Abstract
Glomerular epithelial podocytes are highly specialized cells that play a crucial role in maintaining normal function of the glomerular filtration barrier via their foot processes. Chrysin (5,7-dihydroxyflavone) is a natural flavonoid found in propolis and mushrooms that has anti-inflammatory, antioxidant and anticancer properties. This study aimed to evaluate the renoprotective effects of chrysin on podocyte apoptotic loss and slit diaphragm protein deficiency in high glucose-exposed podocytes and in db/db mouse kidneys. Exposure to high glucose (33 mmol/L) caused glomerular podocyte apoptosis in vitro, which was dose-dependently attenuated by nontoxic chrysin (1–20 μmol/L) through reduction of DNA fragmentation. Chrysin treatment dose-dependently restored the increased Bax/Bcl-2 ratio, and suppressed Apaf-1 induction and the elevated cytochrome c release in high glucose-exposed renal podocytes. In diabetic db/db mice, oral administration of chrysin (10 mg·kg−1·d−1, for 10 weeks) significantly attenuated proteinuria, and alleviated the abnormal alterations in glomerular ultrastructure, characterized by apoptotic podocytes and foot process effacement. In addition, this compound improved the induction of slit diaphragm proteins podocin/nephrin in the diabetic glomeruli. Exposure to high glucose elevated the unfolded protein response (UPR) to ER stress in renal podocytes, evidenced by up-regulation of PERK-eIF2α-ATF4-CHOP. Chrysin treatment blocked such ER stress responses pertinent to podocyte apoptosis and reduced synthesis of slit diaphragm proteins in vitro and in vivo. These observations demonstrate that targeting ER stress is an underlying mechanism of chrysin-mediated amelioration of diabetes-associated podocyte injury and dysfunction.
Similar content being viewed by others
Introduction
Diabetic nephropathy (DN) is characterized by increased albuminuria, one of the most important prognostic risk factors for kidney disease progression1,2. Glomerular visceral epithelial cells, or podocytes, are highly specialized cells in the kidney that play a pivotal role in maintaining the structure and function of the glomerular filtration barrier to prevent escape of plasma proteins from the glomerular circulation3,4. Aberrant structural changes in podocytes have been proposed to be involved in the pathogenesis of albuminuria in diabetes3,5. Reduced podocyte density has been observed in individuals with DN as well as patients who had diabetes for short periods of time and did not present with salient albuminuria6,7. Accordingly, the loss of podocytes in glomeruli can be a credible predictor of DN progression, which is the leading cause of end-stage renal disease4,6,8. Although podocyte depletion is feature of early-phase diabetic kidney disease, the underlying mechanisms of podocyte loss in DN remain poorly understood. However, reactive oxygen species (ROS) contribute to podocyte injury, which leads to apoptosis and cellular depletion under high glucose and experimental DN conditions6,8,9. In addition, the podocyte loss may be attributed to impaired autophagy in diabetes, which ultimately leads to massive proteinuria in DN10,11.
Several studies have suggested that the unfolded protein response (UPR), known as the ER stress response, is activated in diabetic kidneys to restore normal endoplasmic reticulum (ER) function by reducing protein synthesis, enhancing ER folding and down-regulating gene expression involved in multiple pathways12,13. The UPR- and ER-associated degradation networks interact with the intracellular proteolytic pathways to alleviate ER malfunction14. If excessive ER stress continuously occurs, the pro-survival UPR can be superseded by pro-apoptotic cell death15. One investigation showed that oxidative stress induces podocyte injury via activation of ER stress, which then triggers both C/EBP homologous protein (CHOP)-dependent apoptosis and autophagy to cope with the injury16. In addition, the increased oxidants derived from ER stress disrupt the functionality of cellular protein machines, eventually culminating in proteotoxic stress and proteostasis deregulation17. Therapeutic strategies directed to regulate different UPR signaling pathways and target specific UPR components may reduce stress levels18. However, salient side effects are predicted in the chronic administration of synthetic drugs that can modulate the ER proteostasis network.
Based on accumulating evidence that ER stress induction is evident in human glomerulopathies14, the current study investigated whether chrysin antagonized podocyte injury and maintained the structure and function of the glomerular filtration barrier in association with a reduction in ER stress and a boost in ER proteostasis. Chrysin (5,7-dihydroxyflavone, Figure 1A) is a naturally occurring flavone-type flavonoid and is found in chamomile, oyster mushrooms and propolis. Chrysin-mediated biological activities targeting inflammation, apoptosis and oxidative stress have been documented elsewhere19,20. Recent studies have shown that chrysin ameliorates renal toxicity in rats induced by 5-fluorouracil or adenine involved in inflammation and oxidative stress20,21. However, whether chrysin restores optimal structure and function to the glomerular epithelial podocytes in diabetic kidney remains unknown. Our previous study showed that chrysin attenuated renal tubulointerstitial fibrosis via blocking the induction of the epithelial to mesenchymal transition in renal tubular epithelial cells and in db/db mice22. The epithelial to mesenchymal transition and tubulointerstitial fibrosis could be considered as novel routes and potential drug targets for the future therapeutic interventions using chrysin. In this study, ER stress-mediated CHOP-dependent apoptosis was examined in high glucose-exposed podocytes and in diabetic glomerular tissues. The current study also determined the protective effects of chrysin on the podocyte filtration slit structure in diabetic glomeruli. To reveal that chrysin inhibited ER stress-mediated misfolding of slit diaphragm proteins, this study examined the induction of podocin and nephrin in high glucose-exposed podocytes and in diabetic glomerular tissues. Our findings highlight the mechanism by which chrysin combats ER stress to provide a possible new therapeutic target for podocyte loss and glomerular filtration barrier injury.
Chemical structure (A) and cytotoxicity (B) of chrysin and inhibitory effects of chrysin on viability (C) and DNA fragmentation (D) in high glucose-exposed podocytes. Podocytes were incubated in media containing different glucose contents (5.5 mmol/L glucose and 27.5 mmol/L mannitol as osmotic controls and 33 mmol/L glucose in the absence or presence of 1–20 μmol/L chrysin for 3 d). Podocyte viability (mean±SEM, n=5) was measured using the MTT assay and expressed as percent cell survival relative to that of the glucose controls. The DNA fragmentation in podocytes was measured using the TUNEL assay, and nuclear staining was accomplished with DAPI (D). Representative microphotographs were obtained by fluorescence microscopy with a fluorescein green filter. Magnification: ×200. Fluorescence intensity was quantified by using an Axiomager microscope system. *P<0.05 vs 5.5 mmol/L glucose or 27.5 mmol/L mannitol. #P<0.05 vs 33 mmol/L glucose alone.
Materials and methods
Materials
Fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid, and penicillin–streptomycin were purchased from Lonza (Walkersvillle, MD, USA). RPMI-1640 media, mannitol, D-glucose, chrysin and all other reagents, unless stated otherwise, were obtained from Sigma-Aldrich (St Louis, MO, USA). Protein kinase RNA-like endoplasmic reticulum kinase (PERK) inhibitor was supplied by EMD Millipore Corporation (Billerica, MA, USA). Rabbit polyclonal apoptotic peptidase activating factor 1 (Apaf-1), rabbit polyclonal total PERK and rabbit polyclonal glucose-regulated protein 78 (GRP78)/BIP antibodies were supplied by Abcam Biochemicals (Cambridge, UK, USA). Mouse monoclonal antibodies against Bcl-2 and Bax were provided by BD Transduction Laboratories (Franklin Lakes, NJ, USA). Goat polyclonal nephrin and rabbit polyclonal phospho-PERK antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against rabbit polyclonal cytochrome c, mouse monoclonal cytochrome c oxidase IV (COX IV), rabbit polyclonal phospho-eukaryotic initiation factor 2α (eIF2α), mouse monoclonal CHOP and rabbit polyclonal activating transcription factor 4 (ATF4) were purchased from Cell Signaling Technology (Beverly, CA, USA). Rabbit polyclonal podocin and mouse monoclonal β-actin antibodies were obtained from Sigma-Aldrich. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, goat anti-mouse and donkey anti-goat IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Murine kidney podocyte culture
Conditionally immortalized mouse podocytes were purchased from the Cell Line Service (Eppelheim, Germany). Cells were cultured in RPMI-1640 culture media containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in a 37°C humidified atmosphere with 5% CO2. The podocytes were subcultured at 90% confluence for further experiments. To induce hyperglycemic episodes, podocytes were incubated in media containing 33 mmol/L glucose for 48 h and compared with cells cultured in normal media containing 5.5 mmol/L glucose. The cells were also incubated in media containing 5.5 mmol/L glucose and 27.5 mmol/L mannitol as an osmotic control. High glucose-exposed podocytes were treated with chrysin at doses of 1–20 μmol/L for the current experiments.
Cell viability was determined by using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide) assay. Podocytes seeded at a density of 1×104 cells/mL on a 24-well plate were treated with 1–20 μmol/L chrysin in different media conditions. The cells were then treated with 1 mg/mL MTT solution and incubated at 37 °C for 3 h to allow the formation of an insoluble purple formazan product that was dissolved in 250 μL isopropanol. Optical density was measured using a microplate reader at a wavelength of 570 nm. This study found that chrysin at doses of 1–20 μmol/L did not induce cytotoxicity (Figure 1B).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The TUNEL assay is a common method for detecting DNA fragments that result from apoptotic signaling cascades. In the present study, the TUNEL assay was conducted using a commercial fluorometric TUNEL kit (Promega Co, Madison, WI, USA). The podocytes were plated on an 8-well glass chamber slide and incubated for 48 h in media containing 5.5 mmol/L glucose, 5.5 mmol/L glucose plus 27.5 mmol/L mannitol, or 33 mmol/L glucose in the absence or presence of 1–20 μmol/L chrysin. Cells fixed with 4% formaldehyde 4°C for 20 min were permeabilized with 0.2% Triton X-100 and fragmented DNA was labeled with fluorescein-dUTP at 37°C for 1 h. 4′,6-Diamidino-2-phenylindole (DAPI) was used for counterstaining nuclei that were visualized and quantified with an Axiomager optical microscope system.
Western blot analysis
Western blot analysis was conducted using whole cell lysates prepared from 3.5×105 podocytes. Kidney tissue extracts were also prepared from db/db mice that had received 10 mg/kg chrysin. Whole cell lysates and kidney tissue extracts were prepared in a lysis buffer containing 1 mol/L β-glycerophosphate, 1% β-mercaptoethanol, 0.5 mol/L NaF, 0.1 mol/L Na3VO4 and a protease inhibitor cocktail. Cell lysates and kidney tissue extracts containing equal amounts of proteins were electrophoresed on 6%–15% SDS-PAGE and transferred onto a nitrocellulose membrane. Nonspecific binding was blocked with 5% skim milk for 3 h. The membrane was incubated overnight at 4°C with a primary antibody against each target protein and washed in TBS-T buffer [50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, and 0.1% Tween-20] for 10 min. The membrane was then incubated for 1 h with one of the following secondary antibodies conjugated to HRP: goat anti-rabbit IgG, goat anti-mouse IgG, or donkey anti-goat IgG. The level of each target protein was determined by using immobilon Western chemiluminescent HRP substrate (Millipore Corp, USA) and Agfa X-ray film (Agfa-Gevaert, Belgium). Incubation with mouse monoclonal β-actin antibody or mouse monoclonal COX IV antibody was also performed for comparative controls.
In vivo animal experiments
Adult male db/db mice (C57BLKS/+Leprdb Iar; Jackson Laboratory, CA, USA) and their age-matched non-diabetic db/m littermates (C57BLKS/J; Jackson Laboratory) were used in the current study. The mice were kept on a 12-h light/12-h dark cycle at 23±1°C with 50%±10% relative humidity under specific pathogen-free conditions and fed a standard pellet laboratory chow diet (Cargill Agri Purina, Biopia, Korea). The mice were supplied by the animal facility of Hallym University. This study employed db/db mice at 7 weeks of age, as these animals begin to develop diabetes (hyperglycemia) at 7–8 weeks of age. The animals were allowed to acclimatize for one week before the feeding experiments were initiated. The mice were divided into three subgroups (n=7–9 for each subgroup). Mice in the first group were the non-diabetic db/m control mice, and the db/db mice were divided into two subgroups. One group of db/db mice was orally administered 10 mg/kg chrysin daily for 10 weeks. All animal experiments were approved by the Committee on Animal Experimentation of Hallym University and performed in compliance with the University's Guidelines for the Care and Use of Laboratory Animals (hallym 2013-125). No mice died, and no apparent signs of exhaustion were observed during the experimental period. The 24-h urine samples were collected during the 10 week-chrysin supplementation using metabolic cages. Urinary albumin was measured using the Albuwell M ELISA kit (Exocell, Philadelphia, PA, USA) according to a manufacturer's instructions. The 24-h urine volume in the db/db mice was 20-fold higher than that of db/m controls, whereas in chrysin-treated mice, the volume declined by ≈50%22. Fasting blood glucose and blood glycated hemoglobin HbA1C levels in mouse tail veins were measured every other week. The chrysin challenge reduced plasma glucose levels and elevated HbA1C levels in db/db mice22.
For the histological analyses of renal tubule tissues, tubular specimens were obtained at the conclusions of the experiments and fixed in 10% buffered formaldehyde. The paraffin-embedded tubular specimens were cut into 5-μm sections, deparaffinized and stained with Periodic acid-Schiff (PAS) stain to assess interstitial fibrosis. The stained tissue sections were examined using an optical microscope AXIOIMAGER (Zeiss, Gottingen, Germany), and five images were taken for each section.
Transmission electron microscopy
Freshly prepared kidney tissues from db/db mice were immediately fixed for 3–7 day in 2.5% glutaraldehyde in 0.1 mol/L Sorenson's phosphate buffer (pH 7.4) and post-fixed in 1% OsO4, followed by washing in distilled water and en bloc staining in 3% uranyl acetate. Dehydration was carried out using a 70%–100% graded ethanol series. The tissues were then embedded in Epon polymer, and ultrathin sections (70 nm) were obtained with a diamond knife on a Reichert Jung ultramicrotome, stained with uranyl acetate-lead citrate, and observed with a Philips CM-100 transmission electron microscope (FEI instruments, Hillsborough, OR, USA) at an accelerating voltage of 60 kV. A representative set of TEM images taken at magnifications up to ×130 000 were examined and analyzed for cellular morphology, subcellular constituents and structures.
Immunocytochemistry
Immunofluorescent cytochemical staining was performed to examine the induction of podocin and nephrin in podocytes grown on 8-well chamber slides. The podocytes were fixed with 4% formaldehyde for 20 min and permeated with 0.1% Triton X-100 for 10 min on ice. The cells were blocked with 20% FBS for 1 h, and then incubated with a primary antibody against podocin or nephrin and a FITC-conjugated IgG antibody or Cy3-conjugated IgG antibody. Nuclear staining was performed with DAPI. Each slide was mounted in a mounting medium, and images from each slide were taken using an optical Axiomager microscope system (Zeiss, Göttingen, Germany).
Immunohistochemical staining
For the immunohistochemical analysis, paraffin-embedded kidney glomerular tissue sections (5 μm thick) were employed. The sections were placed on glass slides, deparaffinized and hydrated with xylene and graded alcohol. The sections were preincubated in a boiling sodium citrate buffer [10 mmol/L sodium citrate (pH 6.0), 0.05% Tween 20] for antigen retrieval. The tissue sections were incubated overnight with a specific primary antibody against podocin. The tissue sections were then incubated for 1 h with HRP-conjugated anti-rabbit IgG. For podocin visualization, the sections were developed with 3,3′-diaminobenzidine to produce a brown stain, which was counterstained with hematoxylin. The stained tissue sections were observed using an optical Axiomager microscope system. The podocin protein level was quantified using the microscope system image analysis program.
Data analysis
The results are presented as the mean±SEM. Statistical analyses were carried out using the Statistical Analysis software package version 6.12 (SAS Institute, Cary, NC, USA). Significance was determined by one-way ANOVA followed by Duncan's multiple-range test for multiple comparisons. Differences were considered significant at P<0.05.
Results
Chrysin-mediated inhibition of high glucose-induced podocyte injury
Glomerular podocytes form the filtration slit structure that maintains glomerular filtration barrier function and prevents the escape of plasma proteins from the glomerular circulation6. Podocyte injury and loss are common pathological features in various glomerular diseases, including glomerulosclerosis and diabetic nephropathy, and are the leading causes of end-stage renal disease12,23. This study examined whether chrysin inhibited high glucose-induced podocyte injury and apoptosis. Glomerular podocytes were dead ≈10% in media containing 33 mmol/L glucose for 2 d, compared with 27.5 mmol/L (+5.5 mmol/L glucose) mannitol-challenged cells (Figure 1C). When the high glucose-exposed podocytes were treated with nontoxic levels of chrysin (1–20 μmol/L), the viability was gradually recovered to that of the glucose controls.
To assess podocyte apoptosis under high glucose conditions, in situ TUNEL assays to detect DNA fragmentation were conducted. High glucose conditions promoted DNA fragmentation in podocytes, whereas green-stained DNA fragments were dose-dependently reduced in ≥1 μmol/L chrysin-treated cells (Figure 1D). These results indicate that submicromolar chrysin alleviated podocyte apoptosis induced by excess glucose.
Inhibitory effects of chrysin on induction of apoptosis-related proteins
Hyperglycemia has been shown to promote podocyte apoptosis and play a key role in the pathogenesis of diabetic nephropathy4. This study examined whether chrysin modulated the expression of the apoptosis-related proteins Bcl-2 and Bax in high glucose-experienced podocytes. As shown in Figure 2A, high glucose conditions reduced Bcl-2 induction in podocytes and enhanced Bax expression compared with the glucose and osmotic mannitol controls. However, 1–20 μmol/L chrysin treatment restored podocyte Bcl-2 expression under experimental diabetic conditions in a dose-dependent manner (Figure 2A). In contrast, Bax induction was significantly inhibited in the chrysin-treated and high glucose-exposed podocytes.
Western blot analysis showing modulation of Bax and Bcl-2 induction (A) and Apaf-1 induction and cytochrome c release in cytosol (B) by chrysin treatment in 33 mmol/L glucose-challenged renal podocytes. The podocytes were incubated in media containing different glucose contents (5.5 mmol/L glucose and 27.5 mmol/L mannitol as osmotic controls and 33 mmol/L glucose in the absence or presence of 1–20 μmol/L chrysin for 3 d). The cytosolic and mitochondrial fractions were separately prepared by extracting cells with digitonin. Cell lysates or fractions were subject to electrophoresis on 12%–15% SDS-PAGE and Western blot analysis using a primary antibody against Bcl-2, Bax, Apaf-1 or cytochrome c (3 separate experiments). β-Actin was used as internal control in whole extracts, and COX IV was used as an internal control for cytochrome c present in the cytosol and mitochondria. The bar graphs (mean±SEM) represent the quantitative results of blots obtained from a densitometer. *P<0.05 vs 5.5 mmol/L glucose or 27.5 mmol/L mannitol. #P<0.05 vs 33 mmol/L glucose alone.
Apaf-1 plays an essential role in apoptosis by assembling into an oligomeric apoptosome in the presence of cytosolic cytochrome c, which is responsible for activation of caspase 9 that leads to apoptosis24. This study investigated whether chrysin attenuated apoptosis by inhibiting Apaf-1-mediated apoptosome formation. High glucose exposure (33 mmol/L) enhanced Apaf-1 induction in podocytes, whereas chrysin treatment diminished this phenomenon (Figure 2B). It is well known that cytochrome c is released into the cytosol from mitochondria and triggers programmed cell death via apoptosis, which is controlled by multiple regulatory proteins, such as Bcl-2 family members25. The cytosolic and mitochondrial fractions were isolated following the permeabilization of plasma cell membranes with digitonin. Western blot data showed that high glucose exposure promoted cytochrome c release into the cytosol from mitochondria (Figure 2B). In contrast, the mitochondrial level of cytochrome c was reduced in high glucose-exposed cells. When the cells were treated with ≥10 μmol/L chrysin, the cytochrome c release into the cytosol was reduced with a concomitant increase in mitochondrial cytochrome c levels (Figure 2B). These results indicated that chrysin interrupted the mitochondrial apoptotic pathway by inhibiting cytochrome c release into the cytosol. Accordingly, chrysin blunted macrophage apoptosis via blockade of apoptosome formation by inhibiting the release of the cytochrome c-Apaf-1 complex into the cytosol.
Suppression of glomerular and podocyte damage by chrysin
Pathologic alterations in and loss of podocytes are major contributors to glomerular filtration barrier failure and proteinuria in diabetic kidney26. As expected, the urinary excretion of albumin was elevated, possibly due to podocyte injury in diabetic animals (Figure 3A). In contrast, the proteinuria was alleviated by oral supplementation of chrysin to the diabetic mice. Glomerular injury leading to ultrafiltration of excessive amounts of proteins signals to the tubular and interstitial structures and is followed by tubulointerstitial damage and dysfunction27. The PAS staining revealed tubular interstitial fibrosis present in db/db mice, which was ameliorated by supplementation with 10 mg/kg chrysin (Figure 3B).
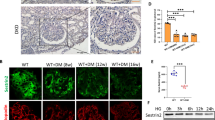
Inhibition of urinary albumin excretion (A) and renal tubular interstitial fibrosis (B) by oral chrysin supplementation in db/db mice. The db/db mice were supplemented with 10 mg/kg chrysin for 10 weeks. Urinary excretion of albumin collected for 24 h was measured using the Albuwell M ELISA kit (A). Real tubular tissue sections were stained using PAS reagents and counterstained with hematoxylin (B). TEM analysis showing the ultrastructures of kidney glomerular tissues and a capillary tuft containing red blood cells, podocytes with primary and secondary processes, and mesangial cells (C). The ultrastructure was significantly normalized after chrysin supplementation. Scale bar=3 μm. The arrows designate podocyte foot process effacement and the asterisk the glomerular basement membrane. Abbreviations: C, capillary tuft; M, mesangial cells; P, podocytes; E, red blood cells. *P<0.05 vs db/m control. #P<0.05 vs db/db mice not treated with 10 mg/kg chrysin.
To examine the impact of chrysin on the abnormal ultrastructure of the glomeruli in diabetic mice, we performed TEM analysis. Under TEM, the glomerular morphology was crushed out of shape in the diabetic mice, compared with that of the non-diabetic animals (Figure 3C). In addition, apoptotic podocytes and significant podocyte foot process effacement were observed in some of the glomeruli from the diabetic mice (arrows). However, such alterations in glomerular ultrastructure were normalized after the oral supplementation of 10 mg/kg chrysin (Figure 3C). The glomerular basal membrane was thickened and had apparent structural aberrations (asterisk). Taken together, these data indicate that chrysin treatment deterred podocyte injuries and proteinuria detected in diabetic mice.
Restoration of slit diaphragm protein synthesis by chrysin treatment
Podocin and nephrin are two essential components of the slit diaphragm of the podocyte foot process28,29. When the podocytes were exposed to 33 mmol/L glucose, the expression of Cy3-reddish nephrin was down-regulated (Figure 4A). Similarly, the induction of FITC-stained podocin was reduced in the high glucose-exposed podocytes (Figure 4B). In contrast, 1–20 μmol/L chrysin treatment improved the induction of these slit diaphragm proteins in a dose-dependent manner. Consistently, immunohistochemical staining for podocin showed heavy brownish staining in non-diabetic mouse glomeruli, whereas there was lack of glomerular podocin expression in the diabetic kidney (Figure 4C). Thus, chrysin may attenuate the severity of foot process effacement through enhancing expression of nephrin and podocin in the glomeruli of diabetic mice (Figure 3C).
Immunostaining showing inhibition of nephrin and podocin induction by chrysin. Podocytes were incubated in media containing different glucose concentrations (5.5 mmol/L glucose and 27.5 mmol/L mannitol as osmotic controls and 33 mmol/L glucose) in the absence or presence of 1–20 μmol/L chrysin for 3 d (A and B). For the immunocytochemical analysis of nephrin, a Cy3-conjugated secondary antibody was used to visualize the nephrin induction, and the nuclear staining was accomplished with DAPI (A). The cells were stained with a FITC-conjugated secondary antibody for podocin visualization (B). Microphotographs were obtained with fluorescence microscopy using an Axiomager microscope system. The db/db mice were supplemented with 10 mg/kg chrysin for 10 weeks. Kidney tissue podocin induction was observed in db/m control and db/db mice by immunohistochemical staining (C). The sections were developed with 3,3′-diaminobenzidine producing a brown staining that was counterstained with hematoxylin. Each photograph was representative of four mice and was obtained with light microscopy and quantified using an Axiomager microscope system. Magnification: ×200. *P<0.05 vs db/m control. #P<0.05 vs db/db mice not treated with 10 mg/kg chrysin.
Blockade of podocyte ER stress by chrysin
There is growing evidence that ER stress is involved in glomerular epithelial cell injury30. This study attempted to address ER stress-mediated podocyte injury and apoptosis under hyperglycemic conditions and to reveal that this phenomenon was blocked by chrysin. High glucose exposure elevated the UPR to ER stress in podocytes through increased phosphorylation of the eIF2α translation initiation factor and increased expression of CHOP (Figure 5A). The activation of eIF2α in podocytes occurred 24 h after the exposure to high glucose and was highly enhanced within 48 h and sustained for 5 d. The CHOP induction was shortly elevated within 48 h after the podocytes were exposed to 33 mmol/L glucose. However, submicromolar chrysin counteracted the activation of eIF2α and the induction of the transcription factors ATF4 and CHOP in the ER promoted by diabetic stimuli (Figure 5B). Consistently, high glucose exposure resulted in a marked activation of the ER stress sensor PERK without a further induction of the ER chaperone GRP78/BIP (Figure 6A). Such PERK activation was attenuated by chrysin in a dose-dependent manner. These results suggest that chrysin may block translation shutoff and allow translational reprogramming to resume (Figure 4A and 4B). Furthermore, this study investigated whether the podocyte injury and slit diaphragm protein malfunction were attributed to the activation of the UPR signaling branch of the PERK-eIF2α-ATF-CHOP pathway (Figure 6B). The induction of the apoptosis-related proteins Bcl-2 and Bax was reversed in 0.1 μmol/L PERK inhibitor-treated podocytes exposed to high glucose conditions (Figure 6B). Additionally, PERK inhibition restored the induction of podocin and nephrin, which was down-regulated in high glucose-exposed podocytes.
Temporal responses of eIF2α activation and CHOP induction (A) and their inhibition by chrysin (B) in 33 mmol/L glucose-challenged renal podocytes. Podocytes were incubated in media containing different glucose concentrations (5.5 mmol/L glucose and 27.5 mmol/L mannitol as osmotic controls and 33 mmol/L glucose in the absence or presence of 1–20 μmol/L chrysin for 3 d). Cell lysates were subject to electrophoresis on 8%–12% SDS-PAGE and Western blot analysis using a primary antibody against phospho-eIF2α, ATF4 or CHOP (3 separate experiments). β-Actin was used as an internal control. The bar graphs (mean±SEM) represent the quantitative results of blots obtained from a densitometer. *P<0.05 vs 0 h (A), or 5.5 mmol/L glucose or 27.5 mmol/L mannitol (B). #P<0.05 vs 33 mmol/L glucose alone (B).
Inhibitory effects of chrysin on GRP78/Bip induction and PERK activation (A) and inhibition of podocyte apoptosis and slit diaphragm protein induction by PERK inhibition (B) in 33 mmol/L glucose-challenged renal podocytes. Podocytes were incubated in media containing different glucose concentrations (5.5 mmol/L glucose and 27.5 mmol/L mannitol as osmotic controls and 33 mmol/L glucose in the absence or presence of 1–20 μmol/L chrysin or 0.1 μmol/L PERK inhibitor for 3 d). Cell lysates were subjected to electrophoresis on 8%–12% SDS-PAGE and Western blot analysis using a primary antibody against GRP78/Bip, PERK, phospho-PERK, Bax, Bcl-2, podocin or nephrin (3 separate experiments). β-Actin was used as an internal control. The bar graphs (mean±SEM) represent the quantitative results of blots obtained from a densitometer. *P<0.05 vs 5.5 mmol/L glucose or 27.5 mmol/L mannitol (A), or 5.5 mmol/L glucose (B). #P<0.05 vs 33 mmol/L glucose alone (A), or 33 mmol/L glucose alone (B).
This study further confirmed that oral supplementation with chrysin inhibited ER stress in diabetic renal cells. Although the GRP78/BIP induction was not altered in db/db mouse kidney, the expression of the UPR-specific eIF2α kinase PERK was abolished, and its activation was substantially enhanced (Figure 7A). Such ER responses were reversed by chrysin supplementation in diabetic mice. In addition, eIF2α activation and the induction of ATF4 and CHOP were highly elevated in the diabetic kidney compared with that in the non-diabetic normal kidney (Figure 7B). When 10 mg/kg chrysin was orally administered to the mice, the activation and induction of ER stress markers was reduced. Moreover, chrysin influenced the expression of the apoptosis-related proteins Bax, Bcl-2 and Apaf-1 in the diabetic kidney (Figure 7C). Chrysin attenuated the diabetic induction of apoptotic Bax and Apaf-1 in renal tissues, whereas the renal Bcl-2 induction was enhanced in chrysin-administered db/db mice (Figure 7C). Accordingly, chrysin may have a potential nephroprotective effect on diabetes-associated podocyte apoptosis and shutdown of slit diaphragm protein synthesis by inhibiting PERK/eIF2α-P/ATF4/CHOP signaling.
Western blot data showing blockade of GRP78/Bip induction and PERK activation (A), eIF2α activation and induction of ATF4 and CHOP (B), and induction of Bax, Bcl-2 and Apaf-1 (C) in chrysin-supplemented db/db mice. The db/db mice were supplemented with 10 mg/kg chrysin for 10 weeks. Kidney tissue extracts were subjected to electrophoresis on 8%–12% SDS-PAGE and Western blot analysis using a primary antibody against GRP78/Bip, PERK, phospho-PERK, phospho-eIF2α, ATF4, CHOP, Bax, Bcl-2 or Apaf-1 (3 separate experiments). β-Actin was used as an internal control. The bar graphs (mean±SEM) represent the quantitative results of blots obtained from a densitometer. *P<0.05 vs db/m control. #P<0.05 vs db/db mice not treated with 10 mg/kg chrysin.
Discussion
Seven major findings were extracted from this study. (1) High glucose exposure caused apoptotic death in glomerular podocytes, which was attenuated by nontoxic chrysin treatment at 1–20 μmol/L through reduction of DNA fragmentation. (2) Chrysin reversed the reduced Bcl-2 expression and enhanced Bax induction in podocytes under in vitro and in vivo diabetic conditions. (3) Proteinuria and tubular interstitial fibrosis were ameliorated by oral supplementation of chrysin to the mice. (4) Oral supplementation of chrysin restored alterations in glomerular ultrastructure with apoptotic podocytes and podocyte foot process effacement observed in the glomeruli of diabetic mice. (5) Chrysin improved the induction of the slit diaphragm proteins podocin and nephrin, which was lost in the glomeruli of diabetic mice. (6) Chrysin diminished the cytochrome c release into the cytosol and/or Apaf-1 induction in podocytes and diabetic renal tissues. (7) The PERK-eIF2α-ATF4-CHOP-dependent ER stress responses were up-regulated in the high glucose-treated podocytes and in diabetic kidney tissues, whereas chrysin counteracted such UPR promoted by diabetic stimuli. Our findings show that chrysin can antagonize ER stress as a possible new therapeutic target for podocyte loss and injury, which leads to malfunction of the glomerular filtration barrier.
There is accumulating evidence that the clinical signature of podocyte injury and reduced podocyte number is proteinuria, regardless of the loss of renal function owing to glomerulosclerosis. Highly specialized glomerular epithelial podocytes play a crucial role in maintaining the function of the glomerular filtration barrier3,4. It has been suggested that aberrant histopathological changes in podocytes are involved in the pathogenesis of albuminuria in diabetes3,5,23. Since the optimal function of podocytes largely depends on their specialized architecture stabilizing glomerular capillaries and the barrier functions of the glomerular filter, podocyte injury is typically associated with marked proteinuria28. The number of podocytes and the induction of molecular components of the slit diaphragm, which is a specialized structure of podocyte-podocyte interactions, all are reduced and damaged in diabetic kidneys29. These findings have furthered drug discoveries that have therapeutic implications in the treatment of the proteinuria and podocyte loss8,28,31. Many current therapies, including corticosteroids and calcineurin antagonists, exhibit potent effects on podocytes, but their nonspecific natures can produce undesirable systemic adverse effects31,32. Natural agents, such as plant compounds of flavonoids and other phenolics with a more specific focus on diabetic renal damage, would cause less treatment-associated morbidity33,34,35. Curcumin inhibits high glucose-induced podocyte apoptosis and diabetic nephropathy via regulation of the functional connections between caveolin-1 phosphorylation and ROS34. This study showed that high glucose/hyperglycemia resulted in podocyte apoptosis via activation of the apoptotic Bax/Bcl-2 and Apaf-1/cytochrome c pathways, which was inhibited by chrysin treatment. In addition, the podocyte loss resulted from the activation of the PERK-CHOP pathway triggered by diabetic stimuli. Furthermore, the proteinuria and tubular interstitial fibrosis were evident in diabetic animals, and such pathological consequences were ameliorated by oral chrysin supplementation. Similarly, astragaloside IV attenuates proteinuria in streptozotocin-induced DN in rats via the inhibition of ER stress-mediated podocyte apoptosis36.
Numerous studies have reported that abnormal changes in the podocyte anatomy disrupt a complex interplay of proteins that comprises the molecular anatomy of the different protein domains in podocytes37,38. Modulation of nephrin expression in the glomerulus is related to the extent of proteinuria in DN29. In this study, the protein induction of nephrin and podocin, which are slit diaphragm proteins, was dampened in high glucose-exposed podocytes and in db/db mouse glomeruli. This study proposed that the reduced expression of these proteins under diabetic conditions could be attributed to the induction of the ER stress response UPR. The UPR is known to be activated in diabetic kidneys to restore normal ER function by reducing protein synthesis and enhancing ER folding for the ER proteostasis network12,13 . The phosphorylation of elF2α responsible for the initiation of translation represses translation during ER stress39. High glucose/hyperglycemia activated elF2α, which is an essential factor for protein synthesis, in high glucose-exposed podocytes and in diabetic renal tissues. Similarly, one investigation showed that the elF2α phosphorylation and consequent general reduction in protein synthesis may be a novel mechanism for limiting complement- or ischemia-reperfusion-dependent glomerular epithelial cell injury29. In addition, PERK/ATF4 activation was observed in the diabetic kidney, indicating that excessive ER stress was a manifest factor in modifying slit diaphragm protein synthesis. Accumulation of misfolded proteins in the ER triggered the PERK activation. If ER stress is undue, the UPR can be superseded by pro-apoptotic cell death15. In the present study, the apoptotic loss was evident in the high glucose-exposed podocytes. Consequently, it is assumed that the protein levels of nephrin and podocin were diminished due to diabetes-associated apoptotic loss of podocytes.
Although podocyte depletion is a common feature during the early stages of diabetic kidney diseases, the underlying mechanisms of such phenomenon in DN remain poorly understood. The UPR interacts in a coordinated manner with protective cellular autophagy to alleviate protein misfolding in proteinuric glomerular diseases12,14,40. Podocytes exhibit high autophagic activity, and autophagy deficiency in podocytes is associated with susceptibility to glomerulopathies with proteinuria and ultrastructural changes including foot process effacement41. Unfortunately, the current study did not examine the crosstalk between UPR and autophagy for the podocyte injury. In addition, it has been shown that podocyte injury via activation of the ER stress response may be caused by oxidative stress, which ultimately leads to apoptosis15. This study did examine the specific mechanism(s) of chrysin targeting of diabetes-associated podocyte injury. Nevertheless, one can assume that the antioxidant activity of chrysin could be a mechanism for the inhibition of ER stress and induction of autophagy. For instance, the antioxidant silybin inhibits high glucose-induced oxidative stress and podocyte injury35. Finally, the anti-inflammatory activity of chrysin may be responsible for the protection against podocyte injury. Luteolin blocks renal inflammatory cell infiltration in streptozotocin-induced mice via inhibition of receptor-interacting protein 140/nuclear factor кB pathway, revealing the beneficial effect on renal damage in DN33.
In summary, this study investigated the capability of chrysin in combating ER stress-mediated podocyte injury and decreased induction of slit diaphragm protein synthesis in high glucose-exposed podocytes and diabetic mice. Nontoxic chrysin treatment deterred high glucose-elicited podocyte apoptosis that resulted in the loss of nephrin and podocin protein expression. Oral administration of chrysin blocked albuminuria, renal tubular fibrosis and podocyte foot process effacement in mouse kidneys. In addition, this compound restored podocin expression in diabetic glomeruli. Furthermore, chrysin restricted ER stress in podocytes and renal cells via inhibition of PERK-elF2α-ATF4-CHOP pathway in diabetic kidneys. Therefore, chrysin acted therapeutically to antagonize podocyte injury, which leads to barrier dysfunction and proteinuria due to aberrant podocyte-podocyte interactions in cellular or animal models of diabetic complications in kidneys.
Abbreviations
Apaf1, apoptotic peptidase activating factor 1; ATF4, activating transcription factor 4; CHOP, C/EBP homologous protein; DN, diabetic nephropathy; ER, endoplasmic reticulum; elF2α, eukaryotic initiation factor 2α; GRP78, glucose-regulated protein 78; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ROS, reactive oxygen species; UPR, unfolded protein response.
Author contribution
Min-Kyung KANG, Eun-Jung LEE, and Young-Hee KANG designed the research; Min-Kyung KANG, Dong Yeon KIM, Sin-Hye PARK, Yun-Ho KIM, and Lucia Dwi ANTIKA conducted the research; Min-Kyung KANG, Eun-Jung LEE, and Yean-Jung CHOI analyzed the data; and Min-Kyung KANG and Young-Hee KANG wrote the paper. Young-Hee KANG had primary responsibility for final content. All authors read and approved the final manuscript.
References
Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF . Diabetic kidney disease: from physiology to therapeutics. J Physiol 2014; 592: 3997–4012.
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008; 233: 4–11.
Garg P, Rabelink T . Glomerular proteinuria: a complex interplay between unique players. Adv Chronic Kidney Dis 2011; 18: 233–42.
Reddy GR, Kotlyarevska K, Ransom RF, Menon RK . The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hyperten 2008; 17: 32–6.
Diez-Sampedro A, Lenz O, Fornoni A . Podocytopathy in diabetes: a metabolic and endocrine disorder. Am J Kidney Dis 2011; 58: 637–46.
Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 2009; 58: 1201–11.
Wolf G, Chen S, Ziyadeh FN . From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005; 54: 1626–34.
Bhatti AB, Usman M . Drug targets for oxidative podocyte injury in diabetic nephropathy. Cureus 2015; 7: e393.
Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 2014; 25: 1237–54.
Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One 2013; 8: e60546.
Yasuda-Yamahara M, Kume S, Tagawa A, Maegawa H, Uzu T . Emerging role of podocyte autophagy in the progression of diabetic nephropathy. Autophagy 2015; 11: 2385–6.
Cunard R . Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med 2015; 4: 715–40.
Taniguchi M, Yoshida H . Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hyperten 2015; 24: 345–50.
Cybulsky AV . The intersecting roles of endoplasmic reticulum stress, ubiquitin- proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 2013; 84: 25–33.
Woehlbier U, Hetz C . Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci 2011; 36: 329–37.
Yuan Y, Xu X, Zhao C, Zhao M, Wang H, Zhang B, et al. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone /mineralocorticoid receptor-induced podocyte injury. Lab Invest 2015; 95:1374–86.
Niforou K, Cheimonidou C, Trougakos IP . Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol 2014; 2: 323–32.
Rivas A, Vidal RL, Hetz C . Targeting the unfolded protein response for disease intervention. Expert Opin Therapeutic Targets 2015; 19: 1203–18.
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E . Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 2014; 728: 107–18.
Rashid S, Ali N, Nafees S, Hasan SK, Sultana S . Mitigation of 5-fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem Toxicol 2014; 66: 185–93.
Ali BH, Adham SA, Al Za'abi M, Waly MI, Yasin J, Nemmar A, et al. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS One 2015; 10: e0125285.
Kang MK, Park, SH, Choi YJ, Shin D, Kang YH . Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med (Berlin) 2015; 93: 759–72.
Lewko B, Stepinski J . Hyperglycemia and mechanical stress: targeting the renal podocyte. J Cell Physiol 2009; 221: 288–95.
Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, Schindler CK, et al. Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ 2001; 8: 1169–81.
Ow YP, Green DR, Hao Z, Mak TW, Gao F . Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol 2008; 9: 532–42.
Wang L, Tang Y, Eisner W, Sparks MA, Buckley AF, Spurney RF . Augmenting podocyte injury promotes advanced diabetic kidney disease in Akita mice. Biochem Biophys Res Commun 2014; 444: 622–7.
Zoja C, Abbate M, Remuzzi G . Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dialysis Transplant 2015; 30: 706–12.
Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y . The role of podocytes in proteinuria. Nephrology 2007; 12: S15–S20.
Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthöfer H, Zaoui P, et al. Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 2002; 45: 1572–6.
Cybulsky AV, Takano T, Papillon J, Kitzler TM, Bijian K . Endoplasmic reticulum stress in glomerular epithelial cell injury. Am J Physiol Renal Physiol 2011; 301: F496–F508.
Mathieson PW . The podocyte as a target for therapies--new and old. Nat Rev Nephrol 2011; 8: 52–6.
Schönenberger E, Ehrich JH, Haller H, Schiffer M . The podocyte as a direct target of immunosuppressive agents. Nephrol Dialysis Transplant 2011; 26: 18–24.
Bao L, Cai X, Dai X, Ding Y, Jiang Y, Li Y, et al. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-γ coactivator 1α in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct 2014; 5: 1872–80.
Sun LN, Liu XC, Chen XJ, Guan GJ, Liu G . Curcumin attenuates high glucose-induced podocyte apoptosis by regulating functional connections between caveolin-1 phosphorylation and ROS. Acta Pharmacol Sin 2016; 37: 645–55.
Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P . The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol 2013; 305: F691–F700.
Wang ZS, Xiong F, Xie, XH, Chen D, Pan JH, Cheng L . Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrol 2015; 16: 44.
Brinkkoetter PT, Ising C, Benzing T . The role of the podocyte in albumin filtration. Nat Rev Nephrol 2013; 9: 328–36.
Pavenstädt H, Kriz W, Kretzler M . Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253–307.
DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M . Phosphorylation of eukaryotic translation initiation factor 2α coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol Cell Biol 2009; 29: 4295–307.
Dodson M, Darley-Usmar V, Zhang J . Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med 2013; 63: 207–21.
Oliva Trejo JA, Asanuma K, Kim EH, Takagi-Akiba M, Nonaka K, Hidaka T, et al. Transient increase in proteinuria, poly-ubiquitylated proteins and ER stress markers in podocyte-specific autophagy-deficient mice following unilateral nephrectomy. Biochem Biophys Res Commun 2014; 446: 1190–6.
Acknowledgements
This work was supported a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No 2015R1A2A2A01006666).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, MK., Park, SH., Kim, YH. et al. Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Acta Pharmacol Sin 38, 1129–1140 (2017). https://doi.org/10.1038/aps.2017.30
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.30
Keywords
This article is cited by
-
The effects of propolis on pro-oxidant–antioxidant balance, glycemic control, and quality of life in chronic kidney disease: a randomized, double-blind, placebo-controlled trial
Scientific Reports (2023)
-
Research progress on endoplasmic reticulum homeostasis in kidney diseases
Cell Death & Disease (2023)
-
Chrysin protects cardiac H9c2 cells against H2O2-induced endoplasmic reticulum stress by up-regulating the Nrf2/PERK pathway
Molecular and Cellular Biochemistry (2023)
-
Development of erianin-loaded dendritic mesoporous silica nanospheres with pro-apoptotic effects and enhanced topical delivery
Journal of Nanobiotechnology (2020)
-
Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats
European Journal of Nutrition (2020)