Abstract
Xyloketal B (Xyl-B) is a novel marine compound isolated from mangrove fungus Xylaria sp. (No 2508). We previously showed that Xyl-B promoted endothelial NO release and protected against atherosclerosis through the Akt/eNOS pathway. Vascular NO production regulates vasoconstriction in central and peripheral arteries and plays an important role in blood pressure control. In this study, we examined whether Xyl-B exerted an antihypertensive effect in a hypertensive rat model, and further explored the possible mechanisms underlying its antihypertensive action. Administration of Xyl-B (20 mg·kg−1·d−1, ip, for 12 weeks) significantly decreased the systolic and diastolic blood pressure in a two-kidney, two-clip (2K2C) renovascular hypertensive rats. In endothelium-intact and endothelium-denuded thoracic aortic rings, pretreatment with Xyl-B (20 μmol/L) significantly suppressed phenylephrine (Phe)-induced contractions, suggesting that its vasorelaxant effect was attributed to both endothelial-dependent and endothelial-independent mechanisms. We used SNP, methylene blue (MB, guanylate cyclase inhibitor) and indomethacin (IMC, cyclooxygenase inhibitor) to examine which endothelial pathway was involved, and found that MB, but not IMC, reversed the inhibitory effects of Xyl-B on Phe-induced vasocontraction. Moreover, Xyl-B increased the endothelial NO bioactivity and smooth muscle cGMP level, revealing that the NO-sGC-cGMP pathway, rather than PGI2, mediated the anti-hypertensive effect of Xyl-B. We further showed that Xyl-B significantly attenuated KCl-induced Ca2+ entry in smooth muscle cells in vitro, which was supposed to be mediated by voltage-dependent Ca2+ channels (VDCCs), and reduced ryanodine-induced aortic contractions, which may be associated with store-operated Ca2+ entry (SOCE). Taken together, these findings demonstrate that Xyl-B exerts significant antihypertensive effects not only through the endothelial NO-sGC-cGMP pathway but also through smooth muscle calcium signaling, including VDCCs and SOCE.
Similar content being viewed by others
Introduction
Hypertension is a major risk factor that predisposes patients to cardiovascular disorders and has high rates of morbidity and mortality. Emerging data implicate increases in systemic oxidative stress1 and vascular inflammation2 in the pathogenesis of hypertension. Endothelial dysfunction, which manifests as impaired nitric oxide (NO) bioactivity, is an important early event associated with impaired vessel diastolic function3 and mainly results from increased nitric oxide (NO) degradation due to interactions between NO and superoxide anions. Pharmacological approaches to restore endothelial function or complement NO have been demonstrated to have antihypertensive effects.
Marine organisms are abundant natural sources of novel bioactive compounds because they can produce a variety of molecules with unique structural features and exhibit various types of biological activities. Xyloketal B (Xyl-B), obtained from the South China Sea Coast, is one of a series of novel ketal compounds isolated from the mangrove fungus Xylaria sp (No 2508)4,5. In previous studies, multiple biomedical activities of Xyl-B have been demonstrated, including neuroprotective effects against toxicity6, antioxidant effects on endothelial cells7 and zebrafish8, anti-glioma effects9 and reduced atherosclerosis plaque formation in apolipoprotein E-deficient mice10. After exploring the underlying mechanisms, we have found that Xyl-B can directly scavenge DPPH free radicals, promote endothelial NO release7 and increase eNOS phosphorylation at Ser-1177 in a concentration- and time-dependent manner10. Furthermore, the effect of Xyl-B on aortic tension was also evaluated, and the data indicated that Xyl-B can improve NO-dependent aortic vasorelaxation in atherosclerotic ApoE−/− mice10 and exert potent vasorelaxant activity on KCl-induced contractions in isolated rat aortic rings11. Based on these findings, we hypothesize that Xyl-B may regulate vascular tone through the endothelial NO system and thus has an effect on blood pressure control.
Therefore, in the present study, we established a 2-kidney, 2-clip (2K2C) renovascular hypertensive rat model to evaluate the effects of Xyl-B in the development of hypertension. In addition, we further explored the possible mechanisms for the antihypertensive effects of Xyl-B, including Xyl-B-mediated vasorelaxation, endothelial nitric oxide synthase (eNOS) bioactivity, the NO-sGC-cGMP pathway and the prostacyclin pathway. Abnormal calcium signaling has been demonstrated to regulate arterial tone in the development of hypertension in both human patients and animal models. Consequently, Ca2+ channel blockers are commonly used as anti-hypertensive agents. Because we found that Xyl-B suppressed KCl- and phenylephrine (Phe)-induced contractions in endothelium-denuded thoracic aortic rings, which could not be completely explained by the endothelium-dependent pathway, the influence of Xyl-B on calcium signaling in vascular smooth muscle cells was also investigated.
Materials and methods
Chemicals and reagents
Xyl-B was isolated by the Department of Applied Chemistry, Sun Yat-sen University (Guangzhou, China). The identity and purity of the compound were characterized by HPLC and 2D-NMR as previously described4. Xyl-B was dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C until use. The final concentration of DMSO in culture media was less than 0.1%. Phenylephrine (Phe), acetylcholine (ACh), sodium nitroprusside (SNP) and NG-nitro-L-arginine methyl ester (L-NAME) were purchased from Sigma (St Louis, MO, USA). Methylene Blue was obtained from Merck (Massachusetts, USA). [3H]L-arginine was obtained from Beijing Institute of Nuclear Industry (Beijing, China). All other reagents used were purchased from Sigma unless otherwise specified.
Animals and experimental design
All experimental animal procedures and protocols were approved by the Sun Yat-sen University Committee for Animal Research and were conducted in accordance with the National Research Council's guidelines. 2K2C stroke-prone renovascular hypertension was induced in male Sprague-Dawley rats (60–80 g, purchased from the Experimental Animal Center of Sun Yat-sen University) as described previously12,13. All rats were randomly divided into 5 groups: (1) the Sham group: sham-operated control group; (2) the Htn group: 2K2C-operated hypertensive group; (3) the propylene glycol-treated hypertensive (Htn+Sol) group: the rats received an intraperitoneal injection of 2 mL of saline solution containing 20% propylene glycol once daily after surgery; (4) the Htn +Xyl-B group: 2K2C rats received an intraperitoneal injection of Xyl-B (20 mg·kg−1·d−1 in 2 mL of saline solution containing 20% propylene glycol) after surgery; and (5) the Htn+Cap group: 2K2C rats were treated with captopril (0.1 mg·kg−1·d−1, ig). The sham-operated animals underwent sham procedures without clip placement. Body weight and SBP or DBP measured by tail cuff plethysmography13 were recorded every two weeks until 12 weeks after operation.
Vascular reactivity experiments in aortic rings
Aortic rings were prepared and vascular reactivity experiments were performed as previously described14. Briefly, Sprague-Dawley rats (200–250 g) were euthanized and their aortas were quickly removed and placed into ice-cold Kreb's solution (composition in mmol/L: NaCl 119, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, NaHCO3 25, CaCl2 2.5, EDTA 0.026, and glucose 5.5) where they were freed of fatty and connective tissues. Aortic ring segments (3–5 mm) were then cut and each segment was mounted onto separate Mulvany myograph chambers, which involved placing each ring onto two stainless hooks in an isometric myograph filled with Kreb's solution (5 mL) aerated with 95% O2/5% CO2 and maintained at 37 °C. Changes in isometric force were recorded online using a Multi-myograph data acquisition system with PowerLab software (AD Instruments, Inc). The rings were stretched to an optimal resting tension (1.5 g) and allowed to stabilize for 2 h. An additional equilibration period of 30 min was allotted before treatment with any drugs. The rings were stimulated with Phe (1 μmol/L) when indicated to induce contraction. To examine the possible mechanisms of the vaso-relaxant effects of Xyl-B, the NO synthase (NOS) inhibitor L-NAME (100 μmol/L), the soluble guanylate cyclase inhibitor methylene blue (MB, 10 μmol/L) or the cyclooxygenase inhibitor indomethacin (IMC, 10 μmol/L) was used. The endothelium-intact aortic rings were pre-incubated with these inhibitors for 30 min before Xyl-B was added.
eNOS activity detection in human umbilical vascular endothelial cells (HUVECs)
Primary culture of HUVECs was performed using collagenase digestion of the human umbilical cord as described previously7. Three to six generations of identified HUVECs were used in this experiment. Cells were pre-incubated with 20 μmol/L of Xyl-B for 0, 15, 30, 45, or 60 min or with 0, 10, 20, 40, or 80 μmol/L of Xyl-B separately for 30 min, and eNOS activity was evaluated by determining the conversion ratio of [3H]L-arginine to [3H]L-citrulline at 15 min or 45 min with a liquid scintillation counter (Packard Inc, CT, USA)15.
Measurement of cyclic GMP in primary cultured VSMCs
Vascular smooth muscle cells (VSMCs) were isolated from adult rat thoracic aortas by the explants method as previously described16. Passages 3 to 6 showing >99% positive immunostaining against α-smooth muscle–actin (α-SMA) were used for the experiments, and cells at 80% confluence were arrested by serum-starvation for 24 h. Cyclic GMP (cGMP) levels in VSMCs were determined using Parameter™ Cyclic GMP Assay kits (R&D Systems, Minnesota, USA) according to the manufacturer's instructions. The cells were divided into a VSMC mono-culture group and a VSMC-HUVEC co-culture group. Each group of cells was treated with DMSO, SNP (10 μmol/L), or Xyl-B (20 and 40 μmol/L) and the incubation time was 30 min. None of the drugs exhibited cytotoxicity at any dose used (evaluated by MTT assay, data not shown).
Determination of single-cell intracellular Ca2+ concentration ([Ca2+]i) in VSMCs
Intracellular Ca2+ was detected by Fluo-3/AM staining as described previously17. Cells plated in 35-mm petri dishes were loaded with Fluo-3/AM (10 μmol/L, Sigma) in Kreb's solution at 37 °C for 30 min and then exposed to KCl (60 mmol/L). Xyl-B was added 5 min prior to KCl stimulation. The dishes were washed twice with Kreb's solution to remove unhydrolyzed indicator before transfer to a chamber where the drugs were added. Cellular real-time fluorescence (Ex488 nm, Em522 nm) was measured using a confocal microscope (FV500-IX 81, OLYMPUS, Japan). A change in fluorescence was expressed as F/F0, where F represents the fluorescence intensity (F) of each pixel in the original fluorescence image and F0 is defined as the F value at the beginning of the images when the cell was assumed to be in a resting state.
Statistical analysis
All values are expressed as the means±SEM. The data were analyzed by a two-tailed unpaired Student's t-test between 2 groups and by one-way ANOVA followed by the Bonferroni post hoc test for 3 or more groups. The analyses were performed using GraphPad Prism Software (GraphPad Software Inc, La Jolla, CA, USA). A P value <0.05 was considered statistically significant.
Results
Xyl-B decreases blood pressure in 2K2C hypertensive rats
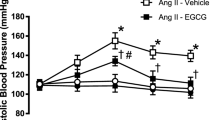
First, we assessed the antihypertensive effects of Xyl-B using the 2K2C hypertensive rat model and blood pressures were measured 0, 2, 4, 6, 8, 10 and 12 weeks after the operation. As shown in Figure 1A and 1B, systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the hypertension (Htn) group increased progressively at 2, 4, 6, 8, 10 and 12 weeks after the operation, which is consistent with our previous report18,19. Xyl-B at 20 mg·kg−1·d−1 was administered to the 2K2C rats intraperitoneally; saline solution containing 20% propylene glycol (2 mL, ip) and captopril (0.1 mg·kg−1·d−1, ig) were used as the solvent and the positive control, respectively. The solvent control (Htn+Sol) group maintained high SBP and DBP values, showing no significant differences from the age-matched Htn group (n=12−14, P>0.05). Xyl-B significantly reduced the SBP and DBP of the 2K2C hypertensive rats at 8, 10 and 12 weeks, with decreases of 59.57%/65.63%, 55.17%/64.10%, 59.15%/68.18% relative to the corresponding values in the positive control (Htn+Cap) group (SBP/DBP, n=12−14, P<0.05 vs the Htn group).
Effect of Xyl-B administration on blood pressure of 2-Kidney 2-Clip (2K2C) hypertensive rats. 2K2C hypertensive rat model was established and 20 mg·kg−1·d−1 of Xyl-B was administered intraperitoneally everyday while saline solution containing 20% propylene glycol and captopril were given as solvent and positive control respectively for 12 weeks. Systolic pressure (A) and diastolic pressure (B) were measured at 0, 2, 4, 6, 8, 10 and 12 weeks after operation. Htn: 2K2C hypertension; Sol: saline solution containing 20% propylene glycol (2 mL, ip); Cap: captopril (0.1 mg·kg−1·d−1, ig). n=12–14. *P<0.05, **P<0.01 vs Htn+Sol group.
Xyl-B reduces aortic tension in response to phenylephrine
Xyloketals have been demonstrated to exert vaso-relaxant activities in KCl-induced vasoconstriction that were stronger in endothelium-intact rings than in denuded rings, suggesting that the vaso-relaxant effect of these compounds may be mediated by endothelium-dependent mechanisms11. Hypertension is closely related to vascular tone, and our previous studies have suggested that Xyl-B plays an important role in endothelial NO synthesis, which mediates vessel relaxation. To further confirm whether Xyl-B protects against hypertension by affecting vascular tension, thoracic aorta constriction in response to Phe was measured. As shown in Figure 2, Xyl-B significantly inhibited Phe-induced vasoconstriction at concentrations of 10−6 mol/L and 10−5 mol/L. Interestingly, the inhibition effect was partly reversed by L-NAME, the inhibitor of endothelial nitric oxide synthetases (eNOS); however, a significant difference was observed in vessel tension between the Xyl-B+L-NAME group and the solvent control group at 10−5 mol/L.
Effect of Xyl-B on phenylephrine (Phe) concentration-response curve for contraction in isolated rat thoracic aorta. Cumulative addition of Phe (10−9–10−5 mol/L) induced a stepwise increase in aortic contraction. (A) Representative tension graph of Phe-induced aorta constriction with or without Xyl-B (20 μmol/L) pre-treatment (10 min) and the influence of L-NAME (10 μmol/L). Vascular tension caused by various concentrations (10−9, 10−8, 10−7, 10−6, 10−5 mol/L) of Phe were recorded. (B) Statistical graph shows Xyl-B decreased Phe-induced tension at 10−6 mol/L and 10−5 mol/L, and this reduction was partly reversed by L-NAME but not totally abolished. Data are expressed as mean±SEM. *P<0.05, **P<0.01 vs DMSO group. #P<0.05 vs Xyl-B group. n=6.
Xyl-B inhibits aortic vasoconstriction through the NO-sGC-cGMP pathway
To identify the pathway through which Xyl-B exerts its antihypertensive effect, we first detected Xyl-B's influence on acetylcholine (ACh)- and sodium nitroprusside (SNP, an exogenous NO provider)-induced vessel diastole in Phe pre-contracted vessels. As shown in Figure 3, Xyl-B enhanced the vaso-relaxant effect of ACh, but not SNP, indicating that Xyl-B decreases vessel tension through the endothelium-dependent NO-related pathway, which is consistent with Figure 2.
(A) Effect of Xyl-B on acetylcholine (ACh) or sodium nitroprusside (SNP) induced vaso-relaxation. Thoracic aorta rings were pre-contracted by Phe (1 μmol/L), then different concentration of (a) ACh (10−9−10−5 mol/L) and (b) SNP (10−12−10−8 mol/L) were cumulatively added. Data were expressed as mean±SEM in concentration-response curve. *P<0.05, **P<0.01 vs DMSO group. n=6. (B) Xyl-B regulates vascular tone through endothelial NO-sGC-cGMP pathway but not PGI2 pathway. (a) Original data show the influence of Indomethacin (IMC 10 μmol/L) and methylene blue (MB, 10 μmol/L) on the inhibition effect of Xyl-B on Phe-induced vasoconstriction. (b) Bar graph showed that IMC did not affect the inhibition effect of Xyl-B on vasocontraction provoked by Phe (1 μmol/L), which was reversed by MB. Data were expressed as mean±SEM. *P<0.05. n=6.
Endothelium-dependent vasodilation is regulated primarily by NO but also by prostacyclin (PGI2) and an unidentified endothelium-derived hyperpolarizing factor. NO and PGI2 are the predominant endogenous endothelial vaso-relaxant substances20. The NO-sGC-cGMP signaling cascade plays an essential role in vascular smooth muscle relaxation, and clinical studies indicate that endothelium-derived NO is involved in normal and pathological blood pressure regulation. PGI2 is produced by the cyclooxygenases, COX-1 and COX-2, which form the prostaglandin endoperoxide PGG2. This is converted to PGH2, which is then transformed enzymatically into PGI2 by prostacyclin synthase. To determine whether the PGI2 pathway is also altered by Xyl-B, indomethacin (IMC, 10 μmol/L) was used to inhibit PGI2 production by depressing COX-1, while methylene blue (MB, 10 μmol/L) was applied to inhibit sGC to interrupt the NO-sGC-cGMP pathway. As shown in Figure 3B, IMC did not alter the inhibitory effect of Xyl-B on vasocontractions provoked by Phe (1 μmol/L), which was significantly reversed by MB, indicating that the NO-sGC-cGMP pathway mediates the anti-hypertensive effect of Xyl-B rather than PGI2.
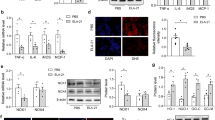
In addition, we detected eNOS activity in HUVECs, which was measured as the conversion% of [3H]L-citrulline transformed from [3H]L-arginine. As shown in Figure 4A, Xyl-B increased endothelial eNOS bioactivity in a dose- and time-dependent manner. Moreover, Xyl-B increased the cGMP level of co-cultured VSMC-HUVECs, but not mono-cultured VSMCs (Figure 4B).
Effects of Xyl-B on eNOS activity in HUVECs and cGMP levels in VSMCs. (A) HUVECs were incubated in with 20 μmol/L of Xyl-B for 0, 15, 30, 45 and 60 min, or incubated for 30 min with Xyl-B of 0, 10, 20, 40, 80 μmol/L. Then eNOS activity was detected by measuring the formation of [3H]L-citrulline from [3H]L-arginine. eNOS activity was represented by conversion %. (B) Vascular smooth muscle cells (VSMCs) were cultured alone or co-cultured with HUVECs. Both mono-cultured VSMCs and co-cultured VSMCs-HUVECs were incubated in the absence (DMSO) or presence of Xyl-B (20 and 40 μmol/L) for 30 min. SNP (10 μmol/L) served as positive control, then cGMP level was detected. Values are mean±SEM. *P<0.05, **P<0.01 vs DMSO group. #P<0.05, ##P<0.01 vs corresponding mono-cultured VSMCs. n=6.
Xyl-B attenuates smooth muscle cell (SMC) Ca2+ signaling
As shown above, Xyl-B inhibited vascular constriction in response to KCl and Phe and this effect could not be completely eliminated by pre-incubation with L-NAME, suggesting that Xyl-B worked through other pathways or factors in addition to NO. As PGI2 production in the endothelium was excluded (Figure 5), the intracellular Ca2+ concentration ([Ca2+]i) of smooth muscle cells, which plays a critical role during vasoconstriction, was speculated to be involved in this process. KCl and Phe elevate the [Ca2+]i in different manners: KCl induces depolarization of SMCs, resulting in voltage-dependent Ca2+ channel activation, while Phe increases the [Ca2+]i by stimulating Ca2+ release from the ER and triggering store-operated Ca2+entry. Our previous data indicated that some xyloketals, including xyloketal B, can attenuate the L-type Ca2+ channel current in cultured newborn mouse hippocampal neurons21. Therefore, to investigate whether Xyl-B has an effect on the [Ca2+]i in SMCs, we first detected the effect of Xyl-B on KCl-induced Ca2+ entry in SMCs and found that Xyl-B significantly suppressed the Ca2+ entry stimulated by KCl (60 mmol/L), as shown in Figure 5A. Then, we determined the influence of Xyl-B on Ca2+ release from the ER using ryanodine (10−7 mol/L). As shown in Figure 5B, Xyl-B pre-incubation obviously decreased ryanodine-induced vasoconstriction.
Xyl-B attenuates smooth muscle cells (SMCs) calcium signaling. (A) Xyl-B inhibits KCl-induced calcium entry in VSMCs. VSMCs were pre-treated with Xyl-B (20 μmol/L) or DMSO for 10 min before stimulation with isotonic 60 mmol/L KCl. Realtime [Ca2+]i was determined using Fluo-3/AM staining and calculated every 30 s (left panel). Peak [Ca2+]i of both Xyl-B and DMSO treated cells were shown in bar graph. *P<0.01 vs DMSO group. Data were obtained from 6 independent experiments. (B) Aortic rings were placed in calcium-free solution with or without Xyl-B (20 μmol/L), then ryanodine (10−7 mol/L) and calcium containing solution were added in order, aortic tension was recorded during this process. Bar graph shows that Xyl-B decreased ryanodine-induced vasoconstriction. Data are expressed as mean±SEM. Results were obtained from 6 independent experiments.
Discussion
This study demonstrates the antihypertensive effects of Xyl-B and the main findings are as follows: (1) 20 mg·kg−1·d−1 of Xyl-B significantly reduced SBP and DBP in the 2K2C hypertensive rat model, providing evidence for Xyl-B's in vivo antihypertensive effect for the first time. (2) Xyl-B decreased vascular tension in response to KCl and phenylephrine in thoracic aortas, which was partly reversed by incubation with L-NAME. (3) Xyl-B inhibited aortic vasocontractions through the endothelial NO-sGC-cGMP pathway rather than the PGI2 pathway. (4) Xyl-B regulated smooth muscle Ca2+ signaling by affecting both voltage-dependent Ca2+ entry (VDCC) and store-operated Ca2+ entry (SOCE) in vascular smooth muscle cells (Figure 6).
Summary of the vasoconstriction signal transduction pathways showing points of intervention by Xyl-B. Vascular tone is the result of joint regulation of endothelium and smooth muscle and endothelium-dependent vasodilation is regulated primarily by NO-sGC-cGMP signaling cascade and prostacyclin (PGI2) in vascular smooth muscle cells. PGI2 is produced by the cyclooxygenases, COX-1 and COX-2, which form the prostaglandin endoperoxide PGG2, PGG2 is converted to PGH2 which is then transformed enzymatically into PGI2 by prostacyclin synthase. Our present study demonstrated that Xyl-B affected eNOS activity and cGMP level, and sGC was also involved but PGI2 pathway was not. Additionally, as Xyl-B's inhibitory effect on vasoconstriction could not be completely explained by endothelial pathway, we furtherly found that Xyl-B regulated vascular tone by affecting both voltage dependent calcium entry (VDCC) and store-operated calcium entry (SOCE) in vascular smooth muscle cells.
The 2K2C model is a widely used renovascular hypertension model12 with significant value for assessing whether a drug has potential antihypertensive effects. Therefore, in this study, 2K2C hypertensive rats were used to evaluate Xyl-B's antihypertensive effects, and the results showed that Xyl-B could significantly decrease SBP and DBP in this model. Our previous studies demonstrated that Xyl-B can directly scavenge free radicals, promote endothelial NO release7 and protect the functions of mitochondria and the endothelium6. We suppose that these endothelium-dependent mechanisms may contribute to the antihypertensive effects of Xyl-B.
The endothelium regulates vascular homeostasis by modulating vasomotor tone through the production of several vasoactive mediators, including NO and prostacyclin22,23. In the development of hypertension, endothelial dysfunction precedes increases in blood pressure and predisposes patients to structural vascular changes24,25. Endothelial dysfunction is characterized by reduced NO levels or bioavailability26. Classically, NO is generated from L-arginine by the binding of Ca2+-calmodulin to eNOS27. NO released from endothelial cells stimulates soluble guanylate cyclase (sGC), leading to a sequential increase in the cGMP level in VSMCs28, which in turn activates cGMP-dependent protein kinase and leads to increased extrusion of Ca2+ from the cytosol in VSMCs and inhibition of the contractile machinery28,29. In the present study, both the eNOS inhibitor L-NAME and the sGC inhibitor MB suppressed the endothelium-dependent vasorelaxation induced by Xyl-B, but the cyclooxygenase blocker (indomethacin) could not. In addition, the cGMP-enhancing effect of Xyl-B was observed in VSMCs co-cultured with HUVECs, but not in the VSMC mono-culture. These data demonstrated that the endothelium-dependent vaso-relaxant activities of Xyl-B are attributed to the NO-sGC-cGMP signaling pathway rather than PGI2.
eNOS activity is known to be regulated by various intracellular signals, resulting in phosphorylation of eNOS30, and eNOS phosphorylation by the upstream PI3K/Akt pathway is required for efficient NO production31,32. Xyl-B has been shown to promote NO release and eNOS bioactivity in HUVECs8, which was confirmed in the present study through detection of the conversion ratio of [3H]L-arginine into [3H]L-citrulline. Our recent study showed that Xyl-B could increase eNOS activity by regulating phosphorylation of Akt and eNOS at Ser1177 and Thr49510. Although many aspects of Xyl-B as a novel drug candidate for protecting the vascular endothelium have been identified and found to be effective for the treatment of cardiovascular diseases such as atherosclerosis7,8,10,11, further experiments are required to confirm the role of the eNOS-dependent mechanism in the antihypertensive action of Xyl-B.
Our previous reports showed marked differences between endothelium-intact (EC50=19 μmol/L) and endothelium-denuded (EC50=41 μmol/L) aortic rings after Xyl-B treatment10,11, suggesting that Xyl-B may exert vaso-relaxant activities by endothelium-dependent mechanisms and by endothelium-independent mechanisms to a lesser extent. Interestingly, when we investigated the endothelial NO pathway using L-NAME, L-NAME could only partly reverse Xyl-B's vaso-relaxant effect on Phe-stimulated pre-contracted aortic rings, but it could not eliminate this effect as a significant difference remained between the solvent control group and the Xyl-B+L-NAME group, indicating that other mechanisms independent of the NO system may be involved.
In addition to endothelial NO, vascular smooth muscle dysfunction is another important cause of hypertension33. Abnormal regulation of intracellular Ca2+ in VSMCs contributes to the pathogenesis of hypertension and also contributes to vascular and cardiac remodeling secondary to hypertension. Two types of Ca2+ channels exist in VSMCs: VDCCs and voltage-independent Ca2+ channels (including receptor-operated Ca2+ channels and store-operated channels)34. Ca2+ channel blockers (CCBs) are clinically useful vasodilators and are widely used in the treatment of hypertension and related cardiovascular diseases35. CCBs lower blood pressure through a well-characterized mechanism of blocking L-type voltage-dependent Ca2+ channels in vascular cells to restore Ca2+ homeostasis36. Our previous data indicated that some xyloketals, including Xyl-B, can attenuate L-type Ca2+ currents in primary cultured hippocampal cells from newborn rats using patch-clamp experiments21, and the current study indicates that Xyl-B dilated Phe- or KCl-induced precontracted thoracic aortic rings. Phe-induced contractions in aortic rings are mainly mediated by Ca2+ entry via voltage-independent Ca2+channels, such as SOCE, while KCl mainly activated the L-type Ca2+channels (VDCCs). In the present study, we detected the effect of Xyl-B on KCl-induced Ca2+ entry in SMCs and on ryanodine-induced aortic contraction. The results not only further confirmed the L-type Ca2+ channel-blocking effect of Xyl-B in smooth muscle cells but also indicated that Xyl-B may affect SOCE, warranting further investigation of the exact Ca2+ signaling mechanism. Additionally, new xyloketals such as xyloketal F and xyloketal A have shown strong inhibitory effects on VDCC in primary cultured rat hippocampal cells (50.33% and 21.47%, respectively)21, but they had no effects on KCl-induced contractions of rat thoracic aortic rings and their effects could be completely inhibited by nifedipine (data not shown). Among the screened xyloketal compounds, only Xyl-B showed significant antihypertensive effects with minor inhibitory effects on VDCCs in newborn rat hippocampal cells (12.05%). These data suggest that the influence of Xyl-B on Ca2+ signaling in different tissues and cell types should be interpreted with caution. Based on the current results, whether regulation of Ca2+ signaling by Xyl-B is the predominant mechanism of its effects in hypertension remains unknown.
Furthermore, during the development of renovascular hypertension, major structural alterations in the heart include left ventricular (LV) hypertrophy and fibrosis13. The cardiovascular remodeling associated with hypertension involves oxidative stress and enhanced matrix metalloproteinase (MMP) expression/activity13. CCBs shows beneficial effects on left ventricular (LV) hypertrophy and fibrosis via antioxidative mechanisms37. The data observed in our current study show that Xyl-B can reverse cardiac remodeling and fibrosis in 2K2C rats (unpublished data). Therefore, Xyl-B not only attenuates the high BP level in hypertension but may also ameliorate the complications of hypertension.
In summary, this is the first study to demonstrate that Xyl-B can reduce blood pressure in 2K2C renovascular hypertensive rats. Our results suggest that Xyl-B induces relaxation in rat aortic rings through an endothelium-dependent pathway mediated by the NO-sGC-/cGMP pathway and through an endothelium-independent pathway involving VDCC blockade mediated by Ca2+ entry in VSMCs. One study limitation is that neither the NO-sGC-/cGMP pathway nor the Ca2+ signaling mechanisms underlying Xyl-B's antihypertensive effects were verified in VSMCs. Therefore, the mechanisms of the antihypertensive action of Xyl-B should be interpreted with caution, and further in vivo studies are warranted to determine the precise mechanism. Taken together, the findings of this study support Xyl-B as a potential new drug candidate for the treatment of hypertension.
Author contribution
Yao-min DU and Yong-cheng LIN conceived and designed the experiments; Li-yan ZHAO, Jie LI, Wei-feng MENG, Guo-hao WANG and Wen-liang CHEN performed the experiments; Li-yan ZHAO, Hong-shuo SUN and Xiong-qing HUANG analyzed the data; Xiao-fei LV and Ji-yan PANG contributed reagents and materials; Li-yan ZHAO and Guan-lei WANG wrote the paper.
References
Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM . Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 2015; 31: 631–41.
Guzik TJ, Touyz RM . Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017; 70: 660–7.
Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T . Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 2008; 10: 1115–26.
Lin Y, Wu X, Feng S, Jiang G, Luo J, Zhou S, et al. Five unique compounds: xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J Org Chem 2001; 66: 6252–6.
Pettigrew JD, Wilson PD . Synthesis of xyloketal A, B, C, D, and G analogues. J Org Chem 2006; 71: 1620–5.
Zhao J, Li L, Ling C, Li J, Pang JY, Lin YC, et al. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res 2009; 1302: 240–7.
Chen WL, Qian Y, Meng WF, Pang JY, Lin YC, Guan YY, et al. A novel marine compound xyloketal B protects against oxidized LDL-induced cell injury in vitro . Biochem Pharmacol 2009; 78: 941–50.
Li ZX, Chen JW, Yuan F, Huang YY, Zhao LY, Li J, et al. Xyloketal B exhibits its antioxidant activity through induction of HO-1 in vascular endothelial cells and zebrafish. Mar Drugs 2013; 11: 504–22.
Chen WL, Turlova E, Sun CL, Kim JS, Huang S, Zhong X, et al. Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways. Mar Drugs 2015; 13: 2505–25.
Zhao LY, Li J, Yuan F, Li M, Zhang Q, Huang YY, et al. Xyloketal B attenuates atherosclerotic plaque formation and endothelial dysfunction in apolipoprotein e deficient mice. Mar Drugs 2015; 13: 2306–26.
Xu Z, Li Y, Xiang Q, Pei Z, Liu X, Lu B, et al. Design and synthesis of novel xyloketal derivatives and their vasorelaxing activities in rat thoracic aorta and angiogenic activities in zebrafish angiogenesis screen. J Med Chem 2010; 53: 4642–53.
Zeng J, Zhang Y, Mo J, Su Z, Huang R . Two-kidney, two clip renovascular hypertensive rats can be used as stroke-prone rats. Stroke 1998; 29: 1708–13; discussion 13–4.
Fang J, Xu SW, Wang P, Tang FT, Zhou SG, Gao J, et al. Tanshinone II-A attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine 2010; 18: 58–64.
Kimura M, Sudhir K, Jones M, Simpson E, Jefferis AM, Chin-Dusting JP . Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice: regulation of nitric oxide production by endogenous sex hormones in males. Circ Res 2003; 93: 1267–71.
Casanello P, Sobrevia L . Intrauterine growth retardation is associated with reduced activity and expression of the cationic amino acid transport systems y+/hCAT-1 and y+/hCAT-2B and lower activity of nitric oxide synthase in human umbilical vein endothelial cells. Circ Res 2002; 91: 127–34.
Xu S, Fu J, Chen J, Xiao P, Lan T, Le K, et al. Development of an optimized protocol for primary culture of smooth muscle cells from rat thoracic aortas. Cytotechnology 2009; 61: 65–72.
Li SJ, Sun NL . Regulation of intracellular Ca2+ and calcineurin by NO/PKG in proliferation of vascular smooth muscle cells. Acta Pharmacol Sin 2005; 26: 323–8.
Shi XL, Wang GL, Zhang Z, Liu YJ, Chen JH, Zhou JG, et al. Alteration of volume-regulated chloride movement in rat cerebrovascular smooth muscle cells during hypertension. Hypertension 2007; 49: 1371–7.
Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, et al. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation 2012; 125: 697–707.
Nava E, Llorens S . The paracrine control of vascular motion. A historical perspective. Pharmacol Res 2016; 113: 125–45.
Wu XY, Liu XH, Lin YC, Luo JH, She ZG, Li HJ, et al. Xyloketal F: A strong L-calcium channel blocker from the mangrove fungus Xylaria sp (#2508) from the South China Sea coast. Eur J Org Chem 2005; 19: 4061–4.
Vane JR, Anggard EE, Botting RM . Regulatory functions of the vascular endothelium. N Engl J Med 1990; 323: 27–36.
Wei W, Chen ZW, Yang Q, Jin H, Furnary A, Yao XQ, et al. Vasorelaxation induced by vascular endothelial growth factor in the human internal mammary artery and radial artery. Vascul Pharmacol 2007; 46: 253–9.
Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, et al. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation 1996; 93: 866–9.
Taddei S, Virdis A, Mattei P, Salvetti A . Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension 1993; 21: 929–33.
Weil BR, Stauffer BL, Greiner JJ, DeSouza CA . Prehypertension is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation in sedentary adults. Am J Hypertens 2011; 24: 976–81.
Fleming I, Bauersachs J, Busse R . Calcium-dependent and calcium-independent activation of the endothelial NO synthase. J Vasc Res 1997; 34: 165–74.
Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res 2000; 87: 825–30.
Chang GJ, Lin TP, Ko YS, Lin MS . Endothelium-dependent and -independent vasorelaxation induced by CIJ-3-2F, a novel benzyl-furoquinoline with antiarrhythmic action, in rat aorta. Life Sci 2010; 86: 869–79.
Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol 2011; 210: 271–84.
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM . Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399: 601–5.
Somani BL, Singh N, Singh VB . Study of Salmonella gallinarum infection in chicks by gas liquid chromatography. Indian J Exp Biol 1975; 13: 503–5.
Hixon ML, Gualberto A . Vascular smooth muscle polyploidization--from mitotic checkpoints to hypertension. Cell Cycle 2003; 2: 105–10.
Guibert C, Ducret T, Savineau JP . Voltage-independent calcium influx in smooth muscle. Prog Biophys Mol Biol 2008; 98: 10–23.
Clunn GF, Sever PS, Hughes AD . Calcium channel regulation in vascular smooth muscle cells: synergistic effects of statins and calcium channel blockers. Int J Cardiol 2010; 139: 2–6.
Godfraind T, Miller R, Wibo M . Calcium antagonism and calcium entry blockade. Pharmacol Rev 1986; 38: 321–416.
Hasegawa H, Takano H, Kohro T, Ueda K, Niitsuma Y, Aburatani H, et al. Amelioration of hypertensive heart failure by amlodipine may occur via antioxidative effects. Hypertens Res 2006; 29: 719–29.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No 81370897 and 81402926), the NSFC-CIHR China-Canada Joint Health Research Initiative Proposal (No 81361128011), the CIHR-NSFC China-Canada Joint Health Research Initiative (CIHR, FRN #132571), the National Key New Drug Creation Program (No 2009ZX09103-039), the Research Funds for Provincial Key Laboratory from the Department of Education of Guangdong Province (No 50000-3211105), the Guangdong Natural Science Foundation (No 2016A030313293), Guangdong Provincial Department of Science and Technology (No 2016A050502023, No 2017A020215104) and the Science and Technology Program of Guangzhou (No 201509010012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, Ly., Li, J., Huang, Xq. et al. Xyloketal B exerts antihypertensive effect in renovascular hypertensive rats via the NO-sGC-cGMP pathway and calcium signaling. Acta Pharmacol Sin 39, 875–884 (2018). https://doi.org/10.1038/aps.2018.12
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2018.12