Abstract
Breast cancer is the most vicious killer for women, and tumor metastasis is one of the leading causes of breast cancer therapy failure. In this study, a new pH-sensitive polymer (polyethylene glycol-block-poly[(1,4-butanediol)-diacrylate-β-N,N-diisopropylethylenediamine], BDP) was synthesized. Based on BDP, docetaxel/silibinin co-delivery micelles (DSMs) was constructed. DSM had a well-defined spherical shape under the transmission electron microscope with average hydrodynamic diameter of 85.3±0.4 nm, and were stable in the bloodstream but could dissociate to release the chemotherapeutic agents in the low pH environment of the endo/lysosomes in the tumor cells. Compared with free drugs, DSM displayed greatly enhanced cellular uptake, higher cytotoxicity and a stronger anti-metastasis effect against mouse breast cancer cell line 4T1. In 4T1 tumor-bearing mice treated with DSM (twice a week for 3 weeks), the inhibition rate on tumor growth and metastasis reached 71.9% and 80.1%, respectively. These results reveal that DSM might be a promising drug delivery system for metastatic breast cancer therapy.
Similar content being viewed by others
Introduction
Breast cancer is one of the leading causes of death in women1,2. It is reported that more than 90% of breast cancer patient deaths are due to tumor metastasis, which generally includes the following steps: invasion, intravasation, circulation, extravasation and colonization in distant organs such as lung, bone, and liver1,3,4,5,6. Currently, chemotherapy is one of the most important treatment strategies for metastatic cancer, but its therapeutic effect is impaired by toxic effects on both cancer cells and normal cells7,8. Fortunately, nanodrug delivery systems can break through various biological barriers by taking advantage of the enhanced penetration and retention (EPR) effects of solid tumors9,10,11,12,13 to increase the intra-tumoral accumulation of drugs, reduce the side effects of chemotherapy3,14,15,16,17,18 and potentially inhibit metastatic breast cancer.
Recently, preclinical studies have shown that silibinin (SIL) can inhibit tumor cell migration and invasion by regulating the epithelial-mesenchymal transition (EMT), protease activation, etc19,20. However, the water solubility of SIL is poor. Meanwhile, docetaxel (DTX) is an efficient chemotherapeutic agent against breast cancer, but side effects such as myelosuppression, short-lasting neutropenia and alopecia limit its application21,22. Therefore, to inhibit the growth of the primary tumor and tumor metastasis, in this work, a new nanodrug co-delivery micellel incorporating DTX and SIL (DSM) with pH-sensitive properties was designed and developed. DSM remained stable in the blood circulation and then dissociated and released DTX and SIL in tumor cells. The influence of DSM on the growth and the metastasis of breast cancer in vitro and in vivo was evaluated. It was expected that DSM could improve the anti-cancer and anti-metastasis effects of breast cancer treatment.
Materials and methods
Materials
DTX was obtained from Knowshine (Shanghai) Pharmachemicals, Inc. SIL was purchased from the Aladdin Reagent Co, Ltd (purity ≥98%, Shanghai, China). Methoxy-polyethyleneglycol amine 5 kDa (mPEG5k-NH2) was bought from Shanghai Seebio Biotech, Inc. 1,4-Butanediol diacrylate (BAB) and β-N,N-diisopropylethyene-diamine (DPA) were purchased from TCI Chemical Industry Co Ltd (Shanghai, China). Coumarin-6 (C6) and Hoechst 33342 were purchased from Sigma-Aldrich (St Louis, MO, USA). Trypsin-EDTA, fetal bovine serum (FBS) and RPMI-1640 were obtained from Gibco Life Technologies (Grand Island, New York, USA). Other reagents were purchased from Sinopharm Chemical Reagent Co, Ltd. All the chemical reagents were of analytical grade and were used without further purification.
Cell culture
The mouse breast cancer cell line 4T1 was obtained from Cell Bank of Shanghai, Chinese Academy of Sciences (Shanghai, China) and was cultured in RPMI-1640 containing 10% FBS, 1.5 g/L sodium bicarbonate, 2.5 g/L glucose, 0.11 g/L sodium pyruvate. The cells were cultured in a humidified atmosphere containing 5% CO2 at 37 °C.
Animals
Female BALB/c nude mice (4–6 weeks, 16–20 g) were obtained from Shanghai Experimental Animal Center (Shanghai, China) and raised in the SPF grade environment at the Animal Care Facility. All the experiments involving animals were carried out under the guideline approved by the Institutional Animal Care and Use Committee (IACUC) of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Synthesis and characterization of polyethylene glycol-block-poly[(1,4-butanediol)-diacrylate-β-N,N-diisopropylethyl- enediamine] (BDP)
BDP was synthesized via two-step reactions. First, 0.50 g of DPA was added dropwise to 1.00 g of BAB under magnetic stirring for 5–10 min. The reaction continued in the dark for 24 h at 60 °C. Then, the product (BAB-DPA) was dialyzed (MWCO 3.5 kDa) in ethanol, and the unreacted reactants were removed 24 h later. Thereafter, 0.10 g of BAB-DPA and 0.17 g of mPEG5k-NH2 were dissolved in 10 mL of dimethyl sulfoxide (DMSO) and stirred at 50 °C for 24 h. The final product was purified by dialysis (MWCO 3.5 kDa) in water for 48 h. Finally, the resulted solution was lyophilized and the copolymer powder was stored at -80 °C. The structure of BDP was tested via 1H NMR. The molecular weight of BDP was confirmed by MALDI-TOF MS.
Preparation and characterization of the DTX/SIL-loading BDP micelle (DSM)
2.5 mg of DTX, 5 mg of SIL and 40 mg of BDP were dissolved in 5 mL of methanol, and DSM was formed by the film dispersion method. The particle size and zeta potential of DSM were determined by a Malven Zetasizer ZEN3690 analyzer (Malven, UK). The morphology of the DSM was characterized by a transmission electron microscope (TEM) (Tecnai F20, FEI, USA). The drug loading (DL) and the encapsulation efficiency (EE) were determined by HPLC assay (C18 column, 5 μm, 4.6 mm×250 mm, Waters, USA). The pH-sensitivity of DSM was verified by the Nile red assay. Nile red-loading micelles were prepared, and their fluorescence with the emission wavelength from 500 to 800 nm at different pH (from 6.0 to 7.4) was measured. The release rates of DTX and SIL from DSM were measured using 0.1 mol/L PBS (pH 7.4) and 0.1 mol/L phosphate-citric acid buffered saline (pH 5.5). The dialysis bags containing DSM (DTX or SIL concentration: 2 mg/mL) were immersed in the release medium and continuously shaken at 37 °C. The release media was replaced by fresh media at certain time points, and the drug concentration in the removed media was measured by HPLC.
Cellular uptake
To compare the internalization of DSM with free drugs, confocal laser scanning microscopy (CLSM) experiments were carried out by replacing DTX and SIL with coumarin-6 (C6), a widely used hydrophobic probe with a high fluorescence emission intensity. 4T1 cells pre-cultured for 24 h in glass-bottomed cell culture dishes (Ø10 mm) in a 24-well plate were treated with the C6-loading BDP micelles (CM) and free C6 at a concentration of 50 ng/well for 2 h at 37 °C. Thereafter, the nuclei were stained with Hoechst 33342 for 30 min in the dark. Then, cells were rinsed twice with PBS and fixed with 4% paraformaldehyde for 20 min. After being fixed onto glass slides with 3 μL sealing liquid, cells were exposed to CLSM examination (Fluoview FV1000, Olympus, Tokyo, Japan).
Wound healing assay
SIL-loading BDP micelles (SM) and blank BDP micelles (BM) were prepared using a similar method as DSM. 4T1 cells were cultured on 12-well plates at a density of 1×105 cells per well to 90% confluence. Next, after removing the medium, a vertical wound was scratched with a pipette tip. Cells were treated with fresh medium containing free SIL, SM and BM at a SIL concentration of 10 μg/mL for 24 h. Untreated cells were used as a control. All cells were photographed using a microscope (Olympus, Japan) before and after adding drugs to see the degree of cell healing.
In vitro migration and invasion assay
For cell migration experiments, 4T1 cells were seeded on 24-well plates at a density of 3×104 cells per well for 24 h. Then, the cells were trypsinized and transferred to the top chambers of Transwell plates, followed by incubation with free SIL, DSM and BM at a SIL concentration of 10 μg/mL in 100 μL of serum-free medium. The lower chambers were filled with 500 μL of medium containing 10% FBS, which was used as a chemoattractant. After 24 h under standard culture conditions, non-invasive cells on the upper surface of the top chambers were removed. The invasive cells on the bottom side were fixed by cold 70% ethanol for 20 min, stained with crystal violet for 20 min, washed by ultra-pure water three times and photographed by a microscope. The cell invasion assay was carried out with the same method as the cell migration assay, except that cells were added on the matrigel layer at a density of 5×104 cells per well.
Cytotoxicity assay
4T1 cells seeded at a density of 5×103 cells per well in 96-well plates were incubated with free SIL, free DTX, DTX-loading BDP micelles (DM), DSM and BM at various concentrations for 24 h. The final concentrations of SIL and DTX were in the range of 2–200 μg/mL and 1–100 μg/mL, respectively. Untreated cells were used as a control. Cell viability was measured by the MTT assay.
In vivo anti-tumor efficacy
The BALB/c nude mice were randomly divided into 4 groups (n=5) and each mouse was injected with 8×105 4T1 cells in the right axilla. When the tumor reached approximately 100 mm3, saline, free drugs, DSM and BM were administered to each group (DTX: 4 mg/kg; SIL: 8 mg/kg) through the tail vein twice a week for three weeks. The volumes of tumors were measured every 3 d to monitor tumor progression, and body weights were examined concurrently. All animals were sacrificed on d 25, and all tumors and lungs were collected and photographed. Lung metastases were examined by visually counting the metastatic nodules and staining the histologic sections with hematoxylin and eosin (H&E) staining.
Statistical analysis
All data was expressed as the mean value±SD. For a two-group comparison, Student's t-test (two-tailed) was used to perform the statistical significance. The difference was considered statistically significant when *P<0.05, very significant when **P<0.01 and extremely significant when ***P<0.001.
Results
Synthesis and characterization of BDP
The successful synthesis of BDP was confirmed by its 1H NMR spectrum (Figure 1). The methyl group of DPA had representative peaks at ppm 0.85–1.12, and the peaks at ppm 3.28–3.71 represented the hydrogen atoms of the methoxyl and the ethylene glycol units of PEG. The molecular weight of BDP measured by MALDI-TOF MS was 8128 Da.
Synthesis of BDP. (A) The scheme of synthesis of BDP. (B) 1H NMR spectrum of BDP. Reaction conditions: (1) in the dark, no solvent, 24 h, 60 °C; (2) in the dark, DMSO, 24 h, 50 °C.
Preparation and characterization of DSM
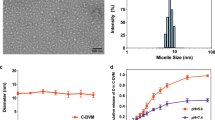
DTX and SIL were co-loaded into DSM by the thin film hydration method. The average hydrodynamic diameter of DSM was 85.3±0.4 nm (Figure 2A), and the polydispersity index (PDI) was 0.190±0.1. TEM images revealed that DSM was obtained with a well-defined spherical shape displaying a narrow size distribution (Figure 2B). The DL and EE of SIL were 4.1%±0.2% and 96.9%±0.3%, respectively. The DL and EE of DTX were 2.8%±0.3% and 97.6%±0.5%, respectively. The fluorescence intensity of Nile red-loaded BDP micelles obviously decreased when the pH reached 6.2, implying that the hydrophobic drug was encapsulated in the core of the micelle when the pH was higher than 6.4 and was rapidly released at pH 6.2 (Figure 2C). The cumulative release rates of DTX and SIL increased with the descending pH of the release medium, from 23.2% and 21.4% in medium at a pH of 7.4, respectively, to 82.3% and 79.7% within 48 h in medium at a pH of 5.5, respectively (Figure 2D).
Characterization of DSM. (A) The particle size distribution of DSM. (B) TEM image of DSM. Scale bar=200 nm. (C) Fluorescence profiles of Nile red-loading BDP micelles at different pH. (D) In vitro release profiles of DTX and SIL from DSM in pH 7.4 and pH 5.5 media with 1% Tween-80. Data are presented as the mean±SD (n=3).
Cellular uptake
The cellular uptake of DSM in 4T1 breast cancer cells was investigated using C6 (a hydrophobic fluorescent dye that has been widely used as a model drug to replace hydrophobic drugs23) loaded BDP micelles. Hoechst 33342 was used for staining nuclei24. Confocal microscopy images show the cellular uptake of DSM and free drugs in 4T1 cells after 2 h incubation (Figure 3). The free C6 exhibits a dim green fluorescence, while CM displays a stronger fluorescence intensity.
Cellular internalization of free drug and the BDP micelle in 4T1 cells after 2 h incubation. Nuclei were stained with Hoechst 33342 (blue). DTX and SIL were replaced with Coumarin-6 (green).
Wound healing, cell invasion and migration assays
A wound healing assay was carried out to evaluate the effects of free SIL, SM and BM on inhibiting the horizontal motility of the 4T1 cells (Figure 4A). DTX was not encapsulated to avoid false positive results caused by its toxicity. After incubation overnight, the scratched space in the control and BM groups was nearly coalescent. However, the free SIL group and the SM group showed uncovered wounds at different levels. Moreover, the wound healing rate of SM was approximately 13.1% (Figure 4B).
Inhibitory effects of different formulations on wound healing of 4T1 cells in vitro. (A) Images depicting wound healing of cells immediately following scratching (1), that received no treatment (control group) (2), that received BM (3), free SIL (4) and SM (5). Scale bar=200 μm. (B) Quantitative analysis of the wound healing rates of different groups. Data are presented as the mean±SD (n=3). **P<0.01.
Cell migration and invasion assays were applied to evaluate whether micelles could affect the vertical motility of 4T1 cells. The results were consistent with those from the wound healing assay. As shown in Figure 5A, 5B, 5C; SM could tremendously depress the invasive and migratory capabilities of 4T1 cells, achieving inhibition rates of 86.9% and 88.4%, respectively.
Inhibitory effects of different formulations on cell migration and invasion of 4T1 cells in vitro. (A) Representative images of cells treated with SM, free SIL and BM, respectively. Scale bar=100 μm. (B, C) Quantitative analysis of migratory (B) and invasive (C) cells of different groups. The number of the control group was set as 100%. Data are presented as the mean±SD (n=3). **P<0.01, ***P<0.001.
Cytotoxicity
A classic syngeneic, highly metastatic breast cancer cell line, 4T1, was chosen as the cell model25,26. The in vitro cytotoxicity of DM, DSM, BM, free SIL and free DTX in 4T1 cells were determined by the MTT assay. The free SIL and BM both displayed negligible anti-proliferative effects on the 4T1 cells, with over 80% cell viability after incubation for 24 h at various concentrations ranging from 2.0 to 200.0 μg/mL (Figure 6). The cytotoxicity of free DTX, DM and DSM increased with increasing DTX concentrations. DM and DSM were more toxic than free DTX. The IC50 values of DM and DSM were 19.1 μg/mL and 18.4 μg/mL, respectively.
In vitro cytotoxicity effects of free SIL, free DTX, DM, DSM and BM at different concentrations on 4T1 cells after a 24 h incubation. Data are presented as the mean±SD (n=3). *P<0.05, ***P<0.001.
In vivo anti-tumor efficacy
The ability of DSM to inhibit the growth of primary tumors was evaluated by the 4T1 mammary tumor metastasis model. Average tumor sizes and body weights were recorded during the 25-d experiment to monitor anti-tumor efficacy. The tumors in the saline and BM groups grew so fast that the mean tumor volume expanded to 2176.09 mm3 and 1944.71 mm3, respectively, by the end of the experiment (Figure 7). The commercial formulation of DTX combined with SIL displayed certain anticancer effects, with a tumor inhibition rate (TIR) of 21.2%. The final tumor volume of the DSM group only increased by 28.1%, and the TIR reached 71.9%. The lungs of the DSM group displayed clean surfaces, with only 6 metastatic nodules per lung (Figure 8). The number of lung metastatic nodules of the DSM group decreased by 80.1% compared with that of the saline group. The proportion of the tumor burden area on the H&E-stained lung section images of the DSM group was also lower than the saline group.
In vivo anti-tumor effects in 4T1 tumor-bearing mice. (A) Images of tumors on d 25 after the first administration. (B, C) Variation profiles of the tumor volumes (B) and body weights (C) of tumor-bearing mice. **P<0.01.
In vivo anti-metastasis effect in 4T1 tumor-bearing mice. (A, B) Images of the lungs (A) and H&E staining of the lung sections (B) at the end of the experiment (1: saline; 2: BM; 3: SIL+DTX; 4: DSM). (C) Quantitative analysis of the pulmonary metastatic nodules. Data are presented as the mean±SD (n=5). **P<0.01.
Discussion
A nanosized DTX and SIL co-delivery system was designed and constructed in this study, aiming to inhibit tumor growth and metastasis to a greater extent. After being loaded into DSM, the pH-sensitive vector, the anticancer effect of DTX and SIL on tumor cell proliferation and tumor cell metastasis was simultaneously enhanced.
The drug release behavior of BDP micelles displayed a pH-responsive profile. The pH-sensitivity of DSM is attributed to the DPA group in BDP, which converted the BAB-DPA block from hydrophobic to hydrophilic at a pH lower than 6.327. Thus, DSM may protect drugs in the blood circulation and then release them once DSM is within endo/lysosomes in the tumor cells. The uptake of CM was higher than the free drug in 4T1 cells, suggesting that the BDP micelle is an effective drug delivery system to facilitate the internalization of drugs into 4T1 cells. SM inhibited the wound healing, migration and invasion of the 4T1 cells more distinctly than free SIL, which could be due to the increased cellular uptake of SIL mediated by the micelle compared to free drugs. In the MTT assay carried out on 4T1 cells, BDP had a good biocompatibility, and the loading of SIL did not increase the cytotoxicity. Encapsulation by the BDP micelle promoted the anti-tumor and anti-metastasis effects of DTX and SIL, which might be caused by a higher intracellular drug concentration and rapid drug release. The BM group showed a similar growth profile as the saline group, indicating that the material comprising the vector barely had any physiological activity. The most efficient anti-tumor activity was achieved by DSM, which might be because the micelle could utilize the EPR effect and mediate more drug distribution in the tumor, enhancing the cellular uptake of the drugs. The better suppression capability on primary tumor growth and the higher intracellular concentration of SIL led to less metastatic foci in the lung. These results revealed the superiority of DSM in suppressing tumor growth and metastasis over the combination of free DTX and SIL.
In summary, the drug release behavior of DSM in the endo/lysosomes was due to the introduction of the pH-sensitive block BAB-DPA. In 4T1 cells, micelles exhibited stronger inhibitory effects on invasion and migration and higher cytotoxicity than free drugs. DSM showed significant anti-tumor and anti-metastasis effects in 4T1 tumor-bearing mice. This pH-sensitive drug co-delivery system could realize satisfactory efficacy for simultaneously inhibiting the growth and metastasis of breast cancer.
Author contribution
Xin-yue DONG performed the experiments and wrote the manuscript; Tian-qun LANG helped with the cellular uptake experiment; Qi YIN and Peng-cheng ZHANG analyzed the data; Ya-ping LI designed and supervised the study. All authors have read and approved the final manuscript.
References
Eckhardt BL, Francis PA, Parker BS, Anderson RL . Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov 2012; 11: 479–97.
Sun HP, Su JH, Meng QS, Yin Q, Zhang ZW, Yu HJ, et al. Silibinin and indocyanine green-loaded nanoparticles inhibit the growth and metastasis of mammalian breast cancer cells in vitro. Acta Pharmacol Sin 2016; 37: 941–9.
Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer 2011; 12: 39–50.
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009; 459: 1005–9.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436: 518–24.
Wan L, Pantel K, Kang Y . Tumor metastasis: moving new biological insights into the clinic. Nat Med 2013; 19: 1450–64.
Wulfkuhle JD, Liotta LA, Petricoin EF . Proteomic applications for the early detection of cancer. Nat Rev Cancer 2003; 3: 267–75.
Steeg PS . Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006; 12: 895–904.
Wang H, Wu Y, Zhao R, Nie G . Engineering the assemblies of biomaterial nanocarriers for delivery of multiple theranostic agents with enhanced antitumor efficacy. Adv Mater 2013; 25: 1616–22.
Chauhan VP, Jain RK . Strategies for advancing cancer nanomedicine. Nat Mater 2013; 12: 958–62.
Mura S, Nicolas J, Couvreur P . Stimuli-responsive nanocarriers for drug delivery. Nat Mater 2013; 12: 991–1003.
He Y, Xia DN, Li QX, Tao JS, Gan Y, Wang C . Enhancement of cellular uptake, transport and oral absorption of protease inhibitor saquinavir by nanocrystal formulation. Acta Pharmacol Sin 2015; 36: 1151–60.
Zhang L, Zhang S, Ruan SB, Zhang QY, He Q, Gao HL . Lapatinib-incorporated lipoprotein-like nanoparticles: preparation and a proposed breast cancer-targeting mechanism. Acta Pharmacol Sin 2014; 35: 846–52.
Fang J, Nakamura H, Maeda H . The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Del Rev 2011; 63: 136–51.
Maeda H . Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev 2015; 91: 3–6.
Jin ZH, Jin MJ, Jiang CG, Yin XZ, Jin SX, Quan XQ, et al. Evaluation of doxorubicin-loaded pH-sensitive polymeric micelle release from tumor blood vessels and anticancer efficacy using a dorsal skin-fold window chamber model. Acta Pharmacol Sin 2014; 35: 839–45.
Zhu JJ, Zhang XX, Miao YQ, He SF, Tian DM, Yao XS, et al. Delivery of acetylthevetin B, an antitumor cardiac glycoside, using polymeric micelles for enhanced therapeutic efficacy against lung cancer cells. Acta Pharmacol Sin 2017; 38: 290–300.
Punfa W, Yodkeeree S, Pitchakarn P, Ampasavate C, Limtrakul P . Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin 2012; 33: 823–31.
Nambiar D, Prajapati V, Agarwal R, Singh RP . In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer Lett 2013; 334: 109–17.
Duan WJ, Li QS, Xia MY, Tashiro S, Onodera S, Ikejima T . Silibinin activated p53 and induced autophagic death in human fibrosarcoma HT1080 cells via reactive oxygen species-p38 and c-Jun N-terminal kinase pathways. Bio Pharm Bull 2011; 34: 47–53.
Fukae M, Shiraishi Y, Hirota T, Sasaki Y, Yamahashi M, Takayama K, et al. Population pharmacokinetic-pharmacodynamic modeling and model-based prediction of docetaxel-induced neutropenia in Japanese patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2016; 78: 1013–23.
Jehn CF, Hemmati P, Lehenbauer-Dehm S, Kummel S, Flath B, Schmid P . Biweekly pegylated liposomal doxorubicin (Caelyx) in heavily pretreated metastatic breast cancer: a phase 2 study. Clin Breast Cancer 2016; 16: 514–9.
Zhang P, Cheetham AG, Lin YA, Cui H . Self-assembled Tat nanofibers as effective drug carrier and transporter. ACS Nano 2013; 7: 5965–77.
Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano 2013; 7: 5858–69.
Marino N, Marshall JC, Collins JW, Zhou M, Qian Y, Veenstra T, et al. Nm23-h1 binds to gelsolin and inactivates its actin-severing capacity to promote tumor cell motility and metastasis. Cancer Res 2013; 73: 5949–62.
Li M, Tang Z, Zhang D, Sun H, Liu H, Zhang Y, et al. Doxorubicin-loaded polysaccharide nanoparticles suppress the growth of murine colorectal carcinoma and inhibit the metastasis of murine mammary carcinoma in rodent models. Biomaterials 2015; 51: 161–72.
Hu JM, Zhang GY, Ge ZS, Liu SY . Stimuli-responsive tertiary amine methacrylate-based block copolymers: Synthesis, supramolecular self-assembly and functional applications. Prog Polym Sci 2014; 39: 1096–143.
Acknowledgements
The National Basic Research Program of China (2014CB931900), the National Natural Science Foundation of China (81521005 and 81230029), and the Youth Innovation Promotion Association of CAS (2015226) are gratefully acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dong, Xy., Lang, Tq., Yin, Q. et al. Co-delivery of docetaxel and silibinin using pH-sensitive micelles improves therapy of metastatic breast cancer. Acta Pharmacol Sin 38, 1655–1662 (2017). https://doi.org/10.1038/aps.2017.74
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.74
Keywords
This article is cited by
-
Lipid-based nanoparticle-mediated combination therapy for breast cancer management: a comprehensive review
Drug Delivery and Translational Research (2023)
-
Lipid/PAA-coated mesoporous silica nanoparticles for dual-pH-responsive codelivery of arsenic trioxide/paclitaxel against breast cancer cells
Acta Pharmacologica Sinica (2021)
-
Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis
Journal of Nanobiotechnology (2020)