Abstract
Microbial agents are regarded as a potential cause of tumors, but their direct effects on tumors, such as myeloma, are not well studied. Our studies demonstrated that expression of HLA-DR and CD40 on the myeloma cell membrane surface is upregulated by interferon-γ and/or microbial antigens (Ags). Unlike prior studies, our study showed that Th2 cells cannot promote myeloma growth directly. However, Bacillus Calmette–Guerin Vaccine (BCGV)-specific Th2 cells stimulated by BCGV-loaded dendritic cells (DCs) promoted myeloma clonogenicity directly when the myeloma cells expressed major histocompatibility complex Class-II molecules (MHC-II) and took up BCGV Ag. B-cell lymphoma 6 (Bcl-6) protein expression and the proportion of HLA-DR+ or CD40+ cells were higher in colonies of Th2 cell-stimulated myeloma cells. Furthermore, anti-HLA-DR or neutralizing CD40 antibody could prevent this increase in Bcl-6 expression and colony number. These results indicate that microbes and microbial Ag-specific Th2 cells may directly impact the biology of myeloma and contribute to tumor progression. Activation may be limited to MHC-II+ myeloma cells that retain B cell and stem cell characteristics. Taken together, our data suggest that factors involved in microbial Ag presentation, such as DCs, Th2 cells and so on, are potential targets for myeloma therapeutic intervention.
Similar content being viewed by others
Introduction
Th2 cells are an important subset of antigen (Ag)-specific T cells. Several studies suggest that Th2 activation by tumor Ags may be a risk factor for multiple myeloma (MM).1, 2 Prior research has shown that the Th2 response can promote MM progression after idiotype-based immunotherapy.3 Thus, Th2 cells may have the ability to induce tumor development. However, myeloma cells express almost no major histocompatibility complex Class-II molecules (MHC-II), leaving open the question about whether Th2 cells can directly promote MM progression. Moreover, the question of whether Th2 cells interact directly with myeloma cells or impact the biology of myeloma cells has not been answered.
Dendritic cells (DCs) are the most efficient Ag presenting cells (APCs) and can be used in Ag-based immunotherapy to activate all T-cell subsets.3, 4 When naïve T cells are stimulated by a Th2-inducing Ag, they acquire the ability to interact with B cells that have specifically taken up Ag.5, 6 Interaction of DCs with B cells may also affect B-cell survival.6, 7 Prior studies have shown a correlation between increased infiltration of human tumors by DCs and adverse prognosis.8, 9, 10 Moreover, it has been demonstrated in vitro that interaction with DCs can directly enhance clonogenicity of human MM.11 Considering that MM is a B-cell malignancy and Th2 cell–B-cell interaction is crucial for regulating B-cell function, we supposed that Ag-specific Th2 cells induced by DCs would promote the survival and growth of myeloma cells that express MHC-II molecules and take up the same Ag.
Previous studies have shown that persistence of microbial Ags in the host is an important growth stimulus for some kinds of tumor,12, 13, 14 but the pathogenesis of these tumors is still largely unknown. DC–Th2–B-cell interactions with microbial Ags stimulate humoral immunity, which ends in B-cell differentiation into plasma cells that produce and secrete specific antibodies to these Ags.6 Malignant plasma cells also clonally produce and secrete a specific immunoglobulin (Ig). However, it is not known whether the monoclonal Igs of myeloma cells are specific for microbial Ags and whether specific Ags can promote the growth of malignant plasma cells as well as the secretion of monoclonal Igs. Therefore, we hypothesized that microbial Ags presented by APCs may be an important aspect of myeloma pathogenesis, which we propose occurs in a multi-step process. First, specific microbial Ags presented to Th2 cells by DCs may persist within DCs or Th2 cells. Then, the specific Th2 cells may stimulate specific monoclonal B cells to proliferate and secrete monoclonal Ig over a long period. Finally, the specific B cells may be transformed to malignant plasma cells, and the specific Ag may continue to promote the growth of malignant plasma cells generated by DC–Th2 cell–myeloma cell interaction.
Materials and methods
Healthy donor samples, patient samples and human myeloma cell lines
Peripheral blood from healthy donors and bone marrow aspirates from 21 patients with MM (Supplementary Tables 1 and 2) were collected after they gave their informed consent, which was obtained in accordance with the Declaration of Helsinki, and after the study protocol was approved by the institutional review board at The First Affiliated Hospital, Sun Yat-Sen University. The human myeloma cell line U266 was kindly provided by Dr Yang Xu (Suzhou University, Jiangsu, China). The K562 cell line was from the American Type Culture Collection (ATCC, Manassas, VA, USA). Some U266 cells were treated with interferon-γ (IFN-γ) (Shanghai Prime Gene Bio-Tech Co., Shanghai, China) and Bacillus Calmette–Guerin Vaccine (BCGV) for 48 h.
Generation of monocyte-derived DCs
Monocyte-derived DCs were generated from peripheral blood monocytes (PBMCs) using an adaptation of protocols described previously.15 In brief, PBMCs were isolated from freshly collected heparin-treated blood by density gradient centrifugation. Subsequently, 5 × 106 cells/ml was cultured for 2–4 h to permit cell adherence, and nonadherent cells were removed. The adherent cells were cultured 5–7 days in RPMI-1640 complete medium supplied with 20 ng/ml of human recombinant granulocyte macrophage colony-stimulating factor (Shanghai Prime Gene Bio-Tech Co.) and 20 ng/ml of interleukin 4 (IL-4; Shanghai Prime Gene Bio-Tech Co.). TNF-α (Shanghai Prime Gene Bio-Tech Co.) and BCGV were added to the culture for an additional 48 h. For some experiments, maturation to DCs was stimulated using purified protein derivative of M. tuberculosis or tetanus toxoid Ag (TTA) instead of BCGV. The purity of mature DCs in vitro was at least 95%, which was confirmed for each experiment.
Isolation of BCGV-specific Th2 (BCGV-Th2) cells
The nonadherent cells removed from PBMCs served as T cells. BCGV-loaded DCs were added to the T cells at a ratio of 1:30 in the presence of granulocyte macrophage colony-stimulating factor (20 ng/ml), IL-4 (20 ng/ml) and IL-2 (10 ng/ml; PeproTech, Rocky Hill, NJ, USA). After 5–7 days, BCGV-Th2 cells were isolated using CD4+ and CD294+ (CRTH2) microbeads (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany) from the DC–T-cell coculture system according to the manufacturer’s instructions.16 The purity of Th2 cells (CRTH2) was always >95%.
Clonogenic assays induced by Th2 cells
BCGV-Th2 cells were added to tumor cells at a ratio of 10:1 (tumor cells: 10 000/well), mixed completely and cocultured for 48 h. The clonogenic growth of tumor cell lines was evaluated by plating tumor cells in quadruplicate 35-mm2 tissue culture dishes containing RPMI-1640 complete medium with 0.9% methylcellulose, 30% fetal bovine serum, 2 mM l-glutamine and 20 μg/ml gentamycin sulfate and incubating these dishes at 37 °C in a 5% CO2 atmosphere. Colonies consisting of >40 cells were counted under a microscope 2–3 weeks after plating.
MHC restriction and neutralizing CD40 on U266 cells
To study the role of MHC-II and CD40 molecules in Th2 cell and MM cell interactions, cells were incubated with mouse MoAb against HLA-DR (IgG2b; 1 μg/ml; Millipore, Billerica, MA, USA), mouse MoAb against CD40 (IgG2b; 5 μg/ml; R&D Systems, Minneapolis, MN, USA), or control IgG2b (5 μg/ml), respectively.17 To assess the Th2-cell contact dependence of tumor cell clonogenicity, Transwell polyester membrane inserts (CLS3460, Corning, Inc., Corning, NY, USA) separating U266 cells from BCGV-Th2 cells were compared with control inserts separating U266 cells from complete RPMI-1640 medium only.
Clonogenic assay of primary tumor cells
Mononuclear cells (MNCs) isolated from bone marrow samples using density gradient centrifugation were treated with IFN-γ and BCGV for 2 days. Then CD138+ and CD138− fractions were isolated from treated-MNCs using CD138 microbeads (Miltenyi Biotec) and an AutoMACS magnetic cell sorter (Miltenyi Biotec). The CD138− fraction was further depleted of normal hematopoietic progenitors using CD34, CD3, CD4 and CD8 microbeads (Miltenyi Biotec). The resulting two fractions (CD138+CD34−CD3−CD4−CD8− and CD138− CD34−CD3−CD4−CD8− cells; 0.5–2.5 × 105/ml) were plated with or without BCGV-Th2 cells at a ratio of 1:2–5 in a methylcellulose culture system (Methocult, Vancouver, BC, Canada), as described above for U266 cells, containing rhIL-6 (10 ng/ml, PeproTech). Tumor colonies were counted after 2–3 weeks of culture. The phenotype of the cells in these colonies was confirmed by flow cytometry.
Flow cytometry analysis
Forward scatter on flow cytometry was used to measure tumor cell size and the change in surface expression of CD80, CD40, HLA-DR, intracellular λ light chain and so on. Data were collected and analyzed using a FACScalibur flow cytometer and Cell Quest software (BD Biosciences, San Jose, CA, USA).
Western blotting
Western blotting was performed on cell lysates from U266 cells. Cells were lysed with sodium dodecyl sulfate (SDS) loading buffer, and an aliquot of each lysate was loaded onto an 10% SDS polyacrylamide gel. After electrophoresis, the proteins in the gel were electrotransferred to nitrocellulose sheets, probed for 2 h with primary antibody to B-cell lymphoma 6 (Bcl-6) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1/500 in PBS-T, 5% milk (Santa Cruz Biotechnology) at room temperature, washed three times with PBS-T, incubated with secondary antibody for 1 h at room temperature, washed with PBS-T three times, dried, incubated with enhanced chemiluminescence reagent (Santa Cruz Biotechnology) for 1 min and visualized in a Kodak Imager (Kodak Film, Kodak, Rochester, NY, USA).
SYBR green PCR assay
RNA was extracted using the Trizol kit (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into first-strand cDNA using random hexamer primers and a reverse transcriptase Superscript II Kit (Invitrogen), according to the manufacturer’s instructions. Bcl-6 was produced from Bcl-6-positive samples by PCR amplification, separated on gel and detected. Then, the target band of Bcl-6 was cut, viewed under long-wave UV, extracted from the gel, purified using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and used as the positive control (OD 260/280>1.8). Sterile distilled water was used as the negative control. The samples were amplified and quantified on a sequence detection system (API 7500; Applied Biosystems, Foster City, CA, USA) using the following thermal cycler conditions: 3 min at 93 °C, 30 s at 93 °C, 45 s at 55 °C, 45 s at 72 °C and 40 cycles. β-actin, a housekeeping gene, was used to normalize each sample. The data were analyzed using the API 7500.
Primers were as follows: Bcl-6, F: 5′-CAGATTTGTACAGGTGGCCCA-3′; R: 5′-AGAGTCTGAAGGTGCCGGAA-3′; β-actin, F: 5′-GCA TGG GTC AGA AGG ATT CCT-3′; R: 5′-TCG TCC CAG TTG GTG ACG AT-3′.
Statistical analysis
Differences between groups were assessed using the Student’s t-test or one-way analysis of variance for multiple comparisons test. The signisficance level was set at P<0.05.
Results
Change in expression of surface markers on U266 cells and primary myeloma cells
Treatment with IFN-γ for 48 h markedly increased surface expression of HLA-DR, CD40 and CD80, but CD40 and CD80 expression were at a low levels, and CD86 and CD54 (markers indicating that Th2 cells have the ability to activate B cells) remained at a high level (Figure 1a). Interestingly, exogenous Ags, such as BCGV, clearly upregulated HLA-DR and CD40 expression (Figure 1b). Similar results were also observed in primary myeloma cells (Table 1). Therefore, exogenous Ag can directly alter the expression of surface molecules on MM cells, which suggests the possibility that MM cells can be activated by Th2 cells.
Surface Ags on U266 cells treated by IFN-γ or BCGV. Surface HLA-DR, CD40, CD80, CD86 and CD54 on tumor cells were examined by flow cytometry. (a) Treatment of U266 cells with IFN-γ for 48 h markedly increased HLA-DR, CD40 and CD80 expression, but CD40 and CD80 expressions were at a low level; *P<0.05. U266 cells expressed high levels of CD86 and CD54. (b) Expression of HLA-DR, CD40 and CD80 was significantly increased by treating U266 cells with BCGV; *P<0.05.
Stimulation of clonogenic growth of U266 cells by Th2 cells
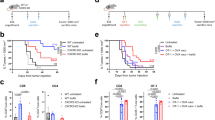
To assess whether U266 cells can respond to Th2 cells, IFN-γ and BCGV-treated U266 cells (treated-U266 cells) were cultured 5 days in the presence of BCGV-specific Th2 cells (BCGV-Th2 cells). Colorimetric assays showed that BCGV-Th2 cells weakly stimulated the proliferation of treated-U266 cells, but clonogenic assays showed that BCGV-Th2 cells significantly increased the clonogenicity of treated-U266 cells in a Th2 cell:tumor cell ratio-dependent manner (Figures 2a and c). In contrast, BCGV-Th2 cells had no impact on the clonogenicity of untreated-U266 cells and K562 cells and U266 treated by IFN-γ alone (Figures 2b), and non-BCGV-specific Th2 cells and BCGV-specific CD8+T cells had no impact on treated-U266 cells (Figure 2f). Similarly, the same results were also observed not only in the assays of human myeloma cell line RPMI8266 but also in the assays of U266 with purified protein derivative or TTA instead of BCGV (see Supplementary Figures 1–3). BCGV-Th2 cell-mediated enhancement of tumor clonogenicity in this system required close contact between tumor cells and Th2 cells, as the effect was not evident when the two cell populations were separated by a Transwell membrane (Figure 2e). Therefore, interactions of treated-MM cells and BCGV-Th2 cells can directly promote MM clonogenicity. Additionally, clonogenic assays showed that either BCGV-Th2 cells or DCs could significantly increase the clonogenicity of treated-U266 cells, but the number of tumor colonies promoted by BCGV-Th2 cells was higher than that of tumor colonies promoted by DCs (Figure 3a).
Clonogenicity of human tumor cells. Colonies were enumerated microscopically after 2–3 weeks of culture. IFN-γ and BCGV-treated U266 cells were plated with or without BCGV-Th2 cells in a clonogenic assay at a ratio of 1:10 (tumor cells: 10 000/well). Results are the mean±s.e.m. (error bars) of the aggregate of three separate experiments; *P<0.05. (a) Th2 cells versus no Th2 cells. Clonogenicity was significantly enhanced by BCGV-Th2, but reduced by anti-HLA-DR or anti-CD40; #P<0.05. (b, d, g) Untreated-U266 cells or K562 cells or U266 treated by IFN-γ alone were not impacted by BCGV-Th2 cells. (c) Treated-U266 cells were plated with BCGV-Th2 cells at an increasing Th2 cell/tumor cell ratio in a clonogenic assay. Clonogenicity was increased by Th2 cells in a Th2 cell:tumor cell ratio-dependent manner. (e) Requirement for cell–cell contact. BCGV-Th2 cells were either plated along with treated-tumor cells (Th2/tumor ratio of 10:1) or separated from treated-tumor cells by a transwell insert membrane. Treated-tumor cells alone were plated in the clonogenic assay and counted as before. (f) To test for allogeneic effects, non-Ag-Th2 or Ag-CD8+T cells were plated with treated-tumor cells. Both non-Ag-Th2 and Ag-CD8+T cells had no impact on tumor clonogenicity.
BCL-6 expression of clonogenicity promoted by DCs versus by Th2 cells. (a) IFN-γ and BCGV-treated U266 cells were plated with BCGV-Th2 cells or DCs in a clonogenic assay at a ratio of 1:10 (tumor cells: 10 000/well). Results are the mean±s.e.m. (error bars) of the aggregate of three separate experiments; *P<0.05, #P<0.05. (b) The expression of Bcl-6 and β actin in U266 cells was analyzed by western blotting.
Bcl-6 expression in tumor colonies
Previous studies have suggested an important role for Bcl-6 in survival and self-renewal of germinal center B cells. Thus, the expression of Bcl-6 in these tumor cells was assessed by western blotting and PCR analysis. Both results confirmed that Bcl-6 expression was increased in colonies of Th2 cell-induced tumor cells (Figure 4), suggesting an important role for Bcl-6 in the clonogenicity of treated-U266 cells. In tumor clonogenic assay promoted by DCs versus by Th2 cells, western blotting showed that expression of Bcl-6 produced by BCGV-Th2 cells was stronger in tumor colonies than that of Bcl-6 produced by DCs (Figure 3b).
Bcl-6 expression of U266 cells from colonies. (a) The expression of Bcl-6 and β actin in U266 cells was analyzed by western blotting. (b) The expression of Bcl-6 mRNA in U266 cells was analyzed by SYBR Green I PCR and normalized to the expression of the housekeeping gene, which was significantly enhanced by BCGV-Th2; *P<0.05, but reduced by anti-HLA-DR or anti-CD40; #P<0.05.
The effect of blocking MHC and CD40 on the interaction between Th2 cells and U266 cells
To gain insight into the role of MHC-II and CD40 in the interaction between Th2 cells and U266 cells, MoAbs against MHC-II (anti-HLA-DR MoAb) or CD40 were used to inhibit the enhancement of BCGV-Th2 cell-stimulated clonogenicity of treated-U266 cells. Pretreatment with the antibodies reduced or abolished the enhancement in clonogenicity (Figures 2a and 5). Likewise, anti-HLA-DR abolished and anti-CD40 reduced the increase in Bcl-6 expression (Figure 4). A mouse isotypic control IgG had no effect (Figures 2a and 4). Therefore, MHC-II and CD40 are two key molecules affecting Th2 cell–myeloma cell interactions.
Appearance of colonies of U266 cells. The clonogenicity assays were performed in methylcellulose. Treated-tumor cells were plated with BCGV-Th2 cells (Tumor+Th2), without BCGV-Th2 cells (tumor Alone), with BCGV-Th2 cells and anti-HLA-DR (Anti-HLA-DR), with BCGV-Th2 cells and anti-CD40 (anti-CD40), with BCGV-Th2 cells and control IgG2b (control IgG2b). Micrographs show the appearance of colonies at low power. (Upper panel) Appearance of colonies of treated-U266 cells (tumor cells: 10 000/well). (Lower panel) Appearance of colonies of tumor cells from bone marrow (tumor cells: 100 000/well).
Analysis of tumor colonies
The enhanced clonogenicity stimulated by BCGV-Th2 cells was evidenced by an increase in the number and size of individual colonies (Figures 5 and 6a), and the lower forward scatter of BCGV-Th2 cell-induced tumor cells on flow cytometry suggested their size was smaller than that of non-Th2 cells-inducer tumor cells (Figure 6b). These results suggest an effect on cloning efficiency or survival. Moreover, we verified that CD40 and HLA-DR were upregulated in a higher percentage of U266 cells after interaction with Th2 cells (Figure 6e). We further demonstrated that the cloning efficiency was higher in tumor cells from Th2-induced colonies, treated with IFN-γ and BCGV, then cocultured with fresh BCGV-Th2 cells again, than in tumor cells from Th2-induced colonies, treated with IFN-γ and BCGV without repeat coculture with BCGV-Th2 cells (Figure 6c). The increase in cloning efficiency was also reduced or abolished by culture in the presence of anti-CD40 or anti-HLA-DR but not in the presence of mouse isotypic control IgG (Figure 6c). In replating assays of clonogenicity, cells from colonies of U266 cells (first treated with IFN-γ and BCGV for 48 h and then induced by Th2 cells) had higher cloning efficiency than U266 cells from suspension cultures in the same culture medium with or without BCGV-Th2 cells (Figure 6d). Therefore, Th2 cell-induced change in the biology of myeloma cells may account for the increased ability of myeloma cells to form colonies.
Analysis of tumor colonies in clonogenic assays. (a) Colony diameter was measured in 100 random colonies per group after a 3-week period of culture. The average diameter of colonies was significantly larger in cocultures of tumor cells with Th2 cells than in cultures of tumor cells without Th2 cells; *P<0.05. However, this increase was eliminated by anti-HLA-DR and anti-CD40; #P<0.05. Results are the mean±s.e.m. (error bars) of the aggregate of three separate experiments. (b) Flow cytometric analysis of the size of tumor cells in colonies after culture with or without BCGV-Th2 cells for 3 weeks. (c, d) Replating assays of clonogenicity. The tumor cells from BCGV-Th2 cell-induced U266 colonies treated again with IFN-γ and BCGV for 48 h and cocultured again with BCGV-Th2 cells for 3 weeks had higher cloning efficiency; *P<0.05. However, anti-CD40 MoAb reduced and anti-HLA-DR MoAb abolished colony formation; #P<0.05. Results are the mean±s.e.m. (error bars) of the aggregate of three separate experiments. (e) Tumor colonies from clonogenic assays after a 3-week culture period were harvested and stained with HLA-DR PE, CD40-FITC, CD4-FITC and Ig lambda FITC for flow cytometric evaluation.
Enhancement of clonogenicity of primary myeloma cells by BCGV-Th2 cells
To extend these observations from U266 cells to primary myeloma cells, bone marrow samples from patients with myeloma were examined. Primary myeloma cells were cultured in the presence or absence of autologous or allogeneic Th2 cells. The number of tumor colonies formed by MNCs (primary myeloma cells from 12 patients, respectively) treated with IFN-γ and BCGV and cocultured with BCGV-Th2 cells was higher in 9/12 patients (6/8 patients using allogeneic Th2 cells and 3/4 patients using autologous Th2 cells) than the number formed by same cells treated in the same way but without MNC-Th2 cell coculture. This increase in tumor colony number was reduced or abolished by the presence of anti-CD40 or anti-HLA-DR in the culture (Figure 7). Therefore, BCGV-Th2 cells can also enhance the clonogenicity of treated-primary myeloma cells.
Clonogenicity of primary myeloma cells. Assay of cells from primary myeloma bone marrow samplesc (n=12). Tumor cells were cultured in the presence or absence of allogeneic (n=8) or autologous Th2 cells (n=4) at a ratio of 1:5 in the clonogenic assay. Patient 1 (tumor cells: 250 000/well); Patient 3, 4, 8 and 9 (tumor cells: 100 000/well); Patient 5, 10, 11 and 12 (tumor cells: 500 00/well). Clonogenicity was markedly enhanced by BCGV-Th2 cells, *P<0.05, but reduced by anti-HLA-DR or anti-CD40; #P<0.05. (a) Clonogenicity was in six allogeneic assays (6/8). (b) Clonogenicity was in three allogeneic assays (3/4).
Discussion
The immune system may facilitate the growth of some tumors. Several studies have suggested a role for tumor-associated DCs or macrophages as a driver of growth in breast cancer, lymphoma and myeloma.11, 18 As malignant myeloma cells are B cells or plasma cells, we thought that Th2 cells probably promote myeloma growth. Prior studies reported enhanced myeloma growth after immunotherapy with tumor Ag-pulsed DCs.3, 11 However, our in vitro data indicated that Th2 cells do not promote proliferation and clonogenicity of myeloma cells directly. We think that the absence of MHC class II molecules and/or other molecules on myeloma cell membrane may account for this limitation. Promotion of tumor growth Th2 cells should require myeloma cell expression of MHC class II molecules, costimulatory molecules B7 (CD80, CD86), CD40, adhesion molecules (CD54) and so on, which are required in Th2 cell-induced proliferation of B cells.19, 20, 21, 22, 23, 24, 25, 26 Our studies showed that myeloma cells express membrane markers of B cells including high levels of CD86 and CD54 and detectable levels of HLA-DR, CD40 and CD80, which can be upregulated by IFN-γ. These data are consistent with the findings of prior studies.17 Surprisingly, we found that microbial Ags, such as BCGV, TTA and so on, could induce expression of MHC-II and CD40 on the myeloma cell surface. In our studies, BCGV-specific Th2 cells induced by BCGV-loaded-DCs significantly enhanced the clonogenicity of myeloma cells induced to express MHC-II, CD40 and CD80 by IFN-γ and BCGV. These data suggest that a small proportion of malignant plasma cells retain B-cell functions, may possess stem cell characteristics, and must interact with Th2 cells to form colonies. This malignant plasma cell subpopulation may account for the development and recrudescence of malignant plasma cell tumors.
In our studies, blockade of T-cell receptor (TCR)–MHC-II interaction by anti-MHC-II MoAb or blockade of the CD40L–CD40 interaction by anti-CD40 MoAb inhibited Th2 cell-mediated enhancement of the clonogenicity of human myeloma cells, suggesting that the Th2 cell–myeloma cell interaction is like the Th2 cell–B-cell interaction in that CD40L ligands and TCR on the Th2-cell surface recognize the specific Ag peptide borne by the MHC-II on the myeloma cell. Prior studies have confirmed that myeloma cells can function as APCs and can present Ag peptide to CD4+T cells, which are MHC class-II restricted.17 In our studies, promotion of tumor colony formation by Th2 cells only occurred if TCR recognized the specific Ag peptide bound to the MHC-II of the tumor cell. The fact that anti-MHC-II MoAb abolished the enhancement of tumor clonogenicity but anti-CD40 MoAb merely reduced the enhancement indicates that tumor colony growth promotion by Th2 cells is mainly MHC class-II restricted. However, our data do not exclude the possibility that other molecules such as adhesion molecules CD54 (CD54–CD11 a interaction) or costimulatory molecules B7 (B7–CD28 interaction) on myeloma cells may also be important in Th2 cell–myeloma interactions. Th2-mediated regulation of myeloma growth may also subvert Th1-cell-mediated immunity and require the participation of Th2-cell-derived cytokines, such as IL-4, IL-6 and IL-10.
In most cases, myeloma cells, like U266 cells, express almost no Bcl-6.11, 27, 28, 29 However, in our study, Bcl-6 was highly expressed in cells from Th2 cell-induced MM colonies. Normally, Bcl-6 contributes to the definition of the germinal center phenotype by repressing multiple DNA damage and cell proliferation checkpoint genes.30, 31, 32 The level of Bcl-6 is downregulated during the differentiation of B cells from memory B cells and plasmablastic cells.33 The Bcl-6-mediated loss of checkpoint function may result in a potentially hazardous state of physiological genomic instability, which may cause B cells to undergo malignant transformation.34, 35, 36 These findings are consistent with the findings of a previous study that showed the reactivation of the B-cell program after Bcl-6 was exogenously expressed in myeloma cell lines.37 So, it is concluded that upregulation of Bcl-6 in myeloma cells may protect the cells from apoptosis and idiovariability and thereby facilitate the formation of malignant clones. We further observed that anti-CD40 reduced but failed to abolish the upregulation of Bcl-6. Therefore, other pathways besides the CD40 signaling pathway should exist to promote Bcl-6 expression in Th2 cell-induced tumor colonies. In our study, Bcl-6 expression was upregulated and the percentages of HLA-DR+ cells and CD40+ cells were higher in Th2 cell-induced tumor colonies, suggesting that the differentiation state of myeloma cells is plastic and can be modified by Th2 cell–myeloma cell interaction.
Microbial Ags are regarded as a potential cause of some tumors. Prior studies have argued that carcinogenesis results from indirect or direct interaction of inflammatory cells and mediators with epithelial cells, stromal cells and extracellular matrix components, which in turn stimulate angiogenesis.38, 39, 40 Nevertheless, the direct effects of microbial Ags on tumor cells have not been well studied. Unlike previous studies, our study showed that microbial Ags, such as BCGV and TTA, enhance myeloma clonogenicity, not just the level of humoral immunity.12, 17, 41, 42 To our knowledge, our findings provide the first evidence that microbial Ags presented by APCs (DCs and myeloma cells) to Th2 cells cause malignant plasma cell disease and participate in MM pathogenesis. In our studies, we analyzed whether allogeneic Th2 cells or autogeneic Th2 cells could drive myeloma cell colony formation, indicating that Th2 cells may be one of the risk factors for patients with MM. The result is consistent with previous reports that early stage and non-advanced MM patients were with Th1 high proportion, whereas advanced MM patients were with a predominated Th2-like response in PBMC after stimulation by monoclonal IgG or microbial Ags.43, 44, 45 So, microbial Ags-Th2 cells may deeply involve in pathogenesy, recurrence and development of MM. However, our findings do not exclude other mechanisms of MM pathogenesis. In conclusion, our study has presented evidence for a novel mechanism of MM pathogenesis (that is, tumor generation from a small number of MHC-II+ malignant plasma cells with B cell and stem cell characteristics that can form colonies efficiently upon Th2-cell induction). Specifically, when the MHC-II+ malignant plasma cells take up microbial Ag they act as APCs. DCs presenting the same microbial Ag activate Th2 cells to recognize malignant plasma cells and induce them to proliferate and form colonies. Thus, every factor involved in Ag presentation, such as microbial Ags, DCs, Th2 cells and so on, may be potential targets to be further studied for therapeutic intervention.
References
Zheng C, Huang D, Liu L, Wu R, Bergenbrant Glas S, Osterborg A et al. Interleukin-10 gene promoter polymorphisms in multiple myeloma. Int J Cancer 2001; 95: 184–188.
Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M . Elimination of CD4(+) T cells enhances anti-tumor effect of locally secreted interleukin-12 on B16 mouse melanoma and induces vitiligo-like coat color alteration. J Invest Dermatol 2000; 115: 1059–1064.
Hong S, Qian J, Yang J, Li H, Kwak LW, Yi Q . Roles of idiotype-specific t cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res 2008; 68: 8456–8464.
Steinman RM, Banchereau J . Taking dendritic cells into medicine. Nature 2007; 449: 419–426.
Randolph DA, Huang G, Carruthers CJ, Bromley LE, Chaplin DD . The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science 1999; 286: 2159–2162.
MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E et al. Extrafollicular antibody responses. Immunol Rev 2003; 194: 8–18.
Garcia De Vinuesa C, Gulbranson-Judge A, Khan M, O'Leary P, Cascalho M, Wabl M et al. Dendritic cells associated with plasmablast survival. Eur J Immunol 1999; 29: 3712–3721.
Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R et al. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res 2005; 11: 2576–2582.
Bahlis NJ, King AM, Kolonias D, Carlson LM, Liu HY, Hussein MA et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood 2007; 109: 5002–5010.
Josselin N, Libouban H, Dib M, Ifrah N, Legrand E, Basle MF et al. Quantification of dendritic cells and osteoclasts in the bone marrow of patients with monoclonal gammopathy. Pathol Oncol Res 2009; 15: 65–72.
Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med 2006; 203: 1859–1865.
Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI . Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci USA 2008; 105: 4358–4363.
Kinlen L . Infections and immune factors in cancer: the role of epidemiology. Oncogene 2004; 23: 6341–6348.
Karin M, Lawrence T, Nizet V . Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 2006; 124: 823–835.
Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods 1996; 196: 137–151.
Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S . CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol 2000; 30: 2972–2979.
Yi Q, Dabadghao S, Osterborg A, Bergenbrant S, Holm G . Myeloma bone marrow plasma cells: evidence for their capacity as antigen-presenting cells. Blood 1997; 90: 1960–1967.
Pollard JW . Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78.
Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC . Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell 1996; 87: 319–329.
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG . Follicular helper T cells: lineage and location. Immunity 2009; 30: 324–335.
Kassiotis G, O’Garra A . Establishing the follicular helper identity. Immunity 2009; 31: 450–452.
Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 1997; 6: 303–313.
Lauritzsen GF, Weiss S, Dembic Z, Bogen B . Naive idiotype-specific CD4+ T cells and immunosurveillance of B-cell tumors. Proc Natl Acad Sci USA 1994; 91: 5700–5704.
Herve M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med 2007; 204: 1583–1593.
Niu H, Cattoretti G, Dalla-Favera R . BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. J Exp Med 2003; 198: 211–221.
Epron G, Ame-Thomas P, Le Priol J, Pangault C, Dulong J, Lamy T et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia 2011; 26: 139–148.
Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood 2000; 95: 2630–2636.
Chen H, Campbell RA, Chang Y, Li M, Wang CS, Li J et al. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood 2009; 113: 1992–2002.
Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2006; 66: 6675–6682.
Ranuncolo SM, Polo JM, Melnick A . BCL6 represses CHEK1 and suppresses DNA damage pathways in normal and malignant B-cells. Blood Cells Mol Dis 2008; 41: 95–99.
Ranuncolo SM, Polo JM, Dierov J, Singer M, Kuo T, Greally J et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol 2007; 8: 705–714.
Polo JM, Juszczynski P, Monti S, Cerchietti L, Ye K, Greally JM et al. Transcriptional signature with differential expression of BCL6 target genes accurately identifies BCL6-dependent diffuse large B cell lymphomas. Proc Natl Acad Sci USA 2007; 104: 3207–3212.
Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E et al. Survival and proliferation factors of normal and malignant plasma cells. Int J Hematol 2003; 78: 106–113.
Polo JM, Ci W, Licht JD, Melnick A . Reversible disruption of BCL6 repression complexes by CD40 signaling in normal and malignant B cells. Blood 2008; 112: 644–651.
Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 2005; 7: 445–455.
Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 2001; 412: 341–346.
Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM et al. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 2004; 119: 75–86.
Moss SF, Blaser MJ . Mechanisms of disease: Inflammation and the origins of cancer. Nat Clin Pract Oncol 2005; 2: 90–97.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ et al. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol 2004; 164: 1131–1139.
Moss Steven F, Martin J . Blaser. Mechanisms of Disease: inflammation and the origins of cancer. Oncology 2005; 2: 90–97.
Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A et al. Asymmetric T lymphocyte Division in the initiation of adaptive immune responses. Science 2007; 315: 1687–1691.
Yi Q, Osterborg A, Bergenbrant S, Mellstedt H, Holm G, Lefvert AK . Idiotype-reactive T-cell subsets and tumor load in monoclonal gammopathies. Blood 1995; 86: 3043–3049.
Yi Q, Osterborg A . Idiotype-specific T cells in multiple myeloma: targets for an immunotherapeutic intervention. Med Oncol 1996; 13: 1–7.
Ostad M, Andersson M, Gruber A, Sundblad A . Expansion of immunoglobulin autoreactive T-helper cells in multiple myeloma. Blood 2008; 111: 2725–2732.
Acknowledgements
We thank Jing Zhang, Ying Li, Chunhuai Wang, Bo Lu and Huizhen Chen for preparing bone marrow and blood sample. We also thank Huiru Xu, Shan Huang, Xianfeng Zha, Shaohua Chen and Lijian Yang for administrative support of this work. This work was supported by a grant from the Nature Science Program Foundation of Guangdong Province (No. 06021319 and No. 8151008901000064).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Tian, F., li, J., Li, Y. et al. Enhancement of myeloma development mediated though myeloma cell-Th2 cell interactions after microbial antigen presentation by myeloma cells and DCs. Blood Cancer Journal 2, e74 (2012). https://doi.org/10.1038/bcj.2012.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2012.19