Abstract
Aim:
Honokiol (HNK) is a natural compound isolated from the magnolia plant with numerous pharmacological activities, including inhibiting epithelial-mesenchymal transition (EMT), which has been proposed as an attractive target for anti-tumor drugs to prevent tumor migration. In this study we investigated the effects of HNK on EMT in human NSCLC cells in vitro and the related signaling mechanisms.
Methods:
TNF-α (25 ng/mL) in combination with TGF-β1 (5 ng/mL) was used to stimulate EMT of human NSCLC A549 and H460 cells. Cell proliferation was analyzed using a sulforhodamine B assay. A wound-healing assay and a transwell assay were performed to examine cell motility. Western blotting was used to detect the expression levels of relevant proteins. siRNAs were used to knock down the gene expression of c-FLIP and N-cadherin. Stable overexpression of c-FLIP L (H157-FLIP L) or Lac Z (H157-Lac Z) was also performed.
Results:
Treatment with TNF-α+TGF-β1 significantly enhanced the migration of A549 and H460 cells, increased c-FLIP, N-cadherin (a mesenchymal marker), snail (a transcriptional modulator) and p-Smad2/3 expression, and decreased IκB levels in the cells; these changes were abrogated by co-treatment with HNK (30 μmol/L). Further studies demonstrated that expression level of c-FLIP was highly correlated with the movement and migration of NSCLC cells, and the downstream effectors of c-FLIP signaling were NF-κB signaling and N-cadherin/snail signaling, while Smad signaling might lie upstream of c-FLIP.
Conclusion:
HNK inhibits EMT-mediated motility and migration of human NSCLC cells in vitro by targeting c-FLIP, which can be utilized as a promising target for cancer therapy, while HNK may become a potential anti-metastasis drug or lead compound.
Similar content being viewed by others
Introduction
Lung cancer is the primary cause of cancer-associated mortality worldwide amongst men and is the second highest cause among women, accounting for 25% of all cancer deaths. Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all cases1. Cancer metastasis is still one of the major causes of treatment failure and death in lung cancer patients, and there is no effective method to directly target metastasis for these patients except traditional chemotherapy. Moreover, the underlying mechanism has not been fully clarified until now. Recently, it was reported that epithelial-to-mesenchymal transition (EMT), a biological process that occurs in epithelial carcinomas, including lung cancer, plays an important role in enhancing cancer motility and migration. EMT is involved in various functions and leads to the dissociation of cell-cell adhesions, disappearance of apical-basolateral polarity, reorganization of spindle-cell shape, promotion of cell motility, and acquisition of mesenchymal markers2. It is well known that transforming growth factor-β (TGF-β) is a major inducer of EMT in cancer cells during tumor progression3. Tumor necrosis factor-α (TNF-α) is able to accelerate the EMT process induced by TGF-β4. Thus, the present study used TGF-β and TNF-α as co-stimulators of the EMT process.
Honokiol (HNK) (Figure 1A), a natural compound derived from the magnolia plant and used in traditional Chinese medicine, has been tested in various preclinical studies. Among its numerous pharmacological effects, its anti-tumor effects, especially its ability to inhibit epithelial-to-mesenchymal transition (EMT), have aroused much interest. In fact, it has been reported that HNK inhibits EMT in breast cancer5,6, gastrointestinal cancer7,8, renal cancer9, glioblastoma cells10, and oral cancer11. However, no study has examined the inhibition of EMT by HNK in lung cancer. Cellular Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (c-FLIP) is the major protein that protects cells from apoptosis induced by the extrinsic death receptors12. C-FLIP has been shown to be overexpressed in various tumors, such as colon cancer, gastric cancer, pancreatic cancer, prostate cancer, and others13. Moreover, cancer-associated gene (CAGE) increases c-FLIP (L) and snail to enhance cell migration14. All of these reports have recommended c-FLIP as a useful therapeutic target for the innovative treatment of cancer patients. It has been reported that HNK down-regulates c-FLIP levels and potentiates the anti-tumor effects of TRAIL in lung cancer in vitro15, suggesting that c-FLIP is involved in exerting inhibitory effects on proliferation by HNK in NSCLC cells.
Effects of TNF-α+TGF-β1+HNK and HNK on the proliferative activity of A549 and H460 cells. (A) The chemical structure of HNK. A549 (B) and H460 (C) cells were treated with TNF-α+TGF-β1+HNK (TNF-α+ TGF-β1 pretreated for 30 min) and HNK for 48 h. The proliferative activities of A549 and H460 cells were investigated in an SRB assay. Data are shown as the mean±SD. n=3. *P<0.05, **P<0.01 compared with the HNK group.
In our study, we hypothesized and provided strong evidences that HNK inhibits EMT in NSCLC cells by targeting c-FLIP through N-cadherin/snail signaling. Our analyses showed that the reduction of c-FLIP levels played a key role in impeding cancer cell migration in NSCLC cells and that N-cadherin and snail are downstream targets of c-FLIP. In addition, we also determined that Smad and NF-κB signaling were involved in the process of TNF-α+TGF-β1-induced EMT inhibition by HNK in NSCLC cells. Furthermore, NF-κB signaling was also modulated by c-FLIP.
Materials and methods
Antibodies and reagents
Mouse monoclonal antibodies against human c-FLIP were purchased from ENZO Life Sciences (New York, NY, USA). Rabbit anti-snail and N-cadherin monoclonal antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal antibodies against β-actin were from ZSGB-BIO (Beijing, China). TNF-α and TGF-β1 were purchased from Peprotech (Rocky Hill, NJ, USA) and prepared initially as 0.1 mg/mL stock solutions by dissolving in 0.1% BSA buffer or dissolving in 1 mg/mL BSA buffer including 4 mmol/L HCl as a 20 μg/mL stock solution, respectively. Both stock solutions were kept at −80 °C. Honokiol (HNK) was purchased from Shanghai Ziyi-reagent Company (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO) as a 20 mmol/L stock solution at −20 °C. All stock solutions were diluted with RPMI-1640 medium to final concentrations before the experiments were performed.
Cell culture
The NSCLC cell lines NCI-A549, NCI-H460, H157-LacZ, and H157-FLIPL were maintained in our laboratory in RPMI-1640 medium (ThermoFisher Scientific Inc, Waltham, MA, USA) containing 10% (v/v) fetal calf serum (Gibco BRL, Life Technologies Inc, Carlsbad, CA, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL) (North China Pharmaceutical Inc, China) in a 5% CO2/95% O2 incubator at 37 °C. The cells were cultured as a monolayer in culture flasks to ∼90% confluence for experiments.
Cell survival analysis
Cell viability was examined in a sulforhodamine B (SRB; Sigma Chemical Company, St Louis, MO, USA) assay. Cells were seeded in a 96-well plate at 5×103 cells/well and exposed to the indicated concentrations of chemicals overnight for 48 h. After treatment, cold 10% (w/v) trichloroacetic acid (TCA; Sigma Chemical Company, St Louis, MO, USA) was used to fix the cells for 60 min at 4 °C, and then the supernatant was discarded. The plates were washed with double-distilled water 5 times and air dried. Then, the cells were stained with SRB solution [0.4% (w/v) in 1% acetic acid] for 5 min. After staining, 1% acetic acid was used to wash the plates 5 times, and the plates were air dried. Tris (10 mmol/L, pH 10.5) was added to the plates to solubilize the SRB dye in the cells, and the absorbance was examined at 570 nmol/L on a microplate reader (MultiskanMK3, Thermo Fisher Scientific Inc, Waltham, MA, USA).
Wound-healing assay
The cells were plated into 6-well plates, and a sterile pipette tip was used to create wounds in confluent cell monolayers. The cells were washed 3 times with PBS to remove floating cells and debris. Normal medium containing TNF-α, TGF-β1 and other treatments were added to the wells, and the plates were maintained at 37 °C. Wounds were assayed at the indicated time points. All experiments were repeated 3 times under each condition.
In vitro migration assay
Migration of A549 and H460 cells was assessed using cell culture inserts (Corning Incorporated, Corning, NY, USA) containing a polycarbonate filter with an 8-μm pore size. A549 (5×105 cells/mL) and H460 (1×106 cells/mL) cell suspensions in a volume of 100 μL of complete medium were added to the upper compartment of the chamber, and 0.6 mL of complete medium supplemented with 20% FBS was added to the lower compartment of the chamber. Then, both sides of the chamber were exposed to TGF-β1, TNF-α, and HNK as indicated. After the culture system was incubated at 37 °C for 24 h, the inserts were fixed with methanol for 30 min followed by staining with 0.1% (w/v) crystal violet. A cotton swab was used to remove the non-migrant cells from the upper surface of the membrane. The migrated cells on the bottom of the membrane were photographed using an inverted microscope (Leica Microsystems Wetzlar GmbH, Germany). Finally, the membrane was dipped into 33% acetic acid to solubilize the crystal violet, and the solution was measured at 570 nm with a microplate reader (MultiskanMK3, Thermo Fisher Scientific Inc, Waltham, MA, USA). The experiments were conducted in triplicate and repeated 3 times.
Western blotting analysis
Cells were exposed to reagents for the indicated times, digested with trypsin (Gibco BRL, Life Technologies Inc, Carlsbad, CA, USA) and then collected. The cells were lysed in cell lysis buffer [50 mmol/L Tris-HCl; pH 8.0; 2% NP-40; 150 mmol/L NaCl; 0.2% SDS; 0.5% sodium deoxycholate; 1% PMSF (Beyotime, Haimen, China)], and the supernatant was harvested after centrifugation. The BCA protein assay kit (Thermo Fisher Scientific Inc, Waltham, MA, USA) was used to determine the protein concentration. Total protein (35–45 μg) for each sample was loaded onto a 10% or 15% polyacrylamide gel, separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Nu Protein Enterprises Inc, Toronto, Ontario, Canada). The blotted membranes were then incubated with primary antibodies followed by secondary antibodies (diluted 1:5000) conjugated with horseradish peroxidase (ZSGB-BIO Inc, Beijing, China). Specific antigen-antibody complexes were visualized by enhanced chemiluminescence with the ECL detection system (EMD Millipore, Billerica, MA, USA). A ChemiImager 5500 (Alpha Innotech Corp, San Jose, CA, USA) was used to capture the images, and a Gel-Pro analyzer was used to examine the integral optical density of each group. All experiments were performed in triplicate.
siRNA transfection
N-cadherin siRNA (sense: 5′-CCAAUCAACUUGCCAGAAAdTdT-3′, anti-sense: 3′-dTdTGGUUAGUUGAACGGUCUUU-5′), c-FLIP siRNA (sense: 5′-GGUUGAGUUGGAGAAACUAdTdT-3′, anti-sense: 3′-dTdTCCAACUCAACCUCUUUGAU-5′) and negative siRNA purchased from Ruibo Biotechnology (Guangzhou, China) were transfected using the RuiboFECT™ CP Transfection Kit according to the instructions. The experiment was specifically designed as follows. First, A549 and H460 cells were plated in a 6-well plate for 24 h, and the medium was replaced with antibiotic-free medium for transfection. After siRNA was dissolved in the transfection solution, which was added to the plates, the cells were incubated at 37 °C for another 24 h and treated with TNF-α+TGF-β1 for 48 h. Finally, the efficiency of the changes induced by RNAi was measured by Western blotting.
Statistical analysis
All of the experiments were conducted independently at least three times. All the data were represented as the mean±SD. Student's t-test was used to evaluate statistical significance. For multiple comparisons, one-way ANOVA was used. P<0.05 was considered significant.
Results
Effects of HNK in combination with TNF-α and TGF-β1 on the inhibition of proliferation in NSCLCs
TNF-α and TGF-β1 have been applied to stimulate the EMT of cancer cells in previous studies16,17; therefore, an SRB assay was used to examine the proliferation of A549 and H460 cells to observe the effects of TNF-α and TGF-β1 on the proliferation inhibition induced by HNK. In the experiment, the cells were exposed to 25 ng/mL TNF-α and 5 ng/mL TGF-β1 combined with the indicated concentrations of HNK (Figure 1B, 1C). The results showed that HNK reduced the growth of A549 and H460 cells in a dose-dependent manner, as previously reported18. At the same time, TNF-α+TGF-β1 enhanced the inhibitory effects exerted by HNK on the proliferation of A549 cells, but there was no significant difference in cell growth for H460 cells between the three agents and HNK alone. Based on the results, we chose TNF-α (25 ng/mL), TGF-β1 (5 ng/mL), and HNK (30 μmol/L) for further experiments.
HNK inhibits the migration of A549 and H460 cells induced by TNF-α+TGF-β1
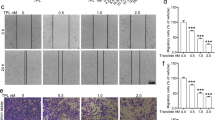
It has been reported that HNK inhibits the migration of NSCLC cells by targeting β-catenin signaling activation mediated by PGE219. Wound-healing assays and a transwell co-culture system were used to examine the effects of HNK in combination with TNF-α+TGF-β1 on the inhibition of motility and the migration abilities of cancer cells. In the wound-healing assay, TNF-α+TGF-β1 treatment resulted in a decrease in the wound area through the movement of A549 and H460 cells into the scarred region compared to the control, and the morphology of A549 cells was particularly elongated (Figure 2A). For A549 cells, after 35 h of culture, the motility indices of different groups treated with TNF-α+TGF-β1, TNF-α+TGF-β1+HNK and HNK were 2.31, 0.88 and 0.82, respectively. There was a significant difference between the TNF-α+TGF-β1, the control (P<0.01) and the TNF-α+TGF-β1+HNK (P<0.01) groups. For H460 cells, after 53 h of culture, the motility indices of different groups treated with TNF-α+TGF-β1, TNF-α+TGF-β1+HNK and HNK were 1.95, 0.98 and 0.43, respectively. There was a significant difference between the HNK groups and the control (P<0.05).
HNK inhibits the migration of A549 and H460 cells induced by TNF-α+TGF-β1. A549 and H460 cells were treated with TNF-α+TGF-β1 (TNF-α 25 ng/mL, TGF-β1 5 ng/mL), TNF-α+TGF-β1+HNK, or HNK (30 μmol/L) and assessed with the scratch test and transwell assays. (A) The results of the scratch test after cells were treated for 35 h (A549 cells) and 53 h (H460 cells). Data are the mean±SD. n=3. *P<0.05, **P<0.01 compared with the TNF-α+TGF-β1 group. #P<0.05 compared with the blank group. (B) The results of the transwell assays are shown after cells were treated for 15 h (A549) and 18 h (H460). TNF-α+TGF-β1 pretreatment was administered for 30 min. The results of the transwell assays were measured with a microplate reader. Data are shown as the mean±SD. n=3. *P<0.05, **P<0.01 compared with the TNF-α+TGF-β1 group.
Similarly, in the transwell assay, TNF-α+TGF-β1 treatment resulted in a high migration index compared to the control in A549 and H460 cells (Figure 2B). The migration indices of A549 in the TNF-α+TGF-β1, TNF-α+TGF-β1+HNK and HNK groups were 1.50, 1.07 and 0.88, respectively. There was a significant difference between the TNF-α+TGF-β1 group and the control (TNF-α+TGF-β1 versus control, P<0.01) or TNF-α+TGF-β1+HNK (TNF-α+TGF-β1 versus TNF-α+TGF-β1+HNK, P<0.05) groups. The migration indices of H460 in the TNF-α+TGF-β1, TNF-α+TGF-β1+HNK and HNK groups were 1.29, 1.11 and 0.92, respectively. There was a significant difference between the TNF-α+TGF-β1 group and the control (P<0.05).
HNK and TNF-α+TGF-β1 affect the expression levels of EMT-associated proteins and c-FLIP in A549 and H460 cells
c-FLIP influences the expression of N-cadherin and snail, while HNK modulates the expression level of c-FLIP and inhibits the motility and migration of non-small cell lung cancer cells. To investigate the relationship between c-FLIP and EMT, we first assessed the effects of the expression levels of N-cadherin, snail and c-FLIP after HNK or TNF-α+TGF-β1 treatment for the indicated times on the cells via Western blotting. The results showed that TNF-α+TGF-β1 increased N-cadherin, snail, and c-FLIP expression levels (Figure 3A, 3B) from 12 h to 72 h in A549 and H460 cells, but HNK significantly decreased these protein levels (Figure 3C, 3D). Based on the results, the changes in the indicators at 48 h were obvious; thus, we chose 48 h as a time point for the following experiments.
Effects of TNF-α+TGF-β1 or HNK on the expression of EMT-related proteins and c-FLIP in A549 and H460 cells. A549 and H460 cells were treated with TNF-α+TGF-β1 (A) or HNK (B) for different periods of time (12, 24, 48, and 72 h). Protein levels were investigated in Western blotting assays. Data are shown as the mean±SD. n=3. *P<0.05, **P<0.01 compared with the control.
HNK reduces the increases in N-cadherin, snail, and c-FLIP expression induced by TNF-α+TGF-β1
Based on the above results, TNF-α+TGF-β1 increased N-cadherin, snail, and c-FLIP expression, while HNK decreased these protein levels in A549 and H460 cells. Thus, we examined whether HNK influenced the expression of EMT-associated proteins and c-FLIP after TNF-α+TGF-β1 pretreatment of cells for 30 min. As shown in Figure 4A and 4B, HNK significantly lowered the levels of N-cadherin, snail, and c-FLIP detected by Western blot. The elevation of these proteins induced by TNF-α+TGF-β1 in NSCLC cells was reduced after the cells were exposed to HNK, TNF-α, and TGF-β1 for another 48 h (after the pretreatment time), which further validated the above results.
Effects of TNF-α+TGF-β1 or HNK treatment in A549 and H460 cells on the expression of related proteins. A549 (A) and H460 (B) cells were treated with TNF-α+TGF-β1 or HNK for 48 h. The related proteins were investigated using Western blotting assays. The optical densities of the bands relative to the untreated group was measured, and the data are shown as the mean±SD. n=3. **P<0.01 compared with the TNF-α+TGF-β1 group. #P<0.05, ##P<0.01 compared with the blank control.
C-FLIP regulates the expression of N-cadherin and snail
Based on the above results, TNF-α+TGF-β1 significantly enhanced the migration of A549 and H460 cells and increased the expression of N-cadherin, snail and c-FLIP, while HNK clearly suppressed the motility of the cells and reduced protein levels. Furthermore, HNK lowered the migratory capacity of NSCLC cells and blocked the elevation of these proteins induced by TNF-α+TGF-β1. Thus, this suggested that c-FLIP was involved in the EMT process.
Western blotting was used to examine the expression levels of N-cadherin, snail and c-FLIP after siRNAs were transfected into A549 and H460 cells to elucidate the relationship between c-FLIP, N-cadherin and snail. First, we applied c-FLIP siRNA to knock down gene expression and detected c-FLIP, N-cadherin and snail proteins. The results showed that the long-form (c-FLIP L) and short-form of c-FLIP (c-FLIP S) were significantly decreased after transfection for 72 h, and N-cadherin and snail protein levels in A549 and H460 cells were lowered compared to the control, even with TNF-α+TGF-β1 treatment (Figure 5A). Second, N-cadherin siRNA was used to assess the upstream and downstream cell signaling pathways for c-FLIP, N-cadherin, and snail. As shown in Figure 5B, c-FLIP L levels did not change compared to the control after N-cadherin was knocked down with/without TNF-α+TGF-β1 treatment in A549 and H460 cells. Furthermore, the expression of c-FLIP S was slightly decreased with both conditions for A549 cells and without any treatment for H460 cells, while there was no difference in c-FLIP S levels after N-cadherin siRNA was transfected into H460 cells treated with TNF-α+TGF-β1 for 48 h. For A549 and H460 cells, snail protein levels were clearly reduced, especially in the presence of TNF-α and TGF-β1.
c-FLIP influences the expression of EMT related proteins. A549 and H460 cells were transfected with N-control and c-FLIP or N-cadherin siRNA and then treated with TNF-α+TGF-β1 for another 48 h. After c-FLIP (A) and N-cadherin (B) were knocked down, the expression of N-cadherin, snail and c-FLIP was tested in A549 and H460 cells in each treatment group by performing Western blotting assays. Data are shown as the mean±SD. n=3. *P<0.05, **P<0.01 compared with N-control untreated cells. #P<0.05, ##P<0.01 compared with the TNF-α+TGF-β1 treated N-control group.
At the same time, the cell proliferation of A549 and H460 cells was examined in an SRB assay to determine the effects of c-FLIP and N-cadherin siRNAs on the growth of cancer cells. Figure 6A indicates that control siRNA did not impact the proliferation of A549 and H460 cells compared to the blank group. The rate of cell growth in the c-FLIP siRNA group was less than that in the control group with/without TNF-α and TGF-β1 in A549 and H460 cells (P<0.01). Moreover, after knocking down c-FLIP, the survival of cells treated with TNF-α+TGF-β1 was reduced significantly (P<0.01), although it was greater than that of the N-cadherin siRNA group (P<0.01 in A549 and P<0.01 in H460).
c-FLIP influences the EMT process. (A) After c-FLIP and N-cadherin were knocked down, respectively, A549 and H460 cells were treated with TNF-α+TGF-β1 for 48 h. The proliferative activity of A549 and H460 cells was tested in an SRB assay. **P<0.01 compared with the TNF-α+TGF-β1 treated N-control group. ##P<0.01 compared between the TNF-α+TGF-β1 treated c-FLIP siRNA group and the N-cadherin siRNA group. (B) After c-FLIP was knocked down, A549 cells were employed to measure migration in transwell assays after 24 h (n=3). **P<0.01 compared with N-control untreated cells. ##P<0.01 compared with the TNF-α+TGF-β1 N-control. &&P<0.01 compared with the blank control in the c-FLIP siRNA group.
Transwell assays were performed to further determine if c-FLIP was involved in cancer EMT. As shown in Figure 6B, the number of migrating cells was significantly reduced in the c-FLIP siRNA group compared to the negative control group, demonstrating that c-FLIP knockdown potently influenced A549 cell migration. Additionally, the migration index after c-FLIP siRNA transfection was decreased from 1.00 to 0.60 without TNF-α and TGF-β1 treatment, while the migration indices were 1.18 and 0.89 for the negative control and siRNA group, respectively, with TNF-α and TGF-β1 treatment. There was a statistically significant difference between the siRNA and negative control regardless of the addition of cell cytokines (P<0.01). These results strongly suggested that c-FLIP was a key regulator of A549 cell motility.
Overexpression of c-FLIP L enhances the migration of H157 cells
To further confirm the effects of c-FLIP on the EMT of cancer cells, we generated H157-FLIP L and H157-Lac Z cells with stable overexpression of c-FLIP L (H157-FLIP L) or Lac Z (H157-Lac Z). First, a Western blotting assay was used to examine the overexpression of c-FLIP L in H157-FLIP L in contrast to the H157-Lac Z control (Figure 7A). The results showed that c-FLIP L was overexpressed in H157-FLIP L cells. The migration ability of H157-FLIP L and H157-Lac Z was tested in a wound-healing assay and a transwell assay. As shown in Figure 7B and 7C, H157-FLIP L cells distinctly exhibited more movement and migration than H157-Lac Z cells, and the relative movement area and migration index were, respectively, 1.48 and 4.07 times greater than the control. There was a statistically significant difference in the wound-healing rate and transwell cell numbers between H157-FLIP L and H157-Lac Z (P<0.01).
Overexpression of c-FLIP L enhanced the motility of H157 cells. (A) Western blotting was used to assess the overexpression of c-FLIP L in H157 compared to the Lac Z control. (B) The results of the scratch test after H157 cells were treated for 40 h (n=3). (C) The results of transwell assays employing H157 cells cultured for 18 h were measured using a microplate reader (n=3). **P<0.01 compared with control.
C-FLIP regulates the expression of IκBα but not p-Smad2/3
Further effects exerted by HNK on EMT should be investigated. It was reported that HNK induces osteoblast differentiation in vitro because of its capacity to inhibit basal and TNF-α-induced NF-κB activation and to reduce the suppressive action of TNF-α on BMP-2-induced Smad activation20,21. Based on this, we examined whether Smad and NF-κB signaling were linked with c-FLIP after NSCLC cells were exposed to HNK. In the experiment, A549 and H460 cells were pre-treated with TNF-α+TGF-β1 for 30 min followed by HNK treatment for 48 h. The results showed that no obvious change was observed in the expression levels of Smad-2/3 proteins, while Smad-2/3 phosphorylation levels increased and IκBα expression decreased after TNF-α+TGF-β1 treatment. Meanwhile, HNK decreased the increase in Smad-2/3 phosphorylation and increased the reduction of IκBα expression levels induced by TNF-α+TGF-β1 pretreatment with no obvious change in the expression levels of total Smad-2/3 proteins (Figure 8A). Interestingly, c-FLIP siRNA did not change Smad-2/3 protein and phosphorylation levels, but the expression of IκBα was increased in H460 cells (Figure 8B).
The relationship between c-FLIP and p-Smad2/3 and IκBα was investigated through Western blotting. A549 and H460 cells were treated with TNF-α+TGF-β1 or HNK for 48 h. (A) Related proteins were investigated by Western blotting assay. The optical densities of bands relative to the untreated group were measured, and the data are shown as the mean±SD. n=3. *P<0.05, **P<0.01 compared with control. (B) H460 cells were transfected with c-FLIP siRNA and then treated with TNF-α+TGF-β1 for another 48 h. After c-FLIP was knocked down, the expression of p-Smad2/3, Smad2/3 and IκBα was tested by Western blotting assay. The optical densities of bands relative to the untreated group were measured, and the data are shown as the mean±SD. n=3. **P<0.01 compared with N-control untreated cells. ##P<0.01 compared with the TNF-α+TGF-β1-treated N-control group.
Discussion
Honokiol, a natural product that exerts many pharmacological effects, has attracted attention from scientists in different medical fields. HNK has been applied to treat neurodegenerative diseases, such as Alzheimer's disease22, and has potential anti-diabetic23, anti-acnegenic24, anti-viral25, anti-tumoral26 and other activities. Our previous study showed that HNK effectively inhibits the proliferation of NSCLC cells through the induction of apoptosis and inhibition of autophagy and also at least partially promotes the apoptosis of NSCLC cells induced by TRAIL by reducing c-FLIP expression levels15,18. Given that EMT is a major biological process that results in malignancy27, EMT could be an effective target for drugs by inhibiting cancer and distant metastases and improving disease conditions28. It is known that HNK regulates EMT in various cancers, including lung cancer. Based on these discoveries, we attempted to elucidate whether c-FLIP is involved in the EMT of NSCLC cells and how HNK modulates EMT via the c-FLIP-mediated signaling pathway. A primary goal in our study was to set up an EMT model. As mentioned above, TGF-β1 combined with TNF-α is often used to effectively promote the EMT of cancer cells. Indeed, EMT is a process in cancer cells that leads to local migration and distant metastasis with molecular and phenotypic changes29. Thus, wound-healing assays and transwell assays were used to confirm our model after A549 and H460 cells were treated with TGF-β1 plus TNF-α. We also tested the potential capacity of HNK in combination with TGF-β1 plus TNF-α to inhibit the EMT of cancer cells.
At first, because different cell lines have different drug sensitivities, we examined the anti-tumor activity of HNK in A549 and H460 cells in vitro with TNF-α+TGF-β1 co-treatment to observe the effects of TNF-α and TGF-β1 on the proliferation inhibition of HNK. Based on the results, 25 ng/mL TNF-α plus 5 ng/mL TGF-β1 exerted no obvious changes on the proliferation inhibitory effects of 30 μmol/L HNK in both cell lines. Thus, the indicated concentrations of the three agents were applied in the following experiments to avoid the effects of cell growth on migration and distant metastasis. The results from wound-healing assays and transwell assays demonstrated that TNF-α+TGF-β1 accelerated the migration rate and increased the transwell cell number, while HNK decreased the rate and number of A549 and H460 cells with/without TNF-α+TGF-β1 treatment.
It is well known that c-FLIP is a promising target for cancer therapy due to its ability to prevent cancer cell apoptosis30. Thus, the reduction of c-FLIP is significant for enhancing death receptor-induced apoptosis with certain anticancer drug31. It is therefore of interest to investigate whether c-FLIP is involved in cancer EMT inhibited by HNK. During EMT, there are often increases in snail (a transcriptional modulator) and N-cadherin (a mesenchymal marker)32. In our study, a Western blotting assay was used to determine the expression levels of N-cadherin, snail and c-FLIP in A549 and H460 cells with TNF-α+TGF-β1 or HNK-only treatment at different time points. Our data indicated that TNF-α+TGF-β1 not only promoted cancer cell migration but also increased the expression of N-cadherin, snail and c-FLIP. Furthermore, HNK not only inhibited cancer cell migration but also decreased the expression of the three proteins from 12 h to 72 h. We chose 48 h as our study time point, and our next Western blotting results showed that HNK reduced the TNF-α+TGF-β1-up-regulated expression of N-cadherin, snail and c-FLIP, which revealed that HNK was able to effectively inhibit the EMT of NSCLC cells. Notably, when c-FLIP was knocked down, the expression of N-cadherin or snail was also decreased. When N-cadherin was knocked down by N-cadherin siRNA, the expression level of snail was reduced, but there was no obvious change in c-FLIP L expression. Data from other reports and our study suggest that c-FLIP siRNA reduces the growth of cancer cells33; therefore, the SRB assay was used to confirm these results. Our data demonstrated that c-FLIP siRNA significantly lowered the rate of A549 and H460 cell growth even with TNF-α+TGF-β1 treatment compared to the control. Additionally, there was a decline in the rate of NSCLC cell proliferation after N-cadherin siRNA was applied in the experiment. Thus, siRNA treatments effectively exerted biological roles in our system. Moreover, the results from the transwell assays showed that the migration abilities of cancer cells treated with c-FLIP siRNA were significantly reduced. Taken together, these experiments demonstrated that c-FLIP was involved in the EMT of cancer cells, and the downstream effectors of c-FLIP signaling were N-cadherin and snail.
The study first showed that c-FLIP, the apoptosis-inhibiting protein, modulated the EMT of cancer cells, and HNK markedly inhibited the EMT of cancer cells by decreasing c-FLIP expression in NSCLC. Moreover, based on our results, we found that the snail protein was regulated by N-cadherin. Snail, a transcriptional factor, regulates other related proteins, including epithelial and mesenchymal markers34. Transcription factors are modulated through feedback from their effective proteins. However, it was reported that miR-10b promotes the EMT of Hep-2 cells by increasing N-cadherin expression levels with no changes in snail mRNA and protein levels35. Thus, our results suggesting that snail is influenced by N-cadherin are of interest and enabled the authors to integrate N-cadherin-regulating snail into the EMT signal transduction network. However, our aim in this study was to sufficiently demonstrate that c-FLIP would modulate EMT in NSCLC cells, and by targeting c-FLIP, HNK effectively impeded the EMT of NSCLC cells. We also generated H157 cells that stably overexpressed c-FLIPL to test whether the increase in c-FLIP expression enhanced the movement and migration in H157 cells, and the data suggested that c-FLIP played a crucial role in triggering the EMT of NSCLC cells.
TNF-α and TGF-β1 both induce Smad signaling and activate NF-κB signaling36,37. Our experiments revealed that TNF-α combined with TGF-β1 also increased Smad2/3 phosphorylation and reduced IκBα expression levels. Notably, HNK decreased the expression of p-Smad2/3 and induced IκBα expression after NSCLC cells were exposed to TNF-α and TGF-β1. Thus, HNK inhibited EMT induced by TNF-α+TGF-β1. Concurrently, Smad signaling and NF-κB signaling influenced by TNF-α+TGF-β1 were disrupted by HNK. We wanted to determine whether c-FLIP plays a role in the effects of HNK on these signals. However, when c-FLIP was knocked down with siRNA, we found that the expression of IκBα was increased but the expression of p-Smad2/3 demonstrated no obvious change. In fact, there have been no reports regarding the relationship between c-FLIP and p-Smad2/3, but the results showing the increased expression of IκBα are consistent with the finding that c-FLIP modulates NF-κB signaling38. In conclusion, these results showed that NF-κB signaling occurred downstream of c-FLIP, and Smad may be upstream of c-FLIP.
In our study, we report for the first time that HNK inhibits EMT-mediated motility and migration by targeting c-FLIP in NSCLC cells (Figure 9A). Our findings reveal that c-FLIP plays an important role in promoting the EMT of cancer cells. The expression level of c-FLIP is highly correlated with the movement and migration of NSCLC cells. The results also indicate that the downstream pathways of c-FLIP are NF-κB signaling and N-cadherin/snail signaling, while Smad signaling may be active upstream of c-FLIP (Figure 9B). c-FLIP can be utilized as a promising target for cancer therapy, and HNK may become a potential anti-metastasis drug or lead compound.
Presentation of a schematic mechanism. (A) We have provided sufficient evidences to demonstrate that HNK inhibits EMT by regulating the expression of c-FLIP in NSCLC cells. (B) We also demonstrate that HNK inhibits the expression of p-Smad while increasing the expression of IκBα. However, NF-κB lies downstream of c-FLIP, while Smad singling may lie upstream of c-FLIP.
Author contribution
Shu-zhen CHEN designed the research and wrote and edited the paper; Xiao-qin LV performed the experiments, analyzed the data, drafted the figures and wrote the paper; Xin-ran QIAO participated in some experiments; Ling SU provided the cell lines stably transfected with c-FLIPL and LacZ. Each author approved the final manuscript.
Abbreviations
c-FLIP, cellular Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein; NSCLC, non-small-cell lung cancer; HNK, Honokiol; TNF-α, tumor necrosis factor alpha; TGF-β1, transforming growth factor β1; EMT, epithelial–mesenchymal transition; SRB, sulforhodamine B.
References
Popat S, Mok T, Yang JC, Wu YL, Lungershausen J, Stammberger U, et al. Afatinib in the treatment of EGFR mutation-positive NSCLC--a network meta-analysis. Lung Cancer 2014; 85: 230–8.
Cao H, Xu E, Liu H, Wan L, Lai M . Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol Res Pract 2015; 211: 557–69.
Li L, Qi L, Liang Z, Song W, Liu Y, Wang Y, et al. Transforming growth factor-beta1 induces EMT by the transactivation of epidermal growth factor signaling through HA/CD44 in lung and breast cancer cells. Int J Mol Med 2015; 36: 113–22.
Saito A, Suzuki HI, Horie M, Ohshima M, Morishita Y, Abiko Y, et al. An integrated expression profiling reveals target genes of TGF-beta and TNF-alpha possibly mediated by microRNAs in lung cancer cells. PLoS One 2013; 8: e56587.
Avtanski DB, Nagalingam A, Bonner MY, Arbiser JL, Saxena NK, Sharma D . Honokiol activates LKB1-miR-34a axis and antagonizes the oncogenic actions of leptin in breast cancer. Oncotarget 2015; 6: 29947–62.
Avtanski DB, Nagalingam A, Bonner MY, Arbiser JL, Saxena NK, Sharma D . Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis. Mol Oncol 2014; 8: 565–80.
Liu SH, Lee WJ, Lai DW, Wu SM, Liu CY, Tien HR, et al. Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol Oncol 2015; 9: 834–49.
Pan HC, Lai DW, Lan KH, Shen CC, Wu SM, Chiu CS, et al. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis 2013; 34: 2568–79.
Li W, Wang Q, Su Q, Ma D, An C, Ma L, et al. Honokiol suppresses renal cancer cells' metastasis via dual-blocking epithelial-mesenchymal transition and cancer stem cell properties through modulating miR-141/ZEB2 signaling. Mol Cells 2014; 37: 383–8.
Joo YN, Eun SY, Park SW, Lee JH, Chang KC, Kim HJ . Honokiol inhibits U87MG human glioblastoma cell invasion through endothelial cells by regulating membrane permeability and the epithelial-mesenchymal transition. Int J Oncol 2014; 44: 187–94.
Yao CJ, Lai GM, Yeh CT, Lai MT, Shih PH, Chao WJ, et al. Honokiol eliminates human oral cancer stem-like cells accompanied with suppression of Wnt/beta-Catenin signaling and apoptosis induction. Evid Based Complement Alternat Med 2013; 2013: 146136.
Goldar S, Khaniani MS, Derakhshan SM, Baradaran B . Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev 2015; 16: 2129–44.
Lozneanu L, Cojocaru E, Giusca SE, Carauleanu A, Caruntu ID . Lesser-known molecules in ovarian carcinogenesis. Biomed Res Int 2015; 2015: 321740.
Kim Y, Park H, Jeoung D . CAGE, a cancer/testis antigen, induces c-FLIP(L) and Snail to enhance cell motility and increase resistance to an anti-cancer drug. Biotechnol Lett 2009; 31: 945–52.
Raja SM, Chen S, Yue P, Acker TM, Lefkove B, Arbiser JL, et al. The natural product honokiol preferentially inhibits cellular FLICE-inhibitory protein and augments death receptor-induced apoptosis. Mol Cancer Ther 2008; 7: 2212–23.
Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T . NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett 2010; 295: 214–28.
Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem 2010; 285: 4060–73.
Lv X, Liu F, Shang Y, Chen SZ . Honokiol exhibits enhanced antitumor effects with chloroquine by inducing cell death and inhibiting autophagy in human non-small cell lung cancer cells. Oncol Rep 2015; 34: 1289–300.
Singh T, Katiyar SK . Honokiol inhibits non-small cell lung cancer cell migration by targeting PGE2-mediated activation of beta-catenin signaling. PLoS One 2013; 8: e60749.
Yamaguchi M, Arbiser JL, Weitzmann MN . Honokiol stimulates osteoblastogenesis by suppressing NF-kappaB activation. Int J Mol Med 2011; 28: 1049–53.
Romero D, Al-Shareef Z, Gorrono-Etxebarria I, Atkins S, Turrell F, Chhetri J, et al. Dickkopf-3 regulates prostate epithelial cell acinar morphogenesis and prostate cancer cell invasion by limiting TGF-beta-dependent activation of matrix metalloproteases. Carcinogenesis 2015; 37: 18–29.
Akagi M, Matsui N, Akae H, Hirashima N, Fukuishi N, Fukuyama Y, et al. Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as Alzheimer's disease. J Pharmacol Sci 2015; 127: 155–63.
Rios JL, Francini F, Schinella GR . Natural products for the treatment of Type 2 diabetes mellitus. Planta Med 2015; 81: 975–94.
Sinha P, Srivastava S, Mishra N, Yadav NP . New perspectives on antiacne plant drugs: contribution to modern therapeutics. Biomed Res Int 2014; 2014: 301304.
Liu X, Han Y, Peng K, Liu Y, Li J, Liu H . Effect of traditional Chinese medicinal herbs on Candida spp from patients with HIV/AIDS. Adv Dent Res 2011; 23: 56–60.
Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, Singh AP . Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med 2012; 12: 1244–52.
Ye X, Weinberg RA . Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 2015; 25: 675–86.
Marcucci F, Stassi G, De Maria R . Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov 2016; 15: 311–25.
Liu W, Kovacevic Z, Peng Z, Jin R, Wang P, Yue F, et al. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. Oncotarget 2015; 6: 35522–41.
Zheng Z, Cheng S, Wu W, Wang L, Zhao Y, Shen Y, et al. c-FLIP is involved in tumor progression of peripheral T-cell lymphoma and targeted by histone deacetylase inhibitors. J Hematol Oncol 2014; 7: 88.
Kim HB, Kim MJ, Lee SH, Lee JW, Bae JH, Kim DW, et al. Amurensin G, a novel SIRT1 inhibitor, sensitizes TRAIL-resistant human leukemic K562 cells to TRAIL-induced apoptosis. Biochem Pharmacol 2012; 84: 402–10.
Mitra A, Mishra L, Li S . EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015; 6: 10697–711.
Sun J, Luo H, Nie W, Xu X, Miao X, Huang F, et al. Protective effect of RIP and c-FLIP in preventing liver cancer cell apoptosis induced by TRAIL. Int J Clin Exp Pathol 2015; 8: 6519–25.
Wang J, Zhu X, Hu J, He G, Li X, Wu P, et al. The positive feedback between Snail and DAB2IP regulates EMT, invasion and metastasis in colorectal cancer. Oncotarget 2015; 6: 27427–39.
Zhang L, Sun J, Wang B, Ren JC, Su W, Zhang T . MicroRNA-10b triggers the epithelial-mesenchymal transition (EMT) of laryngeal carcinoma Hep-2 cells by directly targeting the E-cadherin. Appl Biochem Biotechnol 2015; 176: 33–44.
Shiou SR, Yu Y, Guo Y, Westerhoff M, Lu L, Petrof EO, et al. Oral administration of transforming growth factor-beta1 (TGF-beta1) protects the immature gut from injury via Smad protein-dependent suppression of epithelial nuclear factor kappaB (NF-kappaB) signaling and proinflammatory cytokine production. J Biol Chem 2013; 288: 34757–66.
Yang HL, Korivi M, Lin MW, Chen SC, Chou CW, Hseu YC . Anti-angiogenic properties of coenzyme Q0 through downregulation of MMP-9/NF-kappaB and upregulation of HO-1 signaling in TNF-alpha-activated human endothelial cells. Biochem Pharmacol 2015; 98: 144–56.
Gordy C, Liang J, Pua H, He YW . c-FLIP protects eosinophils from TNF-alpha-mediated cell death in vivo. PLoS One 2014; 9: e107724.
Acknowledgements
The research was supported by grants from the National Natural Science Foundation of China (81373437 and 81321004) and the 'Significant New Drug Development' program of the Science and Technology Major Projects of China (2012ZX09301002-001-022-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, Xq., Qiao, Xr., Su, L. et al. Honokiol inhibits EMT-mediated motility and migration of human non-small cell lung cancer cells in vitro by targeting c-FLIP. Acta Pharmacol Sin 37, 1574–1586 (2016). https://doi.org/10.1038/aps.2016.81
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.81
Keywords
This article is cited by
-
Honokiol inhibits breast cancer cell metastasis by blocking EMT through modulation of Snail/Slug protein translation
Acta Pharmacologica Sinica (2019)