison, 3 (8%) of the nonanthracycline-treated patients were transplanted. The preferential use of transplant in anthracycline-treated patients may have accounted, at least in part, for the improved OS observed in these patients. To address this possibility, OS was analyzed in anthracycline-treated patients dichotomized by transplant status. As shown in Figure 3, the survival advantage associated with anthracycline use persisted when transplanted patients were excluded. The median OS (19 months) was superior to the dismal median OS (4.5 months) observed in nonanthracycline-treated patients.
Discussion
The clinical characteristics observed in this cohort of T-cell and natural killer/T-cell lymphoma patients, including the use of anthracycline-based regimens in 65% of patients, are similar to those observed in other large series, and confirms the prognostic utility of the IPI in a uniformly treated cohort of patients.1, 4, 6, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 In contrast to our study, ∼75% of patients evaluated by the ITCLP were from Europe or Asia. Consequently, AITLs, more prevalent in Europe, and adult T-cell leukemia/lymphomas and natural killer/T cell lymphomas, which are more prevalent in Asia, were all well represented in the ITCLP series, representing ∼39% of the cases examined. In contrast, AITLs, adult T-cell leukemia/lymphomas and natural killer/T cell lymphomas collectively represented ∼26% of the cases we examined, and 14% of the cases examined in another North American study performed by the British Columbia Cancer Agency (BCCA).4 PTCL-NOS, comprising ∼25% of cases reported by the ITCLP, is the most common subtype observed in North America, representing 39% of the cases we reviewed, and 59% of those reported by the BCCA.
Anthracycline-based therapies, usually CHOP, have historically been utilized in the treatment of most PTCL subtypes, including PTCL-NOS and AITL. Durable remission rates are not optimal with this approach. In a quest for improved therapies, investigators have adopted various strategies to intensify treatment, building on the CHOP backbone, or have developed nonanthracycline-based alternatives.22, 23, 24, 25, 26 Results from the ITCLP would seem to support the latter approach, as a comparison of outcomes in anthracycline- and nonanthracycline-treated patients failed to demonstrate a significant benefit with anthracycline use.1 In order to further investigate this question, our study examined outcomes, patient characteristics and future consolidation or salvage therapies in both anthracycline- and nonanthracycline-treated patients. In contrast to the ITCLP, our study observed a significant survival benefit with the use of anthracycline-based regimens. The majority of patients treated with a nonanthracycline-based regimen had high-risk features. However, after adjusting for the IPI on multivariate analysis, the association between anthracycline use and improved survival remained statistically significant. When only high-risk patients were analyzed, anthracycline use was associated with a significant improvement in 2-year OS (43% vs 6%). The use of significantly less intense, and largely palliative, regimens in nonanthracycline-treated patients may have contributed to the apparent benefit associated with anthracycline use. However, a minority of high-risk patients were treated with such regimens (31%). The majority of high-risk patients received regimens frequently utilized in patients for whom an anthracycline would be contraindicated (55%) or received more intense regimens usually reserved for salvage therapy after disease relapse/progression (14%). Although it is difficult to conclude that the inferior survival observed in nonanthracycline-treated patients is explained by the selection of palliative regimens in these patients, treatment selection bias may explain, at least in part, the apparent survival advantage associated with anthracycline use. In contrast, the observed disparity in the use of high-dose therapy and stem cell transplantation was more significant. Among the anthracycline-treated patients alive at 2 years, 21% had been transplanted, and three out of the four nonanthracycline-treated patients alive at 2 years had been similarly treated. The improved survival observed in this study in transplanted patients further highlights the utility of this approach in PTCLs and is consistent with the outcomes recently reported in a large phase II study in which the 5-year PFS and OS were 51% and 44%, respectively, following high-dose therapy and autologous stem cell transplantation.27 Despite the survival benefits associated with transplantation, improved survival was observed in anthracycline-treated patients after excluding patients who had undergone transplant. Given the limitations of a retrospective analysis, it is difficult to determine the extent to which treatment selection bias may have contributed to the improved survival observed in anthracycline-treated patients. But, this may certainly explain, in part, the poorer survival observed in nonanthracycline-treated patients.
In contrast to the observations in our study, the ITCLP did not observe a similar benefit with the use of anthracycline-based therapies. At least for PTCL-NOS patients, the ITCLP observed that anthracycline use was associated with improved overall survival that approached, but did not reach, statistical significance. The significant geographic variation in PTCL subtypes and their considerable heterogeneity may suggest that geographic differences in the prevalence of poorly characterized PTCL subtypes, currently classified (by exclusion) as PTCL-NOS, may explain, at least in part, the apparent discrepancy between our study and that of the ITCLP.
This is the largest study of PTCL in North America and further highlights the need for improved therapeutic strategies for these aggressive lymphomas. The introduction of promising novel agents is encouraging, and participation in well-designed clinical trials should be encouraged.28, 29, 30, 31, 32, 33, 34 Nonetheless, CHOP (and CHOP-like regimens22), consolidated with high-dose therapy and autologous stem cell transplantation in appropriately selected patients, is associated with durable remissions in many patients, and remains a rational therapeutic option.
References
Vose J, Armitage J, Weisenburger D . International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–4130.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329: 987–994.
Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM . Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 2004; 15: 1467–1475.
Reiser M, Josting A, Soltani M, Staib P, Salzberger B, Diehl V et al. T-cell non-Hodgkin’s lymphoma in adults: clinicopathological characteristics, response to treatment and prognostic factors. Leuk Lymphoma 2002; 43: 805–811.
Torimoto Y, Sato K, Ikuta K, Hayashi T, Hirayama Y, Inamura J et al. A retrospective clinical analysis of Japanese patients with peripheral T-cell lymphoma not otherwise specified: Hokkaido Hematology Study Group. Int J Hematol 2013; 98: 171–178.
Escalon MP, Liu NS, Yang Y, Hess M, Walker PL, Smith TL et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer 2005; 103: 2091–2098.
Gutierrez-Garcia G, Garcia-Herrera A, Cardesa T, Martinez A, Villamor N, Ghita G et al. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol 2011; 22: 397–404.
Chihara D, Oki Y, Ine S, Yamamoto K, Kato H, Taji H et al. Analysis of prognostic factors in peripheral T-cell lymphoma: prognostic value of serum albumin and mediastinal lymphadenopathy. Leuk Lymphoma 2009; 50: 1999–2004.
Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011; 117: 3402–3408.
Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood 2004; 103: 2474–2479.
Lee Y, Uhm JE, Lee HY, Park MJ, Kim H, Oh SJ et al. Clinical features and prognostic factors of patients with “peripheral T cell lymphoma, unspecified”. Ann Hematol 2009; 88: 111–119.
Asano N, Suzuki R, Kagami Y, Ishida F, Kitamura K, Fukutani H et al. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am J Surg Pathol 2005; 29: 1284–1293.
Lopez-Guillermo A, Cid J, Salar A, Lopez A, Montalban C, Castrillo JM et al. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol 1998; 9: 849–855.
Rudiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol 2002; 13: 140–149.
Niitsu N, Okamoto M, Nakamine H, Aoki S, Motomura S, Hirano M . Clinico-pathologic features and outcome of Japanese patients with peripheral T-cell lymphomas. Hematol Oncol 2008; 26: 152–158.
Ascani S, Zinzani PL, Gherlinzoni F, Sabattini E, Briskomatis A, de Vivo A et al. Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L. Classification. Ann Oncol 1997; 8: 583–592.
Pellatt J, Sweetenham J, Pickering RM, Brown L, Wilkins B . A single-centre study of treatment outcomes and survival in 120 patients with peripheral T-cell non-Hodgkin’s lymphoma. Ann Hematol 2002; 81: 267–272.
Kim K, Kim WS, Jung CW, Im YH, Kang WK, Lee MH et al. Clinical features of peripheral T-cell lymphomas in 78 patients diagnosed according to the Revised European-American lymphoma (REAL) classification. Eur J Cancer 2002; 38: 75–81.
Au WY, Ma SY, Chim CS, Choy C, Loong F, Lie AK et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol 2005; 16: 206–214.
Arrowsmith ER, Macon WR, Kinney MC, Stein RS, Goodman SA, Morgan DS et al. Peripheral T-cell lymphomas: clinical features and prognostic factors of 92 cases defined by the revised European American lymphoma classification. Leuk Lymphoma 2003; 44: 241–249.
Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010; 116: 3418–3425.
Foss FM, Sjak-Shie N, Goy A, Jacobsen E, Advani R, Smith MR et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma 2013; 54: 1373–1379.
Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ et al. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma 2013; 55: 768–772.
Mahadevan D, Unger JM, Spier CM, Persky DO, Young F, LeBlanc M et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer 2013; 119: 371–379.
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 2012; 120: 2973–2980.
d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 2012; 30: 3093–3099.
Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 2012; 30: 631–636.
O'Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 2011; 29: 1182–1189.
Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 2012; 30: 2190–2196.
Zinzani PL, Pellegrini C, Broccoli A, Stefoni V, Gandolfi L, Quirini F et al. Lenalidomide monotherapy for relapsed/refractory peripheral T-cell lymphoma not otherwise specified. Leuk Lymphoma 2011; 52: 1585–1588.
Zinzani PL, Magagnoli M, Bendandi M, Orcioni GF, Gherlinzoni F, Albertini P et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients.[see comment]. Ann Oncol 1998; 9: 1351–1353.
Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 2012; 30: 837–842.
Tobinai K, Takahashi T, Akinaga S . Targeting chemokine receptor CCR4 in adult T-cell leukemia-lymphoma and other T-cell lymphomas. Curr Hematol Malig Rep 2012; 7: 235–240.
Acknowledgements
This work was supported in part by grants from the Leukemia & Lymphoma Society, the Leukemia Research Foundation and the NCI (K08CA172215).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Briski, R., Feldman, A., Bailey, N. et al. The role of front-line anthracycline-containing chemotherapy regimens in peripheral T-cell lymphomas. Blood Cancer Journal 4, e214 (2014). https://doi.org/10.1038/bcj.2014.34
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2014.34
This article is cited by
-
Incidence, Survival Outcome, and Prognostic Nomogram of Patients with Angioimmunoblastic T-cell Lymphoma: a Population-based Analysis
Current Medical Science (2022)
-
Immunohistochemical evaluation and prognostic value of monocarboxylate transporter 1 (MCT1) and 4 (MCT4) in T-cell non-Hodgkin lymphoma
Clinical and Experimental Medicine (2022)
-
Modulation of doxorubicin-induced expression of the multidrug resistance gene in breast cancer cells by diltiazem and protection against cardiotoxicity in experimental animals
Cancer Cell International (2019)
-
Prognostic and therapeutic significance of phosphorylated STAT3 and protein tyrosine phosphatase-6 in peripheral-T cell lymphoma
Blood Cancer Journal (2018)