Abstract
Major depressive disorder is a common but devastating mental disorder, and recent evidence shows that neuroinflammation may play a pivotal role in the etiology of depression. Astragaloside IV (AS-IV) is an active component purifed from Astragalus membranaceus (Fisch) Bge, which has shown anti-inflammatory, anti-oxidative and anti-apoptotic effects. In this study, we explored whether AS-IV produced antidepressant effects via its inhibition of neuroinflammation in mouse models of depression. Depressive-like behaviors including decreased sucrose consumption, reduced locomotor activity and increased immobility time were induced in mice using repeated restraint stress (RRS). We found that administration of AS-IV (16, 32 and 64 mg·kg-1·d-1, ig) significantly attenuated RRS-induced depressive-like behaviors. Furthermore, AS-IV administration significantly reduced the levels of TNF-α and IL-1β, increased PPARγ expression and GSK3β phosphorylation, decreased NF-κB phosphorylation, and reduced NOD-, LRR- and pyrin domain-containingprotein 3 (NLRP3) inflammasome and caspase-1 p20 generation in the hippocampus of the mice. LPS-induced depression-like behaviors were induced by LPS injection (1 mg·kg-1·d-1, ip), which were ameliorated by administration of AS-IV (20, 40 mg·kg-1·d-1, ig). The results of the LPS-induced mouse model were in accordance with those acquired from the RRS-induced mouse model: LPS injection significantly increased TNF-α and IL-1β expression in the mouse hippocampus, which was reversed by administration of AS-IV. Moreover, administration of AS-IV significantly increased PPARγ expression and GSK3β phosphorylation, and decreased NF-κB phosphorylation and NLRP3 inflammasome. These results suggest that AS-IV is a potential drug against depression, and its antidepressant effects are partially mediated by inhibition of neuroinflammation via the upregulation of PPARγ expression.
Similar content being viewed by others
Introduction
Depression is a devastating mental illness, afflicting nearly 20% of the population in the United States1. Furthermore, depression is even life-threatening because of the increased risk of suicide, which results in disability or mortality, contributing to the health burden and family economic loss around the globe2,3. The currently available antidepressants cannot meet the needs of depressive patients due to the delayed efficacy, severe side effects and partial responsiveness or non-responsiveness1. Accordingly, the development of better treatments with high efficacy and safety is urgently needed.
Accumulating evidence shows that environmental stimuli (including social and psychological stress) activate the inflammatory system, leading to a high risk of vulnerability to depression1,4,5,6. Of note, extensive data have elucidated the existence of a strong connection between inflammation and major depressive disorder (MDD), supported by elevated levels of pro-inflammatory cytokines in people diagnosed with MDD7,8,9. Post-mortem studies have also shown increased levels of the pro-inflammatory cytokines interleukin 1β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α) in brain tissues of MDD patients4,10. After the administration of inflammatory cytokines such as IFNα or their inducers such as endotoxin, healthy individuals develop symptoms of depression4,11,12, which could be blocked by anti-inflammatory interventions such as knockout of cyclooxygenase 2 or IL-1 receptors4,13,14. Clinical trials, along with animal studies, have elaborated that peripheral damage caused by damage-associated molecular patterns activates the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome followed by caspase-1 activation, contributing to the production of inflammation in the periphery or in the central nervous system (CNS)4,11. These data support the idea that inflammatory responses in peripheral tissues may transport inflammation into the brain through several pathways such as saturable transport systems, thereby contributing to the context of depression4,6,11,13, highlighting that neuroinflammation may play a pivotal role in the etiology of depression.
Peroxisome proliferator activated receptor gamma (PPARγ), a ligand-activated transcription factor, belongs to the nuclear receptor superfamily15. Among many ligands of PPARγ, thiazolidinediones, acting as PPARγ agonists, are commonly used to cure type 2 diabetes16. In particular, PPARγ agonists are reported to play a critical role in regulating metabolic disorder, inflammatory responses and oxidative stress16,17. In addition, PPARγ agonists have demonstrated antidepressant effects in previous studies15. Consequently, PPARγ agonists could be potential drug candidates for depression therapy.
GSK3β activation, characterized by inhibition of its phosphorylation18, is reported to be closely related to the regulation of the downstream nuclear transcription factor NF-κB, further modulating the inflammatory response19,20. The production of IL-1β is mediated by the NLRP3 inflammasome, which can be blocked by the NF-κB inhibitor (Bay11-7082)21, indicating that NF-κB activation can evoke inflammatory signals in the periphery and CNS. However, whether PPARγ activation can inhibit the GSK3β/NF-κB/NLRP3 inflammasome axis to fight against depression remains unknown.
Astragaloside IV (AS-IV, Figure 1A) is a monomer purified from Astragalus membranaceus (Fisch) Bge(C41H68O14; Mw=784.98)22 that is widely employed in traditional Chinese medicine to treat neurodegenerative diseases such as Alzheimer's disease23. Laboratory studies demonstrate that AS-IV has anti-oxidative, anti-inflammatory and anti-hypertensive properties24. Moreover, AS-IV has also been reported to ameliorate ischemia reperfusion-induced brain injury, reduce obesity and prevent diabetes, suggesting a potential neuroprotective effect of AS-IV. In addition, AS-IV has been found to be a natural PPARγ agonist17 and may produce anti-depressant effects similar to rosiglitazone, a PPARγ agonist that has been reported to have an antidepressant effect15. However, whether AS-IV can attenuate depressive-like behaviors has never been researched, and its underlying mechanism remains unclear. From this perspective, we aimed to investigate the anti-depressive effect of AS-IV and the associated downstream NF-κB signaling pathway. Our results revealed that AS-IV alleviated depressive-like behaviors in repeated restraint stress (RRS)-induced mice, decreased TNF-α and IL-1β expression, increased PPARγ expression, and inactivated GSK3β, NF-κB and the NLRP3 inflammasome, results that were also consistent with the outcomes observed in lipopolysaccharide (LPS)-induced mice. In conclusion, AS-IV may serve as a potential drug candidate for the treatment of depression.
Materials and methods
Drugs and reagents
Astragaloside IV (AS-IV, purity ≥98%) was obtained from Nanjing Spring & Autumn Biological Engineering Co, Ltd (Nanjing, China). Lipopolysaccharide was purchased from Sigma-Aldrich Co (St Louis, USA). Fluoxetine hydrochloride (Flu) was bought from Changzhou Siyao Pharmaceuticals Co, Ltd (Changzhou, China). The bicinchoninic acid assay kit was acquired from the Beyotime Institute of Biotechnology Co, Ltd (Shanghai, China).
Animals
Male ICR mice, weighing 23-26 g, were purchased from the Experimental Animal Center in Jiangsu Province (Nanjing, China) and housed in cages under standard laboratory conditions (12-h light/dark cycle, 25±2 °C). The mice had free access to food and water. No experiment was carried out before one week of environmental adaptation. All animal experiments were performed in strict accordance to the Provision and General Recommendation of Chinese Experimental Animals Administration Legislation and were approved by the Science and Technology Department of Jiangsu Province.
Repeated restraint stress model
Male ICR mice were randomly divided into seven groups (10 mice in each group): Control group, AS-IV64 group, RRS group, RRS+Flu (20 mg·kg-1·d-1, ig) group, RRS+AS-IV16 (16 mg·kg-1·d-1, ig) group, RRS+AS-IV32 (32 mg·kg-1·d-1, ig) group, and RRS+AS-IV64 (64 mg·kg-1·d-1, ig) group. A repeated restraint stress (RRS) procedure was conducted, as previously reported with minor modifications25. The dose of Flu in mice was set according to a previous report26. Mice, except those in the Control group and AS-IV64 group, were individually subjected to restraint stress (4 h per day) for 9 consecutive days through their placement into a 50-mL plastic tube. After the mice were released from the plastic tube, they were treated with Flu or AS-IV through oral gavage (both diluted in double distilled water). The Control group and RRS group received the same volume of double distilled water. The experimental design is shown in Figure 1B.
LPS model
Male ICR mice were randomly divided into five groups (10 mice in each group): Control group, LPS group, LPS+Flu (20 mg·kg-1·d-1, ig) group, AS-IV20 (20 mg·kg-1·d-1, ig) group, and AS-IV40 (40 mg·kg-1·d-1, ig) group. AS-IV doses were determined by referring to the effects of AS-IV in the RRS mouse model. The dose of LPS and the experimental procedure were carried out according to a previous study27. The mice, except for those in the Control group, received Flu (20 mg·kg-1·d-1, ig), AS-IV (20, 40 mg·kg-1·d-1, ip) or double distilled water for 14 consecutive days, followed by LPS (diluted in physiological saline, 1 mg·kg-1·d-1, ip) injection for two days, as shown in Figure 1C.
Behavioral evaluation
Sucrose preference test (SPT)
Anhedonia is one of the common depressive-like behaviors observed in animal models of depression. SPT is used to assess the degree of anhedonia through observation of sucrose consumption as previously described28. First, after 24 h of water and food deprivation, mice were acclimatized to two bottles of 1% sucrose solution (w/v), one of which was switched to tap water (w/v) six hours later. Another six hours later, the mice were supplied with one bottle of tap water as usual. Then, in the test, after 24 h of water and food deprivation, each mouse was placed in a single cage with one bottle of 1% sucrose solution and one bottle of tap water. Six hours later, the location of the two bottles was switched in case of side preference. The weight of the two bottles was recorded before and after the 12-h test. Sucrose preference was calculated using the following formula: Sucrose Preference (SP)=sucrose intake (g)/[sucrose intake (g)+water intake (g)]×100%.
Open field test (OFT)
To examine the effects of AS-IV on locomotor activity in mice, an OFT was conducted as described previously29. The experimental apparatus was a metallic circular enclosure (30 cm in diameter and 20 cm high). In a quiet environment, mice were individually placed in the center of the enclosure and allowed to explore the bottom of the apparatus painted with 36-square fields for six minutes. The number of crossings during the last four minutes was recorded. The instrument was sprayed with ethanol to eliminate the smell after each trial.
Tail suspension test (TST)
A TST was performed in accordance with the previously reported method30. A mouse's tail, approximately 1 cm from the tip, was fixed onto the test instrument with black tape. Mice were suspended for 6 min, and the total immobility time during the final 4 min was recorded. Mice were considered immobile when they hung passively without struggling.
Forced swimming test (FST)
The FST was performed in accordance with the previously reported method31. Mice were forced to swim in 25±2 °C water for 10 min 24 h before the formal test. On the day of the test, mice were individually placed in a glass cylinder (height, 20 cm; diameter, 14 cm) filled with 25±2 °C water to a depth of 10 cm. Mice were forced to swim for 6 min, and the duration of immobility was recorded during the last 4 min. Mice were considered immobile when they were floating in the water without struggling and only making small movements necessary to keep their head above the water.
Immunohistochemistry
Mice were euthanized with 10% chloral hydrate intraperitoneally. Brains were quickly removed on ice and immersed for fixation in a fixative containing 10% formalin (WH2001, Shanghai Biotech Well Co, Ltd, China) for 24 h. Coronal sections were cut at the level of the dorsal hippocampus and processed for staining with the primary antibody [rabbit anti-TNF-α and anti-IL-1β, Abcam (Cambridge, UK)] according to the method previously described. The positive expression of TNF-α and IL-1β in the CA1 region of the hippocampus was visualized under a light microscope at 20× magnification. Quantitative image analysis was assessed by Image-Pro Plus 6.
Western blot analysis
On the day the mice were sacrificed, the brains were quickly isolated and then frozen at -80 °C. Then, we used the mouse hippocampi for protein analysis. An equal amount of hippocampal tissue in each group was homogenized by centrifugation at 12 000 rpm for 30 min, and then the supernatants were collected and quantified using the bicinchoninic acid assay kit as previously described. An equivalent amount of protein was separated by SDS-PAGE and transferred to PVDF membranes. The PVDF membranes were then blocked with 5% skimmed milk at room temperature for 2 h before incubation with the primary antibodies [anti-PPARγ (ab45036), anti-NLRP3 (ab214185), and anti-caspase-1 (ab108362), Abcam (Cambridge, UK); anti-p-NF-κB (BS4138), anti-NF-κB (BS1253), and anti-caspase-1 p20 (BS7071), anti-β-actin (BS6007M), Bioworld Technology (St Paul, MN, USA); anti-GSK3β (22104-1-AP), Proteintech Group (USA); anti-p-GSK3β (Ser9) (D85E12), Cell Signaling Technology (Beverly, MA, USA)] at 4 °C overnight. The membranes were washed three times for 10 min with TBST the next day and then incubated with streptavidin-HRP (at appropriate dilution) with gentle agitation for 2 h at room temperature. The membranes were detected by an imaging system (Bio-Rad, Hercules, CA, USA) with enhanced chemiluminescence (ECL) kits (Millipore, CA, USA). The quantification was performed by Image-Pro Plus 6.0 (IPP 6.0) software.
Statistical analysis
Data were expressed as the mean±SEM. Significant differences were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's test. The value, P<0.05, was considered statistically significant.
Results
AS-IV protected RRS-induced mice from depression
Effects of AS-IV in the SPT in RRS-induced mice
As shown in Figure 2A, a significant difference was seen between groups in the SPT [F(6, 70)=6.215]. RRS exposure reduced the sucrose consumption of mice (P<0.05), whereas AS-IV and Flu administration greatly increased the sucrose consumption (P<0.05), ameliorating the anhedonia behavior of the mice. Additionally, mice treated with AS-IV (32 mg/kg) consumed more sucrose than mice in the RRS+AS-IV16 group (AS-IV, 16 mg/kg), indicating that the effects of AS-IV were dose-related.
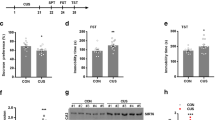
AS-IV alleviated depressive-like behavior in RRS-induced mice. (A) Effects of AS-IV treatment on the sucrose consumption percentage in RRS-induced mice (n=10). (B) Effects of AS-IV on the number of crossings of RRS-induced mice in the open field test (n=8). (C) Effect of AS-IV on the immobility time of RRS-induced mice in the tail suspension test (n=10). (D) Effect of AS-IV on the immobility time of RRS-induced mice in the forced swimming test (n=9). All values are expressed as the mean±SEM. # P<0.05 compared to the Control group; * P<0.05 compared to the RRS group.
Effects of AS-IV in the OFT in RRS-induced mice
We subsequently examined the effect of AS-IV on the spontaneous locomotor activity of mice. The comparison of the number of crossings between the groups was shown in Figure 2B [F(6,56)=3.634, P<0.05]. Compared with the Control group, the RRS group exhibited a significant reduction in the number of crossings. The RRS+AS-IV32 group, in comparison with the RRS group, had significantly more crossings (P<0.05). In addition, there was no difference in the number of crossings between the AS-IV64 group and Control group, indicating that AS-IV produced an anti-depressive effect without exciting the CNS.
Effects of AS-IV in the TST in RRS-induced mice
We then tested the effects of AS-IV on desperate behaviors in mice. First, as shown in Figure 2C [F(6,70)=3.451, P<0.05], RRS exposure increased the immobility time of mice in the TST compared to that in the Control group (P<0.05). Administration of Flu (20 mg/kg) and AS-IV (16, 32, and 64 mg/kg) reversed this increase in immobility time (P<0.05). In particular, AS-IV at 32 mg/kg had a better effect than AS-IV at 16 mg/kg or 64 mg/kg.
Effects of AS-IV in the FST in RRS-induced mice
As shown in Figure 2D [F(6, 63)=5.114], compared to the Control group, the RRS group showed a greater immobility time in the FST (P<0.05); however, AS-IV treatment (16, 32, and 64 mg/kg) decreased the immobility time in mice (P<0.05). The above data indicated that AS-IV and Flu treatment attenuated the RRS-induced depressive-like behaviors in mice.
Effects of AS-IV on TNF-α and IL-1β expression in the hippocam-pus of RRS-induced mice
The immunohistochemistry results are shown in Figure 3. RRS exposure obviously elevated the expression of TNF-α (Figure 3A) and IL-1β (Figure 3B) in the CA1 region of the hippocampus when comparing the expression in the RRS group to that in the Control group (P<0.05). AS-IV (16, 32, and 64 mg/kg) and Flu administration greatly suppressed the increase in the expression of TNF-α and IL-1β (P<0.05). These data demonstrated that AS-IV could repress the neuroinflammation observed in the RRS model, and the effect was similar to that observed with Flu treatment.
Effect of AS-IV on TNF-α and IL-1β expression in the RRS-induced mice. TNF-α and IL-1β expression was examined by immunohistochemistry staining in the CA1 region of the hippocampus. All values are expressed as the mean±SEM of 3 mice per group. # P<0.05 compared to the Control group; * P<0.05 compared to the RRS group. (A) TNF-α level. (B) IL-1β level.
Effects of AS-IV on the PPARγ/NF-κB pathway in the hippocampus of RRS-induced mice
To further elucidate the underlying mechanism of AS-IV in repressing depression, we tested the levels of related proteins in the mouse hippocampus. The expression levels of PPARγ and downstream proteins are displayed in Figure 4. After exposure to RRS, PPARγ expression in the mouse hippocampus of the RRS group was significantly lower than that of the Control group (P<0.05). However, AS-IV (16, 32, and 64 mg/kg) administration reversed this increase observed in the RRS group, as shown in Figure 4A and 4B (P<0.05). Furthermore, compared with the Control group, the RRS group exhibited a remarkable downregulation in the expression of phosphorylated GSK3β (P<0.05), whereas AS-IV (16, 32, and 64 mg/kg) treatment upregulated GSK3β phosphorylation, as shown in Figure 4A and 4C (P<0.05). In addition, the RRS group exhibited a notable elevation in the expression of NF-κB phosphorylation compared to the Control group (P<0.05), whereas AS-IV (16, 32, and 64 mg/kg) and Flu administration altered this effect, as shown in Figure 4A and 4D (P<0.05). These data revealed that the mechanism of the anti-depressive effect of AS-IV was closely related to alterations in PPARγ expression and phosphorylation of GSK3β and NF-κB.
Effects of AS-IV on NLRP3 inflammasome activation in the hippocampus of RRS-induced mice
Hippocampal NLRP3 and active caspase-1 p20 were detected as described in Figure 5. Mice that were exposed to RRS showed an increase in NLRP3 and active caspase-1 expression in the mouse hippocampus compared to those in the Control group, while AS-IV (16, 32, and 64 mg/kg) and Flu administration alleviated these effects, as shown in Figure 5A-5C, suggesting an inhibitory effect of AS-IV treatment on NLRP3 inflammasome activation.
AS-IV prevented the expression of depression-like behaviors in LPS-induced mice
To further demonstrate the antidepressant effect of AS-IV, we employed LPS to induce depressive-like symptoms in mice, in which LPS triggered inflammatory responses through challenging the innate immune system. According to the results of the RRS-induced mouse model, mice in the RRS+AS-IV64 group (AS-IV, 64 mg/kg) did not exhibit better therapeutic effects than those in the RRS+AS-IV32 group (AS-IV, 32 mg/kg) overall; therefore, we only tested the effects of 20 mg/kg and 40 mg/kg of AS-IV on the LPS-induced mouse model.
Effects of AS-IV in the OFT of LPS-induced mice
Experimental procedures were conducted as described for the RRS mouse model. Significant differences between groups are listed in Figure 6A [F(4,50)=13.08]. In the OFT, mice that received an LPS injection showed low locomotor activity, as demonstrated by fewer crossings in the LPS group than in the Control group (P<0.05). In contrast, AS-IV pretreatment (20 and 40 mg/kg) greatly increased the number of crossings in the OFT (P<0.05). The effect was similar to Flu treatment.
AS-IV alleviated the depressive-like behavior in LPS-induced mice. (A) Effects of AS-IV on the number of crossings of LPS-induced mice in the open field test (n=10). (B) Effect of AS-IV on the immobility time of LPS-induced mice in the tail suspension test (n=9). (C) Effect of AS-IV on the immobility time of LPS-induced mice in the forced swimming test (n=10). All values are expressed as the mean±SEM. # P<0.05 compared to the Control group; * P<0.05 compared to the LPS group.
Effects of AS-IV in the TST of LPS-induced mice
Effects of AS-IV on the immobility time in the TST are shown in Figure 6B [F(4,45)=4.086]. Compared to the Control group, the LPS group exhibited a prolonged immobility time in the TST (P<0.05), whereas AS-IV (20 mg/kg) pretreatment reduced the immobility time in the TST (P<0.05). AS-IV (40 mg/kg) and Flu pretreatment also decreased the immobility time but not significantly.
Effects of AS-IV in the FST of LPS-induced mice
As shown in Figure 6C [F(4,50)=12.93], mice subjected to LPS injection demonstrated an elevated immobility time in the FST compared to the Control group (P<0.05), whereas mice that received AS-IV (20 and 40 mg/kg) pretreatment showed reduced immobility time in the FST compared to those in the LPS group (P<0.05). These data indicated that AS-IV administration attenuated the depressive-like behaviors observed in LPS-induced mice.
Effects of AS-IV on TNF-α and IL-1β expression in the hippocampus of LPS-induced mice
TNF-α and IL-1β expression were analyzed by immunocytochemistry in Figure 7. LPS injection significantly enhanced the expression of TNF-α and IL-1β in the CA1 region of the mouse hippocampus (Figure 7A, 7B) (P<0.05). However, AS-IV (20 and 40 mg/kg) and Flu administration eliminated the effects of LPS via inhibiting the levels of TNF-α and IL-1β (P<0.05). The results coincided with the outcomes of the RRS-induced mouse model, strengthening the idea that AS-IV treatment suppressed neuroinflammation.
Effect of AS-IV on TNF-α and IL-1β expression in the LPS-induced mice. TNF-α and IL-1β expression was examined by immunohistochemistry staining in the CA1 region of the hippocampus. All values are expressed as the mean±SEM (n=3). # P<0.05 compared to the Control group; * P<0.05 compared to the LPS group. (A) TNF-α level. (B) IL-1β level.
Effects of AS-IV on the PPARγ/NF-κB pathway in the hippocampus of LPS-induced mice
The expression of PPARγ and the levels of GSK3β and NF-κB phosphorylation were detected by Western blot. As shown in Figure 8A and 8B, LPS injection led to reduced PPARγ expression (P<0.05), and AS-IV and Flu treatments greatly increased the expression of PPARγ (P<0.05). Additionally, in Figure 8A and 8C, GSK3β was activated, as shown by the decrease in its phosphorylated form, in the LPS group(P<0.05), but AS-IV and Flu treatment reversed this effect by increasing GSK3β phosphorylation (P<0.05). In addition, LPS injection activated the translocation of NF-κB, as demonstrated by the elevated phosphorylation of NF-κB in the LPS group (P<0.05), while AS-IV and Flu treatment reversed this effect (P<0.05), as shown in Figure 8A and 8D.
Effects of AS-IV on NLRP3 inflammasome activation in the hippocampus of LPS-induced mice
As shown in Figure 9, the protein levels of NLRP3 and active caspase-1 in the hippocampus of the LPS group were significantly higher than those in the Control group (P<0.05). Treatment with AS-IV (20 and 40 mg/kg) and Flu remarkably mitigated this upregulation of NLRP3 and active caspase-1, as demonstrated in Figure 9A-9C (P<0.05). These data indicated that AS-IV treatment repressed NLRP3 inflammasome activation in the mouse hippocampus.
Discussion
The present study used two types of animal models to explore the antidepressant effects of AS-IV and further identify its underlying mechanism. Remarkably, the antidepressant effects of AS-IV were accompanied by a reduction in pro-inflammatory cytokine levels via regulation of the expression of PPARγ and its downstream proteins. In addition, these results indicated that AS-IV possessed a neuroprotective effect, which was associated with neuroinflammation inhibition via modulation of the PPARγ/NF-κB/NLRP3 inflammasome axis.
Several studies have shown that mice subjected to chronic stress undergo physiological changes and altered behaviors in the hypothalamus, hippocampus, and prefrontal cortex32,33. The repeated restraint stress model used in our study is a well established method of inducing depressive-like symptoms in mice34, mimicking the symptoms of human depression. The RRS-induced mouse model was used to screen the effective doses of AS-IV and explore its antidepressant function and mechanism of action for the first time. However, the LPS-induced mouse model, a typical inflammation animal model of depression, was designed to further explore the mechanism of AS-IV involving neuroinflammation inhibition. The combination of these two animal models can comprehensively explain the function and mechanism of AS-IV under conditions of both restraint and inflammatory stress.
In the RRS-induced animal model, the mice developed depressive-like behaviors, which were consistent with previous studies35. RRS is reported to disrupt HPA axis activity, causing symptoms of depression such as anhedonia and increased immobility in the FST25,35. However, our study demonstrated that AS-IV increased sucrose consumption and the number of crossings in the OFT and reduced the immobility time in the TST and FST in mice, indicating the antidepressant potential of AS-IV.
Moreover, AS-IV suppressed the RRS-induced increase in TNF-α and IL-1β expression in the CA1 region of the hippocampus, suggesting that the antidepressant function of AS-IV was related to neuroinflammation inhibition. Inflammation has been recognized as one of the pathogenic mechanisms of depression in recent studies12,13,20. Neuroinflammation is initially a protective response in the brain; however, an excessive inflammatory response is triggered by stress and subsequently produces deleterious effects, leading to the pathophysiology of stress-related disorders, including depression36. A previous study reported that neuroinflammation of the hippocampus is associated with the severity of depressive symptoms in patients with chronic fatigue syndrome/myalgic encephalomyelitis37, and neuroinflammation in several brain regions, including the hippocampus, is observed both in an animal model of depression and in the clinic38,39, suggesting a relationship between hippocampal damage and mood disorders. Accordingly, our results were in line with these reports, suggesting that AS-IV treatment decreased the expression of inflammatory cytokines and exerted a neuroprotective effect40.
RRS downregulated the expression of PPARγ in the hippocampus, which was restored by AS-IV administration. PPARγ agonists are reported to effectively treat type 2 diabetes due to their insulin-sensitizing and anti-inflammatory properties15. These properties may enable PPARγ agonists to exert beneficial effects in neurodegenerative diseases41,42. The reported neuroprotective effects of PPARγ agonists43 shed light on potential new drugs to treat mood disorders. AS-IV, a natural PPARγ agonist, may be useful for the treatment of depression due to its anti-inflammatory effect44.
A recent study revealed that stress activates GSK3β, which can be inhibited by pretreatment with the selective GSK3 inhibitor TDZD-8, resulting in a reduction in the stress-induced increase in hippocampal cytokines and the prevention of IκBα degradation20. This observation indicated that active GSK3β is required for activation of NF-κB. Additionally, evidence has shown that acute or chronic restraint stress elevated the activation of NF-κB45. In other words, active GSK3β and NF-κB exacerbate the inflammatory response and, in turn, predate the onset of depression. In the present investigation, activation of GSK3β and NF-κB caused by RRS was blocked by AS-IV treatment, along with the upregulation of PPARγ expression in the drug-treated groups. This result indicated that PPARγ activation can ameliorate GSK3β activation, further altering the levels of downstream proteins. The establishment of the link between PPARγ and GSK3β is one of the highlights of this study.
The NLRP3 inflammasome, a cytoplasmic multiprotein complex consisting of NOD-like receptors, adapter apoptosis-associated speck-like protein and pro-caspase-1, once activated, contributes to the active form of caspase-1, which, in turn, cleaves pro-IL-1β, leading to the production of IL-1β, which further regulates immune responses and inflammatory reactions. In addition, the production of IL-1β, mediated by the NLRP3 inflammasome, can be blocked by the NF-κB inhibitor (Bay11-7082)21, suggesting that NF-κB activation modulates the NLRP3 inflammasome. In our study, treatment with AS-IV decreased the expression of the NLRP3 inflammasome, which could be related to the downregulation of NF-κB phosphorylation.
After the first exploration of the effects of AS-IV on depressive-like behaviors in mice, we subsequently used the LPS-induced mouse model that resulted in symptoms resembling those of MDD to confirm the antidepressant function of AS-IV. LPS injection is a well-established approach to induce depressive-like behaviors by activating the immune system to further trigger inflammatory signals46. In light of the above reports, peripherally administered LPS challenges the immune system, resulting in inflammation in the periphery or in the brain11,41. Together, these studies support the hypothesis that inflammation predisposes the occurrence of depression47. LPS exposure reduced the number of crossings in the OFT and prolonged the immobility in the TST and FST, effects that were alleviated by AS-IV pretreatment. These results were in agreement with the outcomes observed in the RRS-induced mice. Immune stimuli such as LPS induce upregulation of NF-κB-dependent NLRP3 expression, further stimulating the activation of the NLRP3 inflammasome21,46. In contrast, AS-IV treatment improved the LPS-induced inflammation observed in the hippocampus, as shown by an increase in PPARγ levels and inactivation of GSK3β, NF-κB and the NLRP3 inflammasome. These data strongly highlighted the antidepressant effects of AS-IV in stress-induced mice.
Taken together, our results demonstrated that AS-IV treatment relieved the depressive-like behaviors observed in both RRS-induced and LPS-induced mice. In this study, we found that neuroinflammation was of significance to the pathogenesis of MDD. As described previously, AS-IV counteracted the neuroinflammation in mice, and this effect may be mediated by the PPARγ/NLRP3 inflammasome axis. Our study provided evidence that AS-IV could be considered as a potential drug candidate for the treatment of depression. More research on the antidepressant effects of AS-IV needs to be further explored.
Author contribution
Mei-ting SONG designed this research; Mei-ting SONG, Jie RUAN, Ru-yi ZHANG and Jie DENG conducted the animal experiments; Mei-ting SONG wrote the manuscript; Zhan-qiang MA and Shi-ping MA revised the manuscript.
Abbreviations
AS-IV, Astragaloside IV; RRS, repeated restraint stress; LPS, lipopolysaccharide; GSK3, glycogen synthase kinase 3; MDD, major depressive disorder; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; IL-1β, interleukin 1β; TNF-α, tumor necrosis factor-α; CNS, central nervous system; PPARγ, peroxisome proliferator-activated receptor gamma; NF-κB, nuclear transcription factor; Flu, fluoxetine; SPT, sucrose preference test; OFT, open field test; TST, tail suspension test; FST, forced swimming test.
References
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–49.
Ferrari AJ, Norman RE, Freedman G, Baxter AJ, Pirkis JE, Harris MG, et al. The burden attributable to mental and substance use disorders as risk factors for suicide: findings from the Global Burden of Disease Study 2010. PLos One 2014; 9: e91936.
Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 2013; 310: 591–608.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2015; 16: 22–34.
Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005; 5: 243–51.
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56.
Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuro-immunological review on cytokines involved in antidepressant treat-ment response. Hum Psychopharmacol 2010; 25: 201–15.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–41.
Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacol 2011; 36: 2452–9.
Wang Y, Xu Y, Sheng H, Ni X, Lu J. Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav Brain Res 2016; 307: 18–24.
de Timary P, Starkel P, Delzenne NM, Leclercq S. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology 2017; 122: 148–60.
Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflamm 2014; 11: 151.
Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci 2015; 18: 1386–93.
Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008; 13: 717–28.
Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, et al. PPAR-gamma agonists for the treatment of major depression: a review. Pharmacopsychiatry 2017; 50: 49–55.
Sun K, Park J, Kim M, Scherer PE. Endotrophin, a multifaceted player in metabolic dysregulation and cancer progression, is a predictive biomarker for the response to PPARγ agonist treatment. Diabetologia 2017; 60: 24–9.
Wang X, Wang Y, Hu J, Yu S, Li B, Cui Y, et al. Astragaloside IV, a natural PPARγ agonist, reduces Aβ production in Alzheimer's disease through inhibition of BACE1. Mol Neurobiol 2017; 54: 2939–49.
Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 2005; 6: 777–84.
Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol Therapeut 2015; 148: 114–31.
Cheng Y, Pardo M, Armini RDS, Martinez A, Mouhsine H, Zagury J, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behavior Immunity 2016; 53: 207–22.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183: 787–91.
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen Y, et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLos One 2012; 7: e39824.
Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 2016; 1. pii: 1003.
Zhang W, Frei B. Astragaloside IV inhibits NF-κB activation and inflammatory gene expression in LPS-treated mice. Mediat Inflamm 2015; 2015: 1–11.
Abe-Higuchi N, Uchida S, Yamagata H, Higuchi F, Hobara T, Hara K, et al. Hippocampal sirtuin 1 signaling mediates depression-like behavior. Biol Psychiatry 2016; 80: 815–26.
Liu XL, Luo L, Mu RH, Liu BB, Geng D, Liu Q, et al. Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice. Sci Rep 2015; 5: 16024.
Liu L, Zhang Q, Cai Y, Sun D, He X, Wang L, et al. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget 2016; 7: 56045–59.
Qin T, Fang F, Song M, Li R, Ma Z, Ma S. Umbelliferone reverses depression-like behavior in chronic unpredictable mild stress-induced rats by attenuating neuronal apoptosis via regulating ROCK/Akt pathway. Behav Brain Res 2017; 317: 147–56.
Rodrigues AL, Da SG, Mateussi AS, Fernandes ES, Miguel OG, Yunes RA, et al. Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci 2002; 70: 1347–58.
Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiatr Res 2003; 37: 249–59.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977; 229: 327–36.
Vyas A, Mitra R, Shankaranarayana RB, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–8.
Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res 2007; 1148: 226–33.
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, et al. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav 2005; 82: 200–6.
Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, et al. Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J Physiol Pharmacol 2013; 17: 393–403.
Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, et al. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci 2010; 30: 15007–18.
Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An 11C-(R)-PK11195 PET study. J Nucl Med 2014; 55: 945–50.
Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Muller UJ, et al. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? Eur Arch Psychiatry Clin Neurosci 2015; 265: 321–9.
Dang R, Zhou X, Tang M, Xu P, Gong X, Liu Y, et al. Fish oil supple-mentation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr 2018; 57: 893–906.
Li M, Ma RN, Li LH, Qu YZ, Gao GD. Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur J Pharmacol 2013; 715: 189–95.
Han Q, Yuan Q, Meng X, Huo J, Bao Y, Xie G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget 2017; 8: 42001–6.
Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, et al. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans 2006; 34: 1341–6.
Aoun P, Watson DG, Simpkins JW. Neuroprotective effects of PPARgamma agonists against oxidative insults in HT-22 cells. Eur J Pharmacol 2003; 472: 65–71.
Zhu R, Zheng J, Chen L, Gu B, Huang S. Astragaloside IV facilitates glucose transport in C2C12 myotubes through the IRS1/AKT pathway and suppresses the palmitate-induced activation of the IKK/IkappaBalpha pathway. Int J Mol Med 2016; 37: 1697–705.
Gárate I, Garcia-Bueno B, Madrigal JLM, Caso JR, Alou L, Gomez-Lus ML, et al. Stress-induced neuroinflammation: role of the Toll-Like receptor-4 pathway. Biol Psychiat 2013; 73: 32–43.
Zhu W, Cao FS, Feng J, Chen HW, Wan JR, Lu Q, et al. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience 2017; 343: 77–84.
Raison CL, Miller AH. Anti-inflammatory agents as antidepressants: truth or dare. Psychiatric Annals 2015; 45: 255–61.
Acknowledgements
This project was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the National Natural Science Foundation of China (81573701), the College Students Innovation Project for the R&D of Novel Drugs (J1310032) and National Fund for Fostering Talents of Basic Science (NFFTBS).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Song, Mt., Ruan, J., Zhang, Ry. et al. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol Sin 39, 1559–1570 (2018). https://doi.org/10.1038/aps.2017.208
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.208
Keywords
This article is cited by
-
Traumatic Brain Injury-Mediated Neuroinflammation and Neurological Deficits are Improved by 8-Methoxypsoralen Through Modulating PPARγ/NF-κB Pathway
Neurochemical Research (2023)
-
Integrated bioinformatics and network pharmacology identifying the mechanisms and molecular targets of Guipi Decoction for treatment of comorbidity with depression and gastrointestinal disorders
Metabolic Brain Disease (2023)
-
Astragaloside IV attenuates IL-1β-induced intervertebral disc degeneration through inhibition of the NF-κB pathway
Journal of Orthopaedic Surgery and Research (2022)
-
GSK-3β manipulates ferroptosis sensitivity by dominating iron homeostasis
Cell Death Discovery (2021)
-
Xanthohumol Attenuates Lipopolysaccharide-Induced Depressive Like Behavior in Mice: Involvement of NF-κB/Nrf2 Signaling Pathways
Neurochemical Research (2021)