Abstract
Donor lymphocyte infusion (DLI) is commonly used to treat leukemia relapse following stem cell transplantation. In florid relapse, however, the efficacy of DLI is limited with substantial risk of severe graft-versus-host disease (GvHD). Here, we develop a novel risk-adapted strategy characterized by pre-emptive DLI initiated at the time of mixed chimerism, a small starting dose based on donor source, dose-escalation guided by real-time chimerism monitoring and withholding of DLI immediately in patients achieving full donor chimerism. A total of 178 DLIs were given to 38 patients with mixed chimerism; thereafter, 33 patients (86.8%) had donor chimerism successfully increased, including 30 (78.9%) who had chimerism fully converted back to 100% donor. Cumulative incidence of relapse was significantly lower (P=0.00004) and overall survival higher (P=0.0003) in patients with chimerism fully corrected as compared with those of patients whose chimerism remained mixed. Only 13.2% of the patients developed acute grade III-IV GvHD with no associated mortality. In conclusion, the risk-adapted DLI strategy is useful in minimizing the risk of childhood leukemia relapse, GvHD and death.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is curative in patients with high-risk leukemia but is not always successful.1 Despite attempts in enhancing the efficacy of conditioning regimens and graft versus leukemia (GvL) effects, disease relapse still commonly occurs.2, 3 Several approaches have been used to treat leukemia relapse following HSCT, including discontinuation of immunosuppression, re-induction chemotherapy or repeat transplantation.4 Alternatively, donor lymphocyte infusion (DLI) may be considered if cells are available.5
DLI is a form of adoptive immunotherapy to induce GvL activity.6, 7 The efficacy of this approach is dependent upon the type of disease and the dose of infused CD3+ lymphocytes. Encouraging results have been observed in chronic myeloid leukemia (CML) and indolent lymphomas; however, the response is generally limited in florid relapse of acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), and the patients are at risk of severe graft-versus-host disease (GvHD) or marrow aplasia.5, 6, 7, 8, 9 Therefore, several modifications in administering DLI have been investigated in order to optimize the response probability and to minimize complications, including the use of chemotherapy before DLI, escalating lymphocyte dosage, administration of short-term immunosuppression and the use of specific T-cell subsets or gene-modified effector cells;10, 11, 12, 13, 14, 15 however, the efficacy remains limited, and the risk of developing GvHD is still substantial.4, 6, 7 Thus, an international workshop was held by the National Cancer Institute recently to specifically address the biology, prevention and treatment of relapse after HSCT.3, 4, 16, 17
The limitation of DLI in treating pediatric AML or ALL in frank relapse has led to the investigation of pre-emptive DLI at the time of mixed chimerism.18, 19 Previous studies have shown that decreasing donor chimerism heralds leukemia relapse.20, 21 By using serial chimerism analysis, impending hematological relapse could be detected in advance to allow time to collect cells from the donor for DLI.22 Recently, this approach was shown to be feasible in both single center and co-operative group setting.18, 19
Here, we reported the efficacy and toxicity of another risk-adapted approach in administrating DLI as pre-emptive immunotherapy for childhood leukemia based on chimerism and donor source. The data showed that this strategy is successful in more than three-quarters of the recipients with minimal risk of GvHD mortality.
Patients and methods
Patients
Thirty-eight consecutive patients with mixed chimerism off immunosuppressive therapy received a total of 178 DLIs initiated at a median of 3 months after HSCT. All the patients were diagnosed with hematological malignancies and underwent allogeneic HSCT at the St Jude Children’s Research Hospital, Memphis, TN, USA. The patients received DLI between 1 May 2007 and 31 December 2011 after informed written consent was obtained from patients or their legal guardians. The study was approved by our institutional review board.
DLI strategy
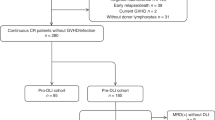
Our risk-adapted strategy is summarized in Figure 1. In brief, the risk of relapse was assessed using serial blood chimerism assays (weekly until day+100, monthly until first annual visit and then yearly). DLI was not given until two consecutive tests revealed mixed donor chimerism (defined as ⩽99% donor) or until a mixed chimerism test was accompanied by evidence of viral reactivation or minimal residual disease (MRD). The starting dose of DLI was based on the relative frequency of alloreactive cells and the associated risk of GvHD according to the donor source (that is, haploidentical>unrelated>matched-sibling). Thus, the first DLI dose was 2.5 × 104, 1 × 106 and 1 × 107 CD3+ cells/kg, respectively. Subsequent doses of DLI were escalated conservatively by a twofold increment. Repeated DLI every 2–4 weeks was given only to patients with persistent mixed chimerism and was stopped immediately when the chimerism converted back to 100% donor, thus limiting the risk of GvHD.
Chimerism assay
Chimerism analysis was performed at the St Jude Molecular Pathology Laboratory, using variable number of tandem repeats (VNTR) analyses as described previously.23, 24 DNA was extracted from whole blood without cell separation. Donor and recipient alleles were screened before transplantation using a panel of 24 VNTR markers established for forensic genetic fingerprinting. The marker that could best discriminate the recipient versus donor pair was then chosen for post-HSCT follow-up. PCR fragments were separated by capillary electrophoresis on an ABI 3130 Genetic Analyzer (Applied Biosystem, Foster City, CA, USA) equipped with the Genescan Analysis Software. The test results were reported as donor percentages using the equation: Percentage of donor=Σ donor peak/Σ donor peak+recipient peak. The sensitivity of this assay for detecting recipient cells was 1%.
Viral surveillance PCRs
Serial quantitative PCR testing for cytomegalovirus (CMV), adenovirus, and Epstein–Barr virus was performed at the St Jude Molecular Microbiology Laboratory as described previously.25 The test was performed at the same time with chimerism assays in the first year after HSCT.
MRD assay
MRD assay in the bone marrow was performed at the St Jude Immunopathology Laboratory.26 Bone marrow was obtained from patients at the time of diagnosis or relapse in order to identify leukemia-specific immunophenotypes. Cells with leukemia-associated immunophenotypes were enumerated by multi-parameter flow cytometry. The assay was able to detect one leukemic cell among 10 000 normal cells.
MRD assay was performed routinely in bone marrow aspirates at 1 month, 3 months and 1 year post HSCT. The assay was also performed monthly in patients with mixed chimerism.
Statistical analysis
The probability of overall survival (OS) was estimated by the Kaplan–Meier method and was compared between the groups by the log-rank test. OS was measured from the day of first DLI to the day of death. Cumulative incidence of relapse (CIR) was estimated after adjusting for competing risk of death by the method of Kalbfleisch and Prentice and was compared between the groups with the use of Gray’s test. Logistic regression analysis was used to test for statistical correlations between chimerism response and primary disease (ALL or AML), age, gender, donor source, type of conditioning regimen and level of donor chimerism at the initiation of DLI. The nominal significance level was set at 0.05. SAS version 9.2 (SAS Institute, Cary, NC, USA) and R-2.15.0 (R Foundation, Vienna, Austria) were used for the statistical analysis.
Results
Patient characteristics
Patients’ characteristics are summarized in Table 1. Thirty-eight patients were treated for ALL (n=14), AML (n=16), acute biphenotypic leukemia (n=3), CML, (n=3), juvenile myelomonocytic leukemia (n=1) and myelodysplastic syndrome (n=1). One-third of the patients were not in remission before transplant, and the majority received a HLA-haploidentical graft (73.7%) with reduced-intensity conditioning regimen (79.0%). All of the haploidentical grafts were CD3+ cell-depleted ex vivo. The primary indication for DLIs was mixed chimerism (median 94% donor, range 15–99%) with or without concurrent viral reactivation or detectable MRD.
Treatment response after DLI administration
The median number of DLIs per patient was 4 (range, 1–14). The median cumulative cell dose in the DLIs was 2 × 106 CD3+ cells/kg (range, 2.5 × 104–72.6 × 107 CD3+ cells/kg). The overall responses after DLI are summarized in Figure 2.
Among the 38 patients, 33 (86.8%) responded to DLI therapy, including 30 patients (78.9%) who had donor chimerisms fully converted back to 100% and 3 patients (7.9%) who had increased donor chimerisms. Three of them (2 complete and 1 partial response) had chimerism level <50% when DLI was initiated (15, 25 and 34% donor). Five patients (13.2%) failed to respond and had further decrease in chimerism.
We did not observe a difference in chimerism response between patients with ALL or AML (P=0.24). There was also no statistical correlation between response rate and the level of donor chimerism at the initiation of DLI (P=0.48), age at HSCT (P=0.52), gender (P=0.74), conditioning regimen (total body irradiation-based versus Fludarabine-based, P=0.1), donor source (haploidentical versus MSD, P=0.12; haploidentical versus matched-unrelated donor, P=0.3) and disease status at HSCT (remission versus non-remission, P=0.73).
Eight patients who received DLI for mixed chimerism were also noted to have concurrent viral reactivation at the time of DLI therapy. All the eight patients and their donors were CMV seropositive before transplantation. Seven had CMV reactivation with their initial CMV copy number ranging between 33 000 and 1 200 000 (median 610 000) per ml of blood, and one had adenovirus reactivation with the initial adenovirus copy number of 6087 per ml of blood. Viral monitoring revealed positive response to the DLI in all the patients. The patient with adenovirus reactivation had a negative adenovirus PCR test, so did three of the seven patients with CMV reactivation. All of the remaining 4 patients with CMV reactivation had a significant decrease in CMV copy numbers by >100-fold after DLI administration. None of these eight patients developed clinical evidence of viral diseases thereafter.
In the 7 patients (4 with AML, 2 ALL and 1 acute biphenotypic leukemia) who had MRD detectable in their marrow at the time of DLI therapy (median, 0.34% MRD; range, 0.004–18%), all had blood donor chimerism successfully converted to 100% after DLI administration; their MRD became negative (and remained negative) in 3, was decreased in 3 but increased in 1 after the DLI treatment. In one of the three patients with partial response, MRD initially became negative after DLI therapy, reappeared 3 months later and is decreasing after additional DLI therapy. The second patient with partial response and decreasing MRD underwent a second allogeneic HSCT and is currently in remission. The third patient with partial response and decreased MRD subsequent died of leukemia relapse after a second allogeneic HSCT. The patient who did not respond to DLI is currently in remission after a repeat allogeneic HSCT. All repeated allogeneic HSCT were done using different donors.
Survival and relapse outcomes based on chimerism response
Of the 38 patients, 11 died of leukemia relapse and 1 succumbed from an anesthetic complication (Supplementary Table S1). The OS of patients with chimerism reverted to 100% post DLI was significantly higher than that of patients who had partial or no response to DLI (80.2±9.3% s.e. versus 0 at 3 years, P=0.0003; Figure 3a). Given that a positive MRD assay could independently affect patient’s survival outcomes, additional analyses were performed after excluding the seven patients with detectable MRD. Similarly, among the remaining 31 patients, the OS of those with chimerism reverted to 100% post DLI remained significantly higher than that of those with partial response or no response (83.1±8.9% versus 0 at 3 years, P=0.0009; Figure 3b). No difference in OS was observed between the patients who had partial response and those with no response.
OS after DLI for mixed chimerism in (a) all patients or (b) patients without detectable MRD. Kaplan–Meier curves were generated separately on patients who successfully had chimerism reversed to 100%, on those who had increased donor chimerism and on those whose chimerism decreased after DLI administration.
CIR after DLI was 33.5±2.1% at 3 years in patients with chimerism reverted to 100% post DLI, significantly lower than those of patients who had partial response (100±11.1%) or no response to DLI therapy (80±8.3%) (P=0.00004; Figure 4a). Additional analysis was performed by excluding the seven patients with detectable MRD. The CIR in the patients with chimerism reverted to full donor chimerism post DLI was statistically significantly lower (16.9±1%) compared with those in the patients who had partial response (100±11.1%) or no response (80±8.3%) (P=0.0001). No difference in relapse was observed between the patients who had partial response and those with no response.
Toxicity and GvHD
The characteristics of the 10 patients who developed GvHD are summarized in Table 2. Using our risk-adapted DLI strategy, none of the four matched-sibling and six matched-unrelated HSCT patients developed acute GvHD after DLI. The rate of GvHD in haploidentical HSCT patients was 35.7%, with only 17.9% having grade III-IV GvHD. The majority of the patients (70%) had single organ involvement. Only two patients had two organ involvement and one patient had three organ involvement.
Currently, 7 of the 10 patients with GvHD are clinically well: one patient died of leukemia, the other died of central line-associated bacteremia after leukemia relapse, and the third died of anesthetic complication. None of the patients died because of DLI toxicity. Chronic GvHD was noted in 3 patients (7.9%): one had extensive GvHD of the liver and skin improving with therapy and the other 2 had skin GvHD that resolved completely with treatment.
Discussion
Although DLI is a treatment option for allogeneic HSCT patients who experienced disease relapse, its clinical success has primarily been limited to those with CML and indolent lymphoma.6, 7 In acute leukemias, small doses of donor lymphocytes are usually not effective, but large doses of cells may increase the risk of fatal GvHD. Here, we developed a simple risk-adapted strategy of DLI without the need of ex vivo manipulation or administration of GvHD prophylaxis. Our approach was successful in more than three-quarters of the recipients with minimal risk of GvHD. The improvement in efficacy may, in part, be related to intensive chimerism monitoring (weekly in the first 100 days and then monthly in the first year), immediate initiation of pre-emptive DLI at the time of mixed chimerism (before detectable MRD) and frequent administration of subsequent doses (every 2–4 weeks). The risk of GvHD was minimized by a small starting dose based on donor source, conservative dose-escalation by only twofold guided by real-time chimerism response and withholding of DLI instantaneously when the chimerism converted back to 100%.
Several studies reported the use of DLI in hematological relapse of pediatric leukemias after allogeneic HSCT; the complete remission rate was only about 20% in AML or JMML and was <10% in ALL.6, 9, 27 Furthermore, the risk of acute GvHD was substantial (between 50–60%).6, 7 In order to minimize this side effect and tip the balance in favor of GvL, several approaches have been investigated, including setting a limit of T-cell dose, selective depletion of certain T-cell subsets, insertion of suicide genes or chimeric receptors into effector cells or activation of donor T cells ex vivo.11, 12, 13, 14, 15, 28 Most recently, a modified DLI regimen was developed using pre-DLI chemotherapy, granulocyte colony-stimulating factor-mobilized peripheral blood stem cells and post-DLI immunosuppression;29 however, the cumulative incidence of acute GvHD was still substantial (53.2% for grades II-IV and 28.4% for grades III-IV). By using our risk-adapted approach (Figure 1), only 13.2% of the entire cohort or 17.9% of the haploidentical HSCT patients experienced grade III-IV acute GvHD, with no associated mortality. Furthermore, none of the 10 patients who received matched-sibling or matched-unrelated DLIs developed acute GvHD. As no patients died of DLI-associated complications (0/37; 95% CI 0–9.4%), the <10% risk of mortality related to DLI compared favorably with the >80% risk of leukemia relapse associated with mixed chimerism (Figure 4).
Because no GvHD prophylaxis is required with our approach, DLIs were also useful for the control of concurrent viral infection.30 Despite the use of contemporary pharmacological interventions, success in eradicating viral infections is limited in these severely immunocompromised patients. Therefore, most of the patients require prolonged courses of anti-viral treatment. The longer the treatment is required, the higher the risk of developing serious side effects, such as myelosuppression and renal dysfunction. Although viral-specific DLIs are useful in treating HSCT patients with viral infections, these cells require specialized laboratory for preparation.31 The use of our DLI administration strategy seemed to be as effective and safe when given concurrently with anti-viral medication and does not require ex vivo manipulation. All the eight patients with viral reactivation achieved significant response to therapy. Further studies with direct assay for virus-specific immunity in larger number of patients are warranted.
The lack of GvHD may not translate to a lack of efficacy in leukemia control, although several studies reported that GvHD appeared to correlate with response to DLI.6, 7 Our strategy of administering DLI was highly effective in general, even with relatively low starting doses, primarily because DLI was given pre-emptively to patients with mixed donor chimerism before frank hematological relapse. In the two largest pediatric multi-center studies published thus far, a total of 25 patients (17 with ALL and 8 with AML) received DLI as frontline treatment for mixed chimerism. Four (24%) of the ALL patients and 4 (50%) of the AML patients survived, compared with 0% survival in those without immunotherapy.18, 19 The poor prognosis of mixed chimerism in these reports was confirmed in our study, as 8/8 patients with no or partial response died shortly with all relapses occurring within 3 months after the onset of mixed chimerism. In contrast, 80% of the 30 patients with complete response survived long term. Thus, our finding underscores the importance of correcting mixed chimerism immediately and maintaining patient’s donor chimerism at 100% continually. For the patients who fail to convert back to 100% donor, our current approach is to perform repeat allogeneic HSCT as soon as possible. Monitoring of donor chimerism by VNTR is technically easier than that for MRD by PCR or flow cytometry, as diagnostic samples may not be available to the transplant centers to establish the leukemia markers.32 Standardization of chimerism monitoring in multi-center setting is feasible and has recently been implemented.33
In summary, our study confirmed the imminent poor prognosis of mixed chimerism and provided the largest data set thus far on the outcomes of pre-emptive DLI for mixed chimerism in childhood leukemia. Based on the favorable results of our regimen, further studies in other pediatric populations are warranted. Our approach should be readily adaptable by all the transplant centers.
References
Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 2011; 118: 223–230.
Pavletic SZ, Kumar S, Mohty M, de Lima M, Foran JM, Pasquini M et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant 2010; 16: 871–890.
Miller JS, Warren EH, van den Brink MR, Ritz J, Shlomchik WD, Murphy WJ et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant 2010; 16: 565–586.
Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JH et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2010; 16: 1467–1503.
Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990; 76: 2462–2465.
Roddie C, Peggs KS . Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther 2011; 11: 473–487.
Deol A, Lum LG . Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev 2010; 36: 528–538.
Collins RH Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Levine JE, Barrett AJ, Zhang MJ, Arora M, Pulsipher MA, Bunin N et al. Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant 2008; 42: 201–205.
Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol 2002; 20: 405–412.
Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, Boulad F et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood 1995; 86: 1261–1268.
Giralt S, Hester J, Huh Y, Hirsch-Ginsberg C, Rondon G, Seong D et al. CD8-depleted donor lymphocyte infusion as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation. Blood 1995; 86: 4337–4343.
Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997; 276: 1719–1724.
Alyea EP, Soiffer RJ, Canning C, Neuberg D, Schlossman R, Pickett C et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood 1998; 91: 3671–3680.
Wehler TC, Nonn M, Brandt B, Britten CM, Grone M, Todorova M et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood 2007; 109: 365–373.
Bishop MR, Alyea EP 3rd, Cairo MS, Falkenburg JH, June CH, Kroger N et al. National Cancer Institute's First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant 2011; 17: 443–454.
Alyea EP, DeAngelo DJ, Moldrem J, Pagel JM, Przepiorka D, Sadelin M et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2010; 16: 1037–1069.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol 2004; 22: 1696–1705.
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 2011; 118: 5681–5688.
Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant 1998; 21: 487–495.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Kremens B, Dilloo D et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transplant 2004; 33: 815–821.
Pulsipher MA, Bader P, Klingebiel T, Cooper LJ . Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant 2009; 15 (1 Suppl): 62–71.
Chen X, Barfield R, Benaim E, Leung W, Knowles J, Lawrence D et al. Prediction of T-cell reconstitution by assessment of T-cell receptor excision circle before allogeneic hematopoietic stem cell transplantation in pediatric patients. Blood 2005; 105: 886–893.
Kreyenberg H, Holle W, Mohrle S, Niethammer D, Bader P . Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Tuebingen experience. Leukemia 2003; 17: 237–240.
Srinivasan A, Wang C, Srivastava DK, Burnette K, Shenep JL, Leung W et al. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19: 94–101.
Leung W, Pui CH, Coustan-Smith E, Yang J, Pei DQ, Gan K et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood 2012; 120: 468–472.
Yoshimi A, Bader P, Matthes-Martin S, Stary J, Sedlacek P, Duffner U et al. Donor leukocyte infusion after hematopoietic stem cell transplantation in patients with juvenile myelomonocytic leukemia. Leukemia 2005; 19: 971–977.
Porter DL, Levine BL, Bunin N, Stadtmauer EA, Luger SM, Goldstein S et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood 2006; 107: 1325–1331.
Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119: 3256–3262.
Breuer S, Rauch M, Matthes-Martin S, Lion T . Molecular diagnosis and management of viral infections in hematopoietic stem cell transplant recipients. Mol Diagn Ther 2012; 16: 63–77.
Sili U, Leen AM, Vera JF, Gee AP, Huls H, Heslop HE et al. Production of good manufacturing practice-grade cytotoxic T lymphocytes specific for Epstein-Barr virus, cytomegalovirus and adenovirus to prevent or treat viral infections post-allogeneic hematopoietic stem cell transplant. Cytotherapy 2012; 14: 7–11.
Kroger N, Bacher U, Bader P, Bottcher S, Borowitz MJ, Dreger P et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant 2010; 16: 1187–1211.
Lion T, Watzinger F, Preuner S, Kreyenberg H, Tilanus M, de Weger R et al. The EuroChimerism concept for a standardized approach to chimerism analysis after allogeneic stem cell transplantation. Leukemia 2012; 26: 1821–1828.
Acknowledgements
This study was supported by funding from the Assisi Foundation of Memphis and the American Lebanese Syrian Associated Charities (ALSAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
PR participated in study design, collection of data and drafting of manuscript; CM and KG collected data; GK performed statistical analyses; CH, BT, MD, AS, DS and AP participated in DLI administration and patient monitoring, CHP took part in study conception and manuscript writing and WL contributed to regimen design, study conception and manuscript writing. All the authors participated in draft revisions and approval of the final manuscript.
Supplementary Information accompanies this paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Rujkijyanont, P., Morris, C., Kang, G. et al. Risk-adapted donor lymphocyte infusion based on chimerism and donor source in pediatric leukemia. Blood Cancer Journal 3, e137 (2013). https://doi.org/10.1038/bcj.2013.39
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2013.39
Keywords
This article is cited by
-
Long-term results and GvHD after prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia
Bone Marrow Transplantation (2022)
-
Outcomes of pediatric patients who relapse after first HCT for acute leukemia or MDS
Bone Marrow Transplantation (2021)
-
Full donor chimerism without graft-versus-host disease: the key factor for maximum benefit of pre-emptive donor lymphocyte infusions (pDLI)
Bone Marrow Transplantation (2020)
-
Improved survival rate in T-cell depleted haploidentical hematopoietic cell transplantation over the last 15 years at a single institution
Bone Marrow Transplantation (2020)
-
Safety and efficacy of fresh whole blood donor lymphocyte infusion in children
Bone Marrow Transplantation (2019)