Abstract
Aberrant activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway has been reported to promote proliferation and survival of Hodgkin and Reed–Sternberg cells of Hodgkin lymphoma (HL). We investigated the activity of the JAK inhibitor AZD1480 in HL-derived cell lines and determined its mechanisms of action. AZD1480 at low doses (0.1–1 μM) potently inhibited STATs phosphorylation, but did not predictably result in antiproliferative effects, as it activated a negative-feedback loop causing phosphorylation of JAK2 and extracellular signal-regulated kinases 1 and 2 (ERK1/2), and increased IP-10, RANTES and interleukin (IL)-8 concentrations in the supernatants. Inhibition of the ERK activity by mitogen-activated extracellular signal regulated kinase (MEK) inhibitors (UO126 and PD98059) enhanced the cytotoxic activity of AZD1480. Interestingly, submicromolar concentrations of AZD1480 demonstrated significant immunoregulatory effects by downregulating T-helper 2 cytokines and chemokines, including IL-13 and thymus- and activation-regulated chemokine, and the surface expression of the immunosuppressive programmed death ligands 1 and 2. Higher concentrations of AZD1480 (5 μM) induced G2/M arrest and cell death by inhibiting Aurora kinases. Our study demonstrates that AZD1480 regulates proliferation and immunity in HL cell lines and provides mechanistic rationale for evaluating AZD1480 alone or in combination with MEK inhibitors in HL.
Similar content being viewed by others
Introduction
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway has been linked to the oncogenic process of a variety of cancers, including Hodgkin lymphoma (HL), making it an appealing target for a pathway-directed cancer therapy.1, 2, 3, 4 JAK/STAT activation is primarily driven by an aberrant deregulation of a network of cytokine and chemokines in the HL microenvironment (autocrine and paracrine loops involving a variety of cytokines such as, interleukin (IL)-6 and IL-13).1, 2, 4 In rare cases, genomic gains of JAK2 and inactivating mutations of suppressors of cytokine signaling (SOCS) proteins have also been linked to the JAK/STAT activation in HL.5, 6 After the cytokine receptor is engaged, members of the JAK family (JAK1, JAK2, JAK3 and tyrosine (Tyr) kinase 2 (TYK2)) are phosphorylated, and in turn, they phosphorylate downstream STAT proteins at Tyr residues. This leads to STAT proteins dimerization and translocation to the nucleus, where they trigger the transcription of target genes involved in cell proliferation and survival.7
Recent studies highlight the importance of the JAK-STAT pathway for mechanisms of immune escape in HL.8, 9 STAT6 activation in Hodgkin Reed–Sternberg cells (HRS) cells leads to the secretion of the immunosuppressive thymus- and activation-regulated chemokine (TARC/CCL17), with consequent attraction and homing of T-helper 2 (Th2) cells in areas surrounding the HRS cells and consequent impairment of immune response.8 Another mechanism of tumor immune evasion is the interaction between the programmed cell death 1receptor in tumor infiltrating T cells with its programmed death ligand (PD-L) 1 and 2 (PD-L1 (CD274, B7-H1) and PD-L2 (CD273, B7-DC)) expressed on the cell surface of a variety of tumor types, including HL, primary mediastinal B-cell lymphoma and anaplastic large T-cell lymphoma.9, 10, 11, 12 The engagement of programmed cell death 1 receptor by PD-L1 and PD-L2, leads to inhibition of T-cell function, promotes apoptosis of cytotoxic T cells and the induction of immunosuppressive T-regulatory cells, leading to a decrease in tumor killing.10 Recently, the JAK/STAT pathway has been shown to be involved in the regulation of PD-L1 and PD-L2 expression in HL and anaplastic large cell lymphoma cells.9, 11, 13
AZD1480 is a novel pyrazol pyrimidine ATP-competitive inhibitor of JAK1 and 2 kinases, with IC50's of 1.3 and <0.4 nM, respectively, in enzyme assays. AZD1480 has been shown to inhibit the STAT3 phosphorylation in vitro and in an in vivo xenograft model of human solid tumors and multiple myeloma.14, 15 At higher concentrations, AZD1480 has also been shown to inhibit other JAK family members and Aurora A kinase in purified enzyme assays.14 Because of the reported addiction of HL cells on JAK/STAT signaling pathway, we investigated the antiproliferative activity of AZD1480 in HL-derived cell lines and examined its mechanism of action with the aim to identify potential predictive molecular markers for response and resistance that can be validated in future in the clinical setting. We report that AZD1480 at low doses (0.1–1 μM) inhibited constitutive STATs phosphorylation in HL cell lines, demonstrating immunoregulatory effects as it downregulated the surface expression of the STAT3-target immunosuppressive cell-surface protein PD-L1 and PD-L2, in addition to downregulation of IL-13, IL-6 and TARC. However, inhibition of STATs phosphorylation resulted in significant antiproliferative activity in only one cell line. In the resistant cell lines, AZD1480 paradoxically activated extracellular signal-regulated kinases 1 and 2 (ERK1/2) and increased the secretion of the chemokines interferon γ-induced protein 10 kDa (IP-10), RANTES and IL-8.
When higher doses (5 μM) were used, its antiproliferative activity was independent of STATs inhibition and due to inhibition of Aurora kinases.
Collectively, these data demonstrate that AZD1480 has a dual mechanism of action, as it regulates immunity and proliferation in HL cell lines. Furthermore, these results provide a framework for investigating AZD1480 alone or in combination with ERK inhibitors in HL.
Materials and methods
Cell lines
The human HRS-derived cell lines HD-LM2, L-428, KM-H2 and L-540 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures (Braunschweig, Germany) in 2009, and were tested and authenticated before using them by the MD Anderson Characterized Cell Lines Core Facility. The phenotypes and genotypes of these cell lines have been previously described.16 The L-428 and KM-H2 cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (GIBCO BRL, Gaithersburg, MD, USA), 1% L-glutamine and penicillin–streptomycin in a humid environment of 5% CO2 at 37 °C. The HD-LM2 and L-540 cell lines were cultured in RPMI 1640 medium supplemented with 20% heat-inactivated fetal bovine serum. Peripheral blood samples were obtained from three healthy donors and peripheral blood mononuclear cells (PBMCs) were isolated from these samples. The protocol was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center; informed consent was obtained from all donors.
Reagents and antibodies
The JAK2 inhibitor AZD1480 was obtained from AstraZeneca, Inc. (Waltham, MA, USA). Nocodazole was purchased from Sigma-Aldrich (St Louis, MO, USA), MG132 was purchased from EMD Chemicals (San Diego, CA, USA), and the mitogen-activated extracellular signal regulated kinase (MEK) inhibitors UO126 and PD98059 were purchased from Cell Signaling Technology (Beverly, MA, USA). For western blotting, antibodies to the following were purchased from Cell Signaling Technology: p-JAK1 (Y1022/1023), JAK1, p-JAK2 (Y1007/1008), JAK2, JAK3, p-TYK2 (Y1054/1055), TYK2, STAT proteins (p-STAT1; Y701), STAT1, p-STAT3 (Y705), STAT3, p-STAT5 (Y694), STAT5, p-STAT6 (Y641), STAT6, p-ERK (Thr 202, Y204), ERK, p-Aurora A (Thr 288), Aurora A, Aurora B, histone H3, caspase 9, cleaved caspase 3, poly (adenosine diphosphate ribose) polymerase, SOCS-3, p-p38 (Thr 180, Y182), p38, p-SHP-2 (Y542) and SHP-2. Antibody to p-JAK2 (Y1007/1008)* was also purchased from Abcam (Cambridge, MA, USA), antibody to p-JAK3 (Y980) from Santa Cruz Biotechnology (Santa Cruz, CA, USA), antibody to p-histone H3 (Ser 10) from Millipore (Temecula, CA, USA) and antibody to β-actin from Sigma. For flow cytometry staining, antibodies to PD-L1 (PE-conjugated B7-H1) were purchased from eBioscience (San Diego, CA, USA) and to PD-L2 (PE-conjugated B7-DC) were from BD Pharmingen (San Jose, CA, USA).
In-vitro proliferation assay
Cells were cultured in 24-well plates at 0.5 × 106 cells/ml. Cell viability was assessed with the nonradioactive cell proliferation MTS [3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium] assay by using the CellTiter 96 AQueous One Solution Reagent (Promega, Madison, WI, USA) as previously described.8 Each measurement was made in triplicate and the mean value was determined. Each results is the mean of three independent experiments.
Western blotting
Preparation of cellular protein lysates, protein quantitation and western immunoblotting were performed as previously described.17, 18
Flow cytometry
Apoptosis was determined by using the Annexin V–FITC apoptosis detection kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. Cell cycle fractions were determined by propidium iodide nuclear staining, as previously described.8 For PD-L1 and PD-L2 expression, after incubation with AZD1480 1 μM for 72 h, cells were harvested, washed twice in cold phosphate-buffered saline, 1% bovine serum albumin, and stained with anti-B7-H1, B7-DC or mouse IgG1 isotype control antibodies PE-conjugated for 20 min. The stained cells were washed twice in cold phosphate-buffered saline, fixed in 1% paraformaldehyde-phosphate-buffered saline and then applied to the flow cytometer. A total of 20 000 events were analyzed. Data were collected on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), using FlowJo software (Tree Star, Ashland, OR, USA) as described previously.8 Each result is the mean of three independent experiments. Bar graphs show the mean of three independent experiments±s.e.m.
Measurement of cytokines and chemokines
HL cell lines were incubated with AZD1480 (1 μM) or dimethyl sulfoxide (0.1%) for 72 h. Supernatants were then collected and examined for production of IL-13, IL-6, IP-10, RANTES and IL-8 by enzyme-linked immunosorbent assay, by using the human cytokine 30-plex panel (Invitrogen, Carlsbad, CA, USA) and for production of TARC by using the Human TARC ELISA kit (RD Systems, Minneapolis, MN, USA), according to the manufacturers’ instructions. Each experiment was performed in triplicate, and each result is the mean of three independent experiments±s.e.m.
Statistical analyses
The effectiveness of the drugs used in this study, alone and in combination, was analyzed by using CalcuSyn software (Biosoft, Ferguson, MO, USA). The combination index (CI) was calculated according to the Chou–Talalay method.17, 18 CI=1 indicates an additive effect of two drugs, CI <1 indicates a synergistic effect, and CI >1 indicates antagonism between the two drugs. Procedures to determine the effects of certain conditions on cell proliferation, cell cycle, apoptosis and cytokine production were performed in three independent experiments. The two-tailed Student's t-test was used to estimate the statistical significance of the differences between results from the three experiments. Significance was set at P<0.05.
Results
Expression of activated JAK2 in cultured HL cells
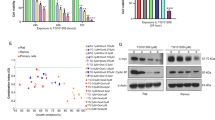
To explore the potential therapeutic value of the JAK2 inhibitor AZD1480 in HL, we initially examined the expression pattern of its protein target, the active, phosphorylated form of JAK2 Y1007/1008, in cultured HL cells. We hypothesized that cells with high levels of p-JAK2 would be more sensitive to the antiproliferative effect of AZD1480 than cells with lower levels of p-JAK2. We found p-JAK2 to be expressed in two of the four HL cell lines (Figure 1a). None of the HL cell lines expressed p-JAK1 Y1022/1023, two cell lines expressed p-TYK2 Y1054/1055, and only one cell line (L-540) expressed p-JAK3 Y980 (Figure 1a). Thus, the activation pattern of the JAK family members is rather heterogeneous in the HL cell lines.
Baseline JAK/STAT pathway activation status and effects of AZD1480 in HL cell lines. (a) Baseline activation of the JAK/STAT pathway in the HL cell lines HD-LM2, L-428, KM-H2 and L-540. PBMCs from three healthy donors were used for comparison. (b) Western blot assay of the four HL cell lines treated with increasing doses of AZD1480 (0.1–5 μm) for 72 h. (c) MTS assay of the four HL cell lines treated with AZD1480. Cells were incubated with increasing concentrations of AZD1480 (0.1–10 μm) for 24, 48 and 72 h. The value for each cell line is the mean of three independent experiments performed in triplicate. Error bars represent s.e.m. (d) IC50 values after HL cell lines were incubated with increasing doses of AZD1480 for 72 h.
Next, we investigated the expression pattern of the active phosphorylated form of downstream (p-STATs). We found p-STAT3, p-STAT5 and p-STAT6 to be expressed in the three cell lines that expressed at least one active phosphorylated member of the JAK family: HD-LM2, L-428 and L-540 (Figure 1a). Consistent with a previous report, the KM-H2 cell line did not express p-JAKs or p-STAT3, pSTAT5 or pSTAT6 proteins;19 neither did the three samples of PBMCs isolated from healthy donors. We found p-STAT1 to be expressed in all HL cell lines and in only one PBMC sample from a healthy donor.
AZD1480 inhibits STAT3, STAT5 and STAT6 phosphorylation in HL cells
The differential expression of JAK/STAT family members in HL cell lines gave us an opportunity to determine the biological effect of AZD1480 in cells that have thee different patterns of expression: HD-LM2 and L-428 (which expressed p-JAK2 and p-TYK2), L-540 (which expressed p-JAK3), and KM-H2 (which lacked p-JAKs expression). Increasing concentrations of AZD1480 (0.1–5 μM) inhibited STAT1, STAT3, STAT5 and STAT6 phosphorylation in all three cell lines that showed evidence of full JAK/STAT activation: HD-LM2, L-428 and L-540 (Figure 1b). These results suggest that in addition to JAK2, AZD1480 may also inhibit JAK3 activity in HL cell lines. Potent inhibition of STAT3 phosphorylation was observed as soon as after 30 min of incubation with AZD1480 and was maintained up to 72 h (Supplementary Figure S1A and B).
Antiproliferative effects of AZD1480 in HL cell lines
Previous studies have demonstrated that HL cell lines are addicted to the JAK/STAT pathway, and selective inhibition of STAT proteins by RNA interference has been shown to result in antiproliferative effects in HL cell lines.2 Because AZD1480 inhibited p-STAT3, p-STAT5 and p-STAT6, we investigated the antiproliferative effect of AZD1480 in HL cells by using the MTS assay. AZD1480 induced antiproliferative effects in a time- and dose-dependent manner (Figure 1c). After 72 h of incubation, the half maximal inhibitory concentration (IC50) values for AZD1480 ranged from 1 to 8 μM; L-540 cells were the most sensitive to AZD1480 (Figure 1c and d). Thus, although submicromolar concentrations of AZD1480 inhibited STAT phosphorylation, these low concentrations were not sufficient to induce a significant antiproliferative activity, especially in the HD-LM2 and L-428 cells. In contrast, at a higher concentration (5 μM), AZD1480 had a stronger effect in all cell lines, including KM-H2, which lacked active JAK/STAT proteins. These data suggest that at this higher concentration (5 μM), AZD1480 induced antiproliferative effects in HL cell lines in a JAK/STAT-independent manner.
To further investigate the mechanisms of the antiproliferative activity of AZD1480, we determined the inhibitor's effect on apoptosis. Consistent with the MTS data in Figure 1c, we found that AZD1480 induced apoptotic cell death in a dose-dependent manner (Figure 2a and b). At the lower concentration of AZD1480 (1 μM), L-540 cells were the most sensitive (43.3% cell death after 72 h of incubation) and L-428 cells were modestly sensitive (27.6% cell death). At the higher concentration (5 μM), AZD1480 induced apoptosis in all four cell lines, but L-540 cells remained the most sensitive. Consistent with induction of apoptosis, AZD1480 activated caspases 9 and 3, and induced poly (adenosine diphosphate ribose) polymerase cleavage. (Figure 2c). This was observed as soon as after 24 h of incubation with AZD1480 in all cell lines, when high doses (5 μM) were used (Supplementary Figure S1C).
AZD1480 induces apoptosis in HL cell lines. (a) Representative experiment demonstrating the effect of two different doses of AZD1480 (1 or 5 μm for 72 h) on apoptosis as determined by annexin V-binding assay. The percentage of dead cells is shown in the upper right quadrant. (b) Summary of the results of dual annexin V and propidium iodide (PI) staining. Each value is the mean of three independent experiments performed in triplicate. *P<0.05; **P<0.005; NS, not significant. Error bars represent s.e.m. (c) Immunoblotting showing activation of the intrinsic apoptotic pathway in HL cell lines incubated with AZD1480 (1–5 μm) for 72 h. Consistent with the data in (a) and (b), cleavage of poly (adenosine diphosphate ribose) polymerase (PARP) and activation of caspases 9 and 3 were observed in all the cell lines exposed to 5 μm AZD1480. In L-540 and L-428, caspase cleavage was observed also with 1 μm AZD1480.
AZD1480 induces paradoxical hyperphosphorylation of JAK2 and TYK2 at the activation loop in HL cells
Although AZD1480 inhibited phosphorylation of STATs, its effect on the phosphorylation of the JAK family members in HL cells is unknown. Only the Tyr-phosphorylated forms of JAKs are known to exhibit kinase activity, and phosphorylation at two tandem Tyr residues in the activation loop appears to be required for enzymatic activity.20 As levels of baseline p-JAK2 expression and p-STATs inhibition did not predict sensitivity to AZD1480 in HD-LM2 and L-428 cells, we investigated the effect of AZD1480 on the phosphorylation status of the JAK family members at the activation loop. To determine the effect of AZD1480 on p-JAKs, we focused our experiments on the three cell lines that expressed active JAK/STAT proteins: HD-LM2, L-428 and L-540 (Figure 3a). In L-540, which was the most sensitive cell line and which expressed only p-JAK3, increasing concentrations of AZD1480 (0.1–5 μM) completely inhibited JAK3 Y980 phosphorylation. In contrast, when HD-LM2 and L-428 cells were incubated with AZD1480, a paradoxical increase in JAK2 Y1007/1008 and TYK2 Y1054/1055 phosphorylation was observed after 72 h of incubation (Figure 3a).
AZD1480 induces paradoxical hyperphosphorylation of JAK2, TYK2 and MAP Kinases (ERK, p38) in HL cells. (a) Representative western blot assay of the three HL cell lines treated with increasing concentrations of AZD 1480 (0.1–5 μm for 72 h). Whole-cell lysates examined for p-JAK2 (two antibodies against Y1007/1008 at the activation loop obtained from different clones (see Materials and Methods)), JAK2, p-TYK2 Y1054/1055, TYK2, p-JAK3 Y980 and JAK3. (b) Representative western blot assay showing increased levels of ERK, p38 and SHP-2 phosphorylation in HD-LM2 and L-428, after treatment for 72 h with increasing doses of AZD1480 (0.1–5 μm). In contrast, L-540 showed a dose-dependent inhibition of ERK and p38 activation without increased SHP-2 phosphorylation. SOCS-3 levels decreased in all the cell lines. (c) After incubation with 1 μm AZD1480 for 72 h, cell culture supernatants were analyzed for IL-8, IP-10 and RANTES levels by enzyme-linked immunosorbent assay. In the bar graphs, each value is the mean of three independent experiments performed in triplicate. *P<0.05; **P<0.005; NS, not significant. (d) Effect of MEK inhibitors used with or without AZD1480 in HL cells. Combination index analysis performed using CalcuSyn software, showing that AZD1480 (1 μm) acted synergistically (CI <1) with UO126 or PD98059 (10–100 μm) in HD-LM2 and L-428 cells at 72 h. No synergistic effect was observed in L-540 cells. (e) Representative western blot assay of HL cells after treatment with 25-μm UO126 or PD98059 with or without 1-μm AZD1480 for 72 h.
The hyperphosphorylation of JAK2 at the activation loop was confirmed using two antibodies obtained from different clones. The phosphorylation of the immediate downstream target STAT3 was abrogated at the same time point in all the three cell lines (Figure 1b), suggesting that the function of the JAKs was effectively inhibited by AZD1480.
When JAKs phosphorylation was analyzed over a shorter time period, a strong increase in JAK2 Y1007/1008 phosphorylation was observed in HD-LM2 and L-428 cells after 30 min of incubation with 1 μM AZD1480 (Supplementary Figure S1A), whereas JAK3 Y980 phosphorylation was abrogated in L-540 cells (Supplementary Figure S1A). The phosphorylation of the immediate downstream target STAT3 was abrogated at the same time point (30 min) in all the three cell lines, suggesting that the function of the JAKs was effectively inhibited after 30 min of incubation with AZD1480 (Supplementary Figure S1A).
AZD1480 induces a negative-feedback loop involving phosphorylation ERK1/2 and p38
As AZD1480 inhibition of STATs activity did not translate into antiproliferative activity in two cell lines, we hypothesized that these cells may depend on other signaling pathways to promote their survival. To test this hypothesis, we examined the effect of AZD1480 on ERK, p38 and phosphatidylinositol-3 kinase/AKT signaling pathways, which are known to promote HL survival. We found that AZD1480 had no effect on phosphatidylinositol-3 kinase/AKT signaling in any of these cell lines (data not shown). In contrast, AZD1480 increased the levels of ERK and p38 phosphorylation in the resistant HD-LM2 and L-428 cells, whereas it inhibited ERK and p38 phosphorylation in the sensitive L-540 cells (Figure 3b). To define the mechanism of ERK and p38 activation in the two resistant cell lines, we first assessed the expression and activation levels of the Src homology 2 domain-containing protein phosphatase 2 (SHP-2) and SOCS-3 (a STAT3 target gene21), two known regulators of JAK/STAT and mitogen-activated protein kinase (MAPK) signaling. In fact, SHP-2 has been reported to be a negative regulator of the JAK/STAT activation, whereas having a positive effect on ERK activation.22, 23, 24, 25 Furthermore, numerous studies have described enhanced SHP-2 and ERK activation after SOCS-3 deletion in both in vitro and in vivo models, postulating a negative-feedback loop between SOCS-3 and SHP-2.22, 23, 26 Consistent with the described changes in ERK phosphorylation status, after 72 h of incubation with increasing doses of AZD1480 (0.1–5 μM), an increase in SHP-2 phosphorylation on Tyr542 (the major site of SHP-2 activation) coupled with SOCS3 downregulation was observed in the HD-LM2 and L-428 cell lines, but not in the L-540 cells (Figure 3b). On the other hand, in the L-540 cells, SOCS3 downregulation was not coupled with SHP-2 hyperphosphorylation, and ERK phosphorylation was in fact inhibited after treatment with AZD1480, suggesting a different model of MAPK activation in this cell line. The activation of p38 signaling observed in the HD-LM2 and L-428 cell lines after incubation with AZD1480 was probably related to the activity of autocrine and paracrine chemokine and cytokine loops, triggered by SHP-2/ERK activation. Over a shorter time period, we observed a strong correlation between SOCS-3 downregulation and SHP-2/ERK activation in the HD-LM2 cell line after 6 h of incubation with AZD1480, whereas in the L-540 cell line, ERK phosphorylation was inhibited without an increase in SHP-2 phosphorylation or a decrease in SOCS-3 levels (Supplementary Figure S1D).
AZD1480 determines increased production of the chemokines IP-10, IL-8 and RANTES in HL cells
The interaction between HRS cells and the surrounding reactive infiltrate provides an environment that supports the growth and survival of HRS cells through a complex network of autocrine and paracrine loops involving a variety of cytokines and chemokines.1, 27 The chemokines IP-10, RANTES and IL-8 are known to be upregulated by MAPK (ERK and p38) activation and have previously been reported to have a role in tumorigenesis and Hodgkin lymphomagenesis by promoting cell proliferation and survival.1, 27, 28, 29, 30, 31 Consistent with the observed changes in SHP2, ERK and p38 phosphorylation, AZD1480 increased the production of IP-10, RANTES and IL-8 in the supernatants of the resistant HD-LM2 and L-428, and not the sensitive L-540 cells. (Figure 3c). This data suggest that the efficacy of AZD1480 may have been attenuated in the HD-LM2 and L-428 cells by an autocrine negative-feedback loop involving cytokines that activate ERK/p38 survival signaling pathway.
MEK inhibitors enhance the efficacy of AZD1480 in the HD-LM2 and L-428 cell lines
To assess whether the observed activation of the SHP-2/ERK pathway is involved in the resistance to JAK/STAT inhibition, HL cells were treated with 1 μM AZD1480 in combination with two commercially available MEK inhibitors (UO126 and PD98059). Cells were incubated with increasing concentrations of UO126 or PD98059 (10–100 μM), with or without 1 μM AZD1480 for 24, 48 and 72 h, and cell viability was assessed using the MTS assay. Both UO126 and PD98059 enhanced the effect of AZD1480 in the HD-LM2 and L-428 cells at 72 h (Figure 3d). This effect was associated with inhibition of AZD1480-induced ERK phosphorylation (Figure 3e).
Immunoregulatory effects of AZD1480 in HL cells
PD-L1 and PD-L2 are members of the B7 family, which inhibit T-cell function by interacting with the programmed cell death 1 receptor expressed on T cells. PD-L1 and PD-L2 have been reported to be regulated by the JAK/STAT pathway or the MAPK pathway, depending on the cellular context.9, 11, 13
Therefore, we assessed the effect of AZD1480 on PD-L1 and PD-L2 expression in HL cells by flow cytometry. After incubation with AZD1480 1 μM for 72 h, we observed a significant downregulation of PD-L1 and PD-L2 in the PD-L1/PD-L2-positive HL cell lines HD-LM2 and L-540 (Figure 4a and b). No significant dowregulation was detected following incubation with MEK inhibitors (UO126 and PD98059 25 μM for 72 h) in the L-540 cell line (data not shown). On the other hand, in the HD-LM2 cell line, an increase in the activation of ERK was observed after incubation with AZD1480, ruling out a role of this pathway in the regulation of PD ligands expression in this cell line (Figure 3b).
Immunoregulatory effects of AZD1480 in HL cells. (a) Representative experiment demonstrating the effect of AZD1480 on PD-L1 and PD-L2 expression in HD cell lines. Cells were incubated with medium or AZD1480 1 μm for 72 h, and PD-L1 and PD-L2 expression levels were examined by flow cytometry. The bar graphs summarize the results from three independent experiments. Each value represents the mean of three independent experiments. *Denotes P-value less than 0.05, and **denotes P-value less than 0.005. (b) Cells were incubated with medium or AZD1480 1 μm for 72 h, and supernatants were examined for cytokine levels by enzyme-linked immunosorbent assay. The bar graphs summarize the results from three independent experiments. Each value represents the mean of three independent experiments. *Denotes P-value less than 0.05 and **denotes P-value less than 0.005.
Given the potent inhibition of STAT proteins phosphorylation observed with low doses AZD1480 (0.1–1 μM), we also assessed the effect of AZD1480 on the expression of STAT-regulated cytokines and chemokines involved in HL cell survival (IL-6) and immune escape (IL-13, TARC), focusing on the three cell lines with baseline JAK/STAT activation. Following incubation with AZD1480 1 μM for 72 h, cell culture supernatants were analyzed by enzyme-linked immunosorbent assay; consistent with the inhibition of STAT3 and STAT6 phosphorylation, decreased levels of IL-6, IL-13 and TARC were observed in HD-LM2, L-428 and L-540 (Figure 4b).
High concentrations of AZD1480 promote early G2/M arrest and cell death in HL cells by inhibiting Aurora kinases
Higher concentrations (5 μM) of AZD1480 promoted a marked increase in the G2/M fraction in all four cell lines after 24 h of incubation, especially pronounced in HD-LM2 and L-428 cells (Figure 5a and b). In contrast, treatment with a lower AZD1480 concentration (1 μM) for 24 h did not significantly affect the cell cycle fractions (Figure 5a and b). As AZD1480 was reported to inhibit Aurora A kinase in enzymatic assays,14 we assessed whether the significant increase in the G2/M fraction observed in HL cell lines after incubation with 5 μM AZD1480 was related to the inhibition of Aurora A.
AZD1480 induces G2/M cell cycle arrest by inhibition of Aurora A in HL cell lines. (a) Cells were incubated with AZD1480 (1 or 5 μm) for 24 h, and the cell cycle was analyzed by flow cytometry. AZD1480 induced an increase in the G2/M fraction only when a high concentration (5 μm) was used. (b) Bar graphs summarizing cell cycle analysis results; each value is the mean of three independent experiments. (c) Baseline expression status of Aurora kinases and histone H3 in HL cell lines. Whole-cell lysates of untreated HL cells were examined by western blotting for Aurora A, Aurora B, histone H3 and p-histone H3 (Ser10). (d) Representative western blot assay showing the effect of treatment with 1 and 5 μm AZD1480 (with or without MG132 20 μm) for 3 h on Aurora A, Aurora B and histone H3 phosphorylation (Ser 10) in HL cells. Cells were pretreated with nocodazole 400 ng/ml for 18 h to achieve a mitotic block.
First, we analyzed the baseline expression of Aurora A and B kinases in exponentially growing HL cells by western blotting. All four cell lines showed overexpression of Aurora A and B, and histone H3, compared with PBMCs from healthy donors (Figure 5c). Aurora A kinase activity depends on autophosphorylation at Thr288 in the activation loop. Thus, to determine the effect of AZD1480 on Aurora A kinase, we evaluated the levels of autophosphorylation at Thr288 by western blotting. Cells were pretreated with nocodazole (400 ng/ml) for 18 h, to achieve a mitotic block, and then exposed to AZD1480 (1 or 5 μM) with or without the proteasome inhibitor MG132 (20 μM; to prevent a potential override of the nocodazole-induced mitotic block by proteasome-dependent mechanisms) for 3 h (Figure 5d). Dose-dependent inhibition of Aurora A was detected after 3 h of incubation in all the four cell lines, and Thr288 autophosphorylation was abrogated when a higher dose of AZD1480 (5 μM) was used (Figure 5d). These findings are consistent with the analysis of the cell cycle fractions, showing major dose-dependent changes in the cell cycle after incubation with AZD1480. In L-428 and L-540 cells, a dose-dependent inhibition of the phosphorylation of histone H3 at Ser10 was observed, suggesting a dual inhibition of Aurora A and B in these cell lines.
Discussion
In this study, we described the pleiotropic activity of AZD1480 in HL-derived cell lines, showing a dual mechanism of action: (1) a direct dose-dependent cytotoxic effect achieved by two independent molecular mechanisms (by targeting the JAK-STAT pathway (at low doses) and the Aurora kinases (at higher doses)), resulting in apoptosis and G2/M cell cycle arrest; (2) an indirect effect on tumor microenvironment achieved through impairment of mechanisms involved in survival and immune evasion, with inhibition of Th2 cytokine and chemokine secretion and downregulation of PD-L1 and PD-L2 expression (Figure 6).
Model for AZD1480 activity in HL cells. A model showing the dual, dose-dependent mechanism of action of AZD1480 in HL cells. At low doses (0.1–1 μm), AZD1480 inhibits the JAK/STAT pathway, showing predominantly immunoregulatory effects (downregulation of IL-6, IL-13, TARC, PD-L1 and PD-L2). At high doses (5 μm), AZD1480 also inhibits the Aurora kinases, promoting G2/M arrest and apoptosis in HL cells.
This study provided several observations that will be important for the development of JAK/STAT pathway-targeted therapy in HL. We demonstrated that the expression of active pJAK2 protein did not predict response to the JAK2 inhibitor AZD1480, and therefore, in the clinical setting, pJAK2 expression may not be a useful biomarker for selecting patients for AZD1480 therapy. Moreover, even though submicromolar concentrations of AZD1480 effectively inhibited the function of the target JAK2 protein, as evident by dephosphorylation of the downstream STAT proteins, these concentrations had no significant antiproliferative effect in the HD-LM2 and L-428 cell lines. Submicromolar concentrations of AZD1480 inhibited the phosphorylation of STAT1, STAT3, STAT5 and STAT6, with no apparent differential effect. This is in contrast with what was recently reported with selective silencing of STAT6 gene expression experiments, as it resulted in activation of STAT1 in the same cell line, which may have contributed to induction of cell death.32 At low concentrations (0.1–1 μM), AZD1480 displayed predominantly immunomodulatory effects, downregulating the expression of Th2 cytokines and chemokines (IL-13 and TARC), and factors involved in mechanisms of immune escape (PD-L1 and PD-L2). Collectively, these data suggest that AZD1480 may enhance anti-tumor immunity by increasing the activity of cytotoxic T cells (through downregulation of PD-L1 and PD-L2, and inhibition of an unfavorable Th2 cytokine profile).
Regarding the mechanism involved in the resistance of the HD-LM2 and L-428 cell lines to low doses of AZD1480, this may be related to a negative-feedback loop involving hyperphosphorylation of JAK2 and activation of secondary ERK and p38 signaling pathways that promote HL survival. In fact, even though the function of JAK2 was effectively inhibited as demonstrated by the abrogation of downstream STATs phosphorylation, we observed a paradoxical increase in the JAK2 and TYK2 phosphorylation status after incubation with AZD1480. Although the mechanism of AZD1480-induced JAK2 phosphorylation is currently unclear, it may be related to the conformational changes and/or induction of negative-feedback loops involving activating cytokines. Similar results were previously reported by Okuzumi et al.33, who described paradoxical hyperphosphorylation of AKT after treatment with an ATP-competitive kinase inhibitor. On the other hand, our data suggest that AZD1480-induced ERK and p38 phosphorylation may involve two major regulators of the JAK and MAPK pathways: SOCS-3 (a known STATs target gene)21 and SHP-2 (a protein phosphatase known to activate ERK signaling).24 According to other reports showing sustained SHP-2 and ERK activation after SOCS-3 deletion in various in vitro and in vivo models,22, 23, 25 we observed a similar regulation of MAPK signaling, at least in the HD-LM2 and L-428 cells that were resistant to AZD1480. These observations are consistent with a model in which SOCS-3, JAK2 and SHP-2 are reciprocally regulated as previously reported.22 It is important to note that these molecular negative-feedback loop events were associated with an increase in the level of IL-8, IP-10 and RANTES cytokines in HL cell lines supernatants. As RANTES may activate and phosphorylate JAK2 and TYK2, it is possible that the increased baseline levels of pJAK2 and pTYK2 in the HDLM2 and L428 cells, but not L-540 cells, may be related to the differential production of RANTES and other cytokines among these cell lines. Similarly, an increased secretion of these cytokines, such as RANTES, in response to AZD1480 treatment, may have also contributed to the observed JAK2 hyperphosphorylation, following incubation with AZD1480.34 This observation should be further investigated in the clinical setting to determine whether changes in the levels of certain cytokines in serial plasma specimens may serve as a surrogate biomarker for resistance to AZD1480 therapy in HL patients. The fact that AZD1480 synergized with two different MEK inhibitors (UO126 and PD98059) in the resistant cell lines characterized by AZD1480-induced ERK hyperactivation strongly supports the hypothesis that ERK hyperactivation may be an important mechanism of resistance to the JAK inhibition in HL.
In this study, we show that higher concentrations of AZD1480 inhibited Aurora kinases and induced G2/M cell cycle arrest and cell death in all HL cell lines, irrespective of JAK/STAT pathway status. Aurora A has a crucial role in mitotic bipolar spindle formation and localizes to centrosomes at the proximal mitotic spindle, whereas Aurora B localizes to kinetochores, phosphorylates histone H3 at Ser10, and has a role in chromosome alignment and cytokinesis.35 Inhibition of Aurora kinases promotes G2/M arrest and apoptosis in multiple human cancers including lymphoid malignancies (multiple myeloma and adult T-cell leukemia),36, 37, 38 but no data are available, which suggest a role of Aurora kinases in Hodgkin lymphomagenesis. We found that Aurora A and B kinases were overexpressed in the four HL cell lines compared with PBMCs from healthy donors. AZD1480 promoted a dose-dependent inhibition of Aurora A autophosphorylation at Thr288 in all the four HL cell lines. We observed a tight correlation between the dose-dependent inhibition of Aurora A and the changes observed in cell cycle fraction and apoptosis in HL cells. A dose-dependent inhibition of histone H3 phosphorylation at ser 10 was detected in two cell lines (L-428 and L-540), suggesting that high doses of AZD1480 may also inhibit Aurora B in these cell lines. Due to the fact that Reed–Sternberg cells account for less than 5% of the entire tumor mass, being very rare in the affected lymph nodes, we were not able to microdissect viable primary HRS cells from patients’ lymph nodes to perform in vitro viability and functional assays.
However, our data clearly demonstrate that AZD1480 inhibits JAK/STAT activation in cultured HL cells at submicromolar concentrations, by blocking the function of JAKs (including JAK3) and determining immunomodulatory effects. Moreover, the two cell lines (HD-LM2 and L-428), which showed MAP kinase hyperactivation following treatment with AZD1480, were resistant to the drug at concentrations clearly able to inhibit STATs phosphorylation. The fact that different MEK inhibitors synergized with AZD1480 in these two resistant cell lines, suggest that this negative-feedback loop activating MAP kinases could be an important mechanism of resistance to AZD1480.
In summary, our results provide preclinical rationale for further clinical investigation of AZD1480 in HL and provide molecular rationale for incorporating biomarker studies according to the primary target inhibition (JAK/STAT vs Aurora kinases). Furthermore, our data demonstrate the importance of evaluating the in vivo effect of small molecule inhibitors on secondary signaling pathways that may mediate resistance to therapy and provide informations on combination strategies.
In fact, these data could be tested in the clinical setting by performing sequential biopsies from patients treated with AZD1480, to determine its in vivo effect on JAK2, ERK, p38 and Aurora A, and to correlate the phosphorylation status of these proteins with the response to AZD1480 therapy. Finally, these data provide a mechanistic rationale for combination strategies aiming at blocking the AZD1480-induced activation of ERK and p38.
References
Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A . The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol 2010; 221: 248–263.
Baus D, Pfitzner E . Specific function of STAT3, SOCS1, and SOCS3 in the regulation of proliferation and survival of classical Hodgkin lymphoma cells. Int J Cancer 2006; 118: 1404–1413.
Holtick U, Vockerodt M, Pinkert D, Schoof N, Sturzenhofecker B, Kussebi N et al. STAT3 is essential for Hodgkin lymphoma cell proliferation and is a target of tyrphostin AG17 which confers sensitization for apoptosis. Leukemia 2005; 19: 936–944.
Skinnider BF, Mak TW . The role of cytokines in classical Hodgkin lymphoma. Blood 2002; 99: 4283–4297.
Kuppers R . The biology of Hodgkin's lymphoma. Nat Rev Cancer 2009; 9: 15–27.
Rui L, Emre NCT, Kruhlak MJ, Chung H-J, Steidl C, Slack G et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell 2010; 8: 590–605.
Levy DE, Darnell Jr JE . Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3: 651–662.
Buglio D, Georgakis GV, Hanabuchi S, Arima K, Khaskhely NM, Liu YJ et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood 2008; 112: 1424–1433.
Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O′Donnell E et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116: 3268–3277.
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206: 3015–3029.
Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci USA 2008; 105: 20852–20857.
Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 2008; 111: 3220–3224.
Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A et al. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci 2009; 100: 2093–2100.
Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A et al. The JAK2 Inhibitor AZD1480 Potently Blocks Stat3 Signaling and Oncogenesis in Solid Tumors. Cancer Cell 2009; 16: 487–497.
Scuto A, Krejci P, Popplewell L, Wu J, Wang Y, Kujawski M et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia 2011; 25: 538–550.
Drexler HG . Recent results on the biology of Hodgkin and Reed-Sternberg cells. II. Continuous cell lines. Leuk Lymphoma 1993; 9: 1–25.
Georgakis GV, Li Y, Rassidakis GZ, Martinez-Valdez H, Medeiros LJ, Younes A . Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin′s lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin Cancer Res 2006; 12: 584–590.
Zheng B, Fiumara P, Li YV, Georgakis G, Snell V, Younes M et al. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood 2003; 102: 1019–1027.
Cochet O, Frelin C, Peyron J-F, Imbert V . Constitutive activation of STAT proteins in the HDLM-2 and L540 Hodgkin lymphoma-derived cell lines supports cell survival. Cell Signal 2006; 18: 449–455.
Chatti K, Farrar WL, Duhe RJ . Tyrosine phosphorylation of the Janus kinase 2 activation loop is essential for a high-activity catalytic state but dispensable for a basal catalytic state. Biochemistry 2004; 43: 4272–4283.
Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997; 387: 917–921.
Boyle K, Zhang JG, Nicholson SE, Trounson E, Babon JJ, McManus EJ et al. Deletion of the SOCS box of suppressor of cytokine signaling 3 (SOCS3) in embryonic stem cells reveals SOCS box-dependent regulation of JAK but not STAT phosphorylation. Cell Signal 2009; 21: 394–404.
Kamezaki K, Shimoda K, Numata A, Haro T, Kakumitsu H, Yoshie M et al. Roles of Stat3 and ERK in G-CSF signaling. Stem Cells 2005; 23: 252–263.
Dance M, Montagner A, Salles JP, Yart A, Raynal P . The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 2008; 20: 453–459.
Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity 2000; 12: 95–105.
Forrai A, Boyle K, Hart AH, Hartley L, Rakar S, Willson TA et al. Absence of suppressor of cytokine signalling 3 reduces self-renewal and promotes differentiation in murine embryonic stem cells. Stem Cells 2006; 24: 604–614.
Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A et al. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer 2008; 122: 769–776.
Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res 2006; 66: 9509–9518.
Waugh DJ, Wilson C . The interleukin-8 pathway in cancer. Clin Cancer Res 2008; 14: 6735–6741.
Li A, Dubey S, Varney ML, Dave BJ, Singh RK . IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 2003; 170: 3369–3376.
Moriai S, Takahara M, Ogino T, Nagato T, Kishibe K, Ishii H et al. Production of interferon-{gamma}-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res 2009; 15: 6771–6779.
Baus D, Nonnenmacher F, Jankowski S, Doring C, Brautigam C, Frank M et al. STAT6 and STAT1 are essential antagonistic regulators of cell survival in classical Hodgkin lymphoma cell line. Leukemia 2009; 23: 1885–1893.
Okuzumi T, Fiedler D, Zhang C, Gray DC, Aizenstein B, Hoffman R et al. Inhibitor hijacking of Akt activation. Nat Chem Biol 2009; 5: 484–493.
Wong M, Uddin S, Majchrzak B, Huynh T, Proudfoot AE, Platanias LC et al. Rantes activates Jak2 and Jak3 to regulate engagement of multiple signaling pathways in T cells. J Biol Chem 276: 11427–11431.
Carmena M, Earnshaw WC . The cellular geography of aurora kinases. Nat Rev Mol Cell Biol 2003; 4: 842–854.
Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood 2010; 115: 5202–5213.
Tomita M, Mori N . Aurora A selective inhibitor MLN8237 suppresses the growth and survival of HTLV-1-infected T-cells in vitro. Cancer Sci 2010; 101: 1204–1211.
Tomita M, Tanaka Y, Mori N . Aurora kinase inhibitor AZD1152 negatively affects the growth and survival of HTLV-1-infected T lymphocytes in vitro. Int J Cancer 2010; 127: 1584–1594.
Acknowledgements
We thank Karen Muller for editorial assistance. This work was supported in part by the National Institutes of Health/NCI SPORE Grant 1P50CA136411-01A1, the MD Anderson's Cancer Center Support Grant CA016672, the Clay Chiles Lymphoma Fund, and the Jack L Stotsky Memorial Fund (all to AY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Derenzini, E., Lemoine, M., Buglio, D. et al. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer Journal 1, e46 (2011). https://doi.org/10.1038/bcj.2011.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2011.46
Keywords
This article is cited by
-
Pathological α-synuclein recruits LRRK2 expressing pro-inflammatory monocytes to the brain
Molecular Neurodegeneration (2022)
-
Interleukin-6 promotes primitive endoderm development in bovine blastocysts
BMC Developmental Biology (2021)
-
Regulation of low-density lipoprotein receptor expression in triple negative breast cancer by EGFR-MAPK signaling
Scientific Reports (2021)
-
A natural compound derivative P-13 inhibits STAT3 signaling by covalently inhibiting Janus kinase 2
Investigational New Drugs (2019)
-
Targeting JAK kinase in solid tumors: emerging opportunities and challenges
Oncogene (2016)