Abstract
Aim:
Increasing evidence has shown that environmental factors such as rotenone and paraquat induce neuroinflammation, which contributes to the pathogenesis of Parkinson's disease (PD). In this study, we investigated the molecular mechanisms underlying the repression by menaquinone-4 (MK-4), a subtype of vitamin K2, of rotenone-induced microglial activation in vitro.
Methods:
A microglial cell line (BV2) was exposed to rotenone (1 μmol/L) with or without MK-4 treatment. The levels of TNF-α or IL-1β in 100 μL of cultured media of BV2 cells were measured using ELISA kits. BV2 cells treated with rotenone with or without MK4 were subjected to mitochondrial membrane potential, ROS production, immunofluorescence or immunoblot assays. The neuroblastoma SH-SY5Y cells were treated with conditioned media (CM) of BV2 cells that were exposed to rotenone with or without MK-4 treatment, and the cell viability was assessed using MTT assay.
Results:
In rotenone-treated BV2 cells, MK-4 (0.5–20 μmol/L) dose-dependently suppressed the upregulation in the expression of iNOS and COX-2 in the cells, as well as the production of TNF-α and IL-1β in the cultured media. MK-4 (5–20 μmol/L) significantly inhibited rotenone-induced nuclear translocation of NF-κB in BV2 cells. MK-4 (5–20 μmol/L) significantly inhibited rotenone-induced p38 activation, ROS production, and caspase-1 activation in BV2 cells. MK-4 (5–20 μmol/L) also restored the mitochondrial membrane potential that had been damaged by rotenone. Exposure to CM from rotenone-treated BV2 cells markedly decreased the viability of SH-SY5Y cells. However, this rotenone-activated microglia-mediated death of SH-SY5Y cells was significantly attenuated when the BV2 cells were co-treated with MK-4 (5–20 μmol/L).
Conclusion:
Vitamin K2 can directly suppress rotenone-induced activation of microglial BV2 cells in vitro by repressing ROS production and p38 activation.
Similar content being viewed by others
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder and is characterized by the preferential loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Both genetic and environmental factors can cause PD1,2,3. Multiple cellular alterations are associated with PD pathogenesis, including the accumulation of toxic proteins4, oxidative stress5, mitochondrial dysfunction6 and neuroinflammation7. It is well documented that environmental factors such as pesticides are causal factors for the development of PD8,9. Epidemiological studies also suggest that exposure to some toxic agents can induce microglial activation and mitochondrial dysfunction, which are currently recognized as prominent features of PD10,11.
Rotenone, a widely used pesticide, can selectively inhibit complex I of the mitochondrial electron transport chain, thereby increasing the production of free hydroxyl radicals and inducing oxidative stress12. Rotenone freely crosses cellular membranes and accumulates throughout the brain to impair the mitochondrial function of neurons, particularly DA neurons, leading to neurodegeneration13,14. Thus, rotenone-treated animals effectively reproduce the pathological features of PD with the loss of SNpc DA neurons15. Our previous study demonstrates that rotenone can act directly on microglial mitochondria to activate microglia and to induce the expression of inflammatory factors via the nuclear factor kappa B (NF-κB) signaling pathway16. Thus, rotenone not only damages DA neurons but also activates microglia by inhibiting mitochondrial complex I.
Interestingly, in the SNpc of PD patients, DA neuronal degeneration is always accompanied by the presence of activated microglia7,17. In a PD animal model induced by rotenone, activated microglia are also observed18. Microglia are a type of glial cell that act as the first and main form of active immune defense in the central nervous system19 and play an important role in neuroinflammation, a process that is associated with the pathogenesis of PD20. Both postmortem brains and various animal models of PD show massive amounts of activated microglia around degenerated neurons21. The activated microglia produce a wide array of prostanoids, free radicals and cytokines, leading to neurodegeneration22. Individuals who take non-steroidal anti-inflammatory drugs show a reduced risk of developing PD23. Therefore, neuroinflammation is closely associated with PD development.
Vitamin K is recognized as a cofactor in the synthesis of the Gla-protein family and is also known to play key roles in physiological regulation, such as cardiovascular and bone metabolism functions24,25. Vitamin K compounds include two naturally occurring forms: vitamin K1 (phylloquinone), which is mainly found in green vegetables, such as spinach, broccoli, kale and Brussels sprouts, and vitamin K2 (menaquinone or MK-n), which is produced mainly by microorganisms. In the human intestine, bacteria convert vitamin K1 into vitamin K2 to meet our daily needs. Menaquinone-4 (MK-4), a subtype of vitamin K2 that contains a geranylgeranyl group (isoprenyl side chain) at the 3-position of 2-methyl-1,4-naphtho-quinone, is derived from the diet, either from animal origin or synthesized from other vitamin K analogues26,27. Increasing evidence suggests that vitamin K2 is associated with inflammatory regulation28,29. However, the mechanisms by which vitamin K2 influences neuroinflammation are still unclear. Recent studies indicate that vitamin K2 serves as a mitochondrial electron carrier that can rescue mitochondrial dysfunction30, suggesting that vitamin K2 has the potential for protecting mitochondria.
Here, we demonstrate an inhibitory effect of MK-4 on rotenone-induced microglial activation by restoration of the mitochondrial membrane potential, thereby decreasing reactive oxygen species (ROS) production and inhibiting NF-κB activation. In addition, MK-4 represses microglial activation-mediated neuronal cell death.
Materials and methods
Reagents
Rotenone (Sigma-Aldrich, Saint Louis, MO, USA) and MK-4 (Sigma-Aldrich) were dissolved in DMSO and 99.5% ethanol as stock solutions, respectively. Before use, the stock (50 mmol/L) was diluted with culture media, in which the final DMSO concentration was lower than 0.01%, and the ethanol concentration was lower than 0.2%.
Cell culture and treatment
BV2 cells, a mouse microglial cell line (a kind gift from Dr Jianqing DING at Shanghai Jiao Tong University, Chna)31, were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) with 100 μg/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA). BV2 cells were treated with rotenone at a concentration of 1 μmol/L with MK-4 at concentrations of 0.5, 1, 5, 10, or 20 μmol/L for 24 h. The cells were washed with culture media and then cultured for another 24 h to produce the conditioned media (CM).
Measurement of ROS generation
A reactive oxygen species assay kit (Beyotime, Shanghai, China) was used to detect the intracellular generation of ROS. The cells were incubated in serum-free media with 10 μmol/L 2′,7′-dichlorofluorescin diacetate (DCF-DA) (Invitrogen) at 37 °C for 20 min. Next, the cells were washed three times with serum-free medium. Samples were analyzed at an excitation wavelength of 488 nm and an emission wavelength of 522 nm using a flow cytometer (Beckman Coulter, Kraemer Boulevard Brea, CA, USA).
Measurement of caspase-1 activity
The Green FLICA™ caspase-1 assay kit (ImmunoChemistry Technologies, Bloomington, MN, USA) was used to detect caspase-1 activation. Cells were incubated with ICT'S green caspase-1 inhibitor probe, FAM-YVAD-FMK, for 1 h at 37 °C. The cells were observed under an inverted IX71 microscope system (Olympus, Tokyo, Japan), and the fluorescence intensity of caspase-1 was analyzed using a multi-detection reader (Molecular Devices) at an excitation of 492 nm and emission of 520 nm.
Measurement of the mitochondrial membrane potential
For the measurement of the mitochondrial membrane potential (ΔΨm), tetramethylrhodamine methyl ester (TMRM) (Sigma-Aldrich) was added to the cell culture medium for 15 min32. To identify the presence of viable mitochondria, the cells were double stained with MitoTracker Green (Beyotime). The cells were then observed under an inverted IX71 microscope system (Olympus). The fluorescence intensity of TMRM was analyzed using a multi-detection reader (Molecular Devices, Sunnyvale, CA, USA) at an excitation of 549 nm and emission of 575 nm.
Subcellular fractionation assay for nuclear extraction
BV2 cells were lysed in a fractionation buffer containing 320 mmol/L sucrose, 3 mmol/L CaCl2, 2 mmol/L MgAc, 0.1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L PMSF and 0.5% NP-40 for 20 min on ice. The cells were centrifuged at 600×g for 15 min at 4 °C, and then the supernatant was collected as the cytoplasmic fraction. The pellet was washed once with the fractionation buffer without NP-40, and then lysed in a nuclear lysis buffer containing 20 mmol/L HEPES (pH 7.9), 25% glycerol, 1.5 mmol/L MgCl2, 280 mmol/L KCl, 0.2 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L PMSF, and 0.3% NP-40.
Immunoblot analysis and antibodies
The BV2 cells were lysed in a 1×SDS lysis buffer (25 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 1% NP-40, and 1% sodium deoxycholate) in the presence of a protease inhibitor cocktail (Roche, Mannheim, Germany). Proteins were separated by 12% SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). An immunoblot analysis was performed using the following primary antibodies: monoclonal anti-GAPDH antibody was from Millipore; monoclonal anti-p38 antibody was from Santa Cruz Biotechnology (Dallas, Texas, USA); polyclonal anti-iNOS antibodies were from ABCAM (Cambridge, UK); polyclonal anti-COX2, anti-histone 2B, anti-IκB, and anti-p65 antibodies were from Epitomics (Cambridge, UK); polyclonal anti-IKK, anti-p-IKK (176/180), and anti-p-p38 antibodies were from Cell Signaling Technology (Danvers, MA, USA). The secondary antibodies, sheep anti-mouse or anti-rabbit IgG-HRP, were purchased from GE Healthcare (Beijing, China).
ELISA assay for TNF-α and IL-1β
The levels of TNF-α or IL-1β in 100 μL of cultured media from BV2 cells were measured using ELISA kits (R&D Systems, Shanghai, China) according to the manufacturer's instructions.
Cell viability assay
Cell viability was assessed using the MTT assay. The BV2 cells were seeded and treated with different concentrations of MK-4 for 24 h. For conditioned medium assays, SH-SY5Y cells were treated with CM from rotenone- and MK-4-treated BV2 cells for 24 h. The cells were incubated with 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) at 37 °C for 2 h. Then, 150 μL of dimethylsulfoxide was added to stop the reaction. The absorbance was measured at 570 nm to determine cell viability.
Propidium iodide staining
SH-SY5Y cells treated with CM were incubated with propidium iodide (PI, Beyotime) for 20 min away from light and visualized using an inverted IX71 microscope system (Olympus).
Immunocytofluorescence
The treated BV2 cells were washed with PBS and fixed with 4% paraformaldehyde in PBS (pH 7.4) for 5 min. Then, the cells were treated with 0.25% Triton X-100 for 5 min and blocked with 4% FBS in PBST. Immunocytochemical staining was performed using polyclonal anti-p65 antibodies. The cells were then incubated with Alexa Fluor 594 donkey anti-rabbit secondary antibodies (Jackson Immuno Research, PA, USA) and DAPI (Invitrogen, Carlsbad, CA, USA).
Statistical analysis
Statistical comparisons between groups and treatments were performed using a one-way analysis of variance (ANOVA). Student's t-tests were used for comparing two groups. A P value of <0.05 was considered statistically significant. The data are presented as the mean±SEM.
Results
MK-4 suppressed the rotenone-induced production of inflammatory factors in BV2 cells
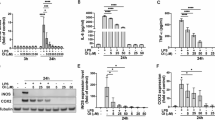
Because our previous studies showed that rotenone induces the production of inflammatory factors in BV2 cells16, and it has been reported that MK-4 represses the production of inflammatory factors33, we tested whether MK-4 could inhibit the rotenone-induced production of inflammatory factors in BV2 cells. We first examined whether MK-4 affects BV2 cell viability. MK-4 itself did not influence BV2 cell viability at concentrations up to 100 μmol/L (Figure 1A). Because MK-4 represses LPS-induced inflammatory factor production at concentrations of 1, 10 or 25 μmol/L33,34, we decided to perform experiments using concentrations of MK-4 that ranged from 0 μmol/L to 20 μmol/L in order to examine its effects on rotenone-induced microglial activation. In BV2 cells that were treated with rotenone, the inflammatory factors iNOS and COX-2 were significantly increased (Figure 1B). The production of IL-1β (Figure 1C) and TNF-α (Figure 1D) was also significantly increased. However, the rotenone-induced increase of inflammatory factors was significantly blocked by MK-4 in a dose-dependent manner (Figure 1B, 1C, and 1D), suggesting that MK-4 can inhibit the production of inflammatory factors induced by rotenone in BV2 cells.
MK-4 suppresses rotenone-induced inflammatory factor expression. (A) Various doses (0.5–100 μmol/L) of MK-4 were administered to BV2 cells for 24 h. Cell viability was measured by the MTT assay. No significant changes were observed in any group. (B) BV2 cells were treated with different doses of MK-4 and 1 μmol/L rotenone for 24 h. An immunoblot analysis showed the expression of iNOS and COX-2, with antibodies used as indicated. The band intensities of iNOS and COX-2 relative to that of GAPDH are shown in the lower two panels. The value of the group without drug (dissolvent only) is normalized as 1. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA. (C and D) The levels of IL-1β (C) and TNF-α (D) in the cultured media were measured using ELISA assays. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA.
MK-4 inhibited the rotenone-induced nuclear translocation of the NF-κB p65 subunit in BV2 cells
NF-κB is an important transcription factor that plays a key role in transactivating inflammatory gene expression by its translocation to the nucleus when microglia are exposed to stimulation16,35,36. Because rotenone activates NF-κB in microglial cells16, and MK-4 inhibits the production of NF-κB-targeted inflammatory factors (Figure 1), we wondered whether MK-4 could inhibit NF-κB nuclear translocation. We first examined the location of the NF-κB p65 subunit. p65 was located in the cytoplasm under normal conditions, whereas it was partially translocated to the nucleus after 1 μmol/L rotenone treatment in BV2 cells (Figure 2A). However, p65 was predominantly located in the cytoplasm after MK-4 treatment at doses greater than 1 μmol/L (Figure 2A), suggesting that MK-4 inhibits rotenone-induced nuclear translocation of NF-κB. To further verify the inhibition of NF-κB nuclear translocation by MK-4, we performed a fractionation assay. Consistent with immunocytochemical data, the nuclear level of p65 was significantly decreased after MK-4 treatment in rotenone-treated BV2 cells (Figure 2B). Thus, our data suggest that MK-4 inhibits rotenone-induced NF-κB nuclear translocation in BV2 cells.
MK-4 inhibits rotenone-induced nuclear accumulation of the NF-κB p65 subunit in BV2 cells. (A) Various doses of MK-4 and 1 μmol/L rotenone were administered to BV2 cells as indicated for 6 h. The cells were fixed and labeled with anti-p65 (red) antibodies. Then, the cells were visualized under a fluorescent microscope. The nuclei were stained with DAPI (1 μg/mL) (blue). Scale bars, 5 μm. (B) The cytoplasmic and nuclear fractions from the treated BV2 cells were immunoblotted with anti-p65, anti-GAPDH or anti-histone 2B antibodies. The band intensities of p65 relative to that of GAPDH (cytoplasmic fraction) or histone 2B (nuclear fraction) are shown in the lower two panels. The value of the group without drug (dissolvent only) is normalized as 1. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA.
MK-4 suppressed the rotenone-induced degradation of IκB by inhibiting IKK and p38 activation
IκB, an inhibitor of NF-κB, is ubiquitinated and subsequently rapidly degraded by the ubiquitin-proteasomal system after its phosphorylation by IκB kinase (IKK), leading to the activation of NF-κB37,38. Because we have shown that MK-4 inhibits rotenone-induced nuclear translocation of the NF-κB subunit p65, we wondered whether MK-4 affects IκB stability. In BV2 cells that were treated with rotenone, IκB was obviously degraded (Figure 3A), and phospho-IKK (p-IKK) was significantly increased (Figure 3B). However, the degradation of IκB and the activation of IKK were significantly inhibited by MK-4 treatment (Figure 3A and 3B).
MK-4 suppresses rotenone-induced degradation of IκB, activation of IKK and p38, and production of ROS. (A) BV2 cells were treated with different doses of MK-4 and 1 μmol/L rotenone for 24 h as indicated. The cells lysates were immunoblotted with anti-IκB antibodies. GAPDH served as the loading control. The band intensities of IκB relative to that of GAPDH are shown in the lower panel. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, analyzed by one-way ANOVA. (B) The cells lysates were immunoblotted with anti-p-IKK or anti-IKK antibodies. A quantitative analysis was performed as described in (A). (C) The cell lysates were immunoblotted with anti-p-p38 or anti-p38 antibodies. A quantitative analysis was performed as described in (A). (D) The cells were stained with an ROS indicator, DCF-DA, for 20 min. The DCF fluorescence of the cells was evaluated using a flow cytometer. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA.
Previous studies have shown that LPS- or rotenone-induced NF-κB activation is dependent on the phosphorylation of p3816,39,40. We therefore examined whether MK-4 affects p38 phosphorylation. In BV2 cells that were treated with rotenone, the phosphorylation of p38 was increased after rotenone treatment; however, the phosphorylation of p38 was significantly decreased by MK-4 in a dose-dependent manner (Figure 3C).
Many studies have reported that p38 is phosphorylated in response to reactive oxygen species (ROS) and that rotenone can induce mitochondrial ROS production41,42. We wondered whether the decreased p38 activation induced by MK-4 in rotenone-treated BV2 cells is associated with ROS production. After the rotenone and MK-4 treatment, cells were incubated with DCF-DA, an ROS indicator that is oxidated by ROS into DCF, a highly fluorescent compound. In BV2 cells, rotenone significantly induced ROS production. However, ROS production was obviously attenuated after MK-4 treatment (Figure 3D).
MK-4 decreased rotenone-induced caspase-1 activation in BV2 cells
Previous studies have reported that the activated NF-κB signaling pathway may induce inflammasome activation by elevating the expression of inflammasome components43. The assembled inflammasome eventually induces an activation of caspase-1 that subsequently cleaves pro-IL-1β into mature IL-1β, a potent activator of the NF-κB signaling pathway, resulting in positive feedback that enhances the immune response37. We therefore examined whether MK-4 represses rotenone-induced caspase-1 activation. In BV2 cells that were treated with rotenone, caspase-1 activation was significantly increased, as indicated by a green fluorescent indicator (FAM-FLICA in vitro caspase-1 kits) that can bind to activated caspase-1 (Figure 4A). However, MK-4 decreased the fluorescence intensity of caspase-1 (Figure 4A and 4B) in a dose-dependent manner, further suggesting that MK-4 can suppress rotenone-induced caspase-1 activation.
MK-4 suppresses rotenone-induced caspase-1 activation in BV2 cells. (A) BV2 cells were treated with 1 μmol/L rotenone and various doses of MK-4 as indicated for 24 h. The cells were stained with green fluorescent probes for activated caspase-1 for 1 h. The cells were visualized under a fluorescence microscope. Scale bars, 5 μm. The fluorescence intensity of caspase-1 was analyzed with a multi-detection reader. (B) The quantitative data from (A) are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA.
MK-4 restored the mitochondrial membrane potential damaged by rotenone
Rotenone, a well-known pesticide and inhibitor of mitochondrial complex I41,44, decreases the mitochondrial membrane potential45, leading to ROS production41,46. We therefore examined whether the decreased ROS production by MK-4 is associated with the mitochondrial membrane potential. In BV2 cells treated with rotenone, there was a significant decrease in the mitochondrial membrane potential, which was visualized by TMRM and MitoTracker Green co-staining. However, in the presence of MK-4, the mitochondrial membrane potential was significantly restored (Figure 5A and 5B), suggesting that MK-4 can maintain mitochondrial integrity to decrease the ROS production induced by rotenone.
MK-4 restores the mitochondrial membrane potential damaged by rotenone. (A) Various doses of MK-4 and 1 μmol/L rotenone were administered to BV2 cells as indicated for 24 h. The cells were stained with TMRM and MitoTracker Green. TMRM was used to detect mitochondrial membrane potential. MitoTracker Green was used to detect the mitochondria. The cells were visualized under a fluorescence microscope. Scale bars, 5 μm. (B) The fluorescence intensity of TMRM was analyzed with a multi-detection reader. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA.
MK-4 exhibited protective effects against microglia-mediated SH-SY5Y cell death
Activated microglial cells secrete inflammatory molecules that are toxic to neuronal cells20,47,48. We previously reported that rotenone induces an activation of BV2 cells, leading to the secretion of inflammatory molecules16. To determine whether MK-4 influences microglia-mediated neuronal cell death, we performed in vitro assays to examine the effects of CM from rotenone-treated BV2 cells, with or without MK-4 treatment, on SH-SY5Y cells using PI staining and the MTT assay. The CM collected from BV2 cells that were treated with or without MK-4 was used for the culture of SH-SY5Y cells. The CM from rotenone-treated BV2 cells showed a significant level of toxicity when applied to the SH-SY5Y cells; however, the toxicity of the CM from BV2 cells that were treated with rotenone in combination with MK-4 was obviously reduced (Figure 6A and 6B), suggesting that there are protective effects of MK-4 against microglia-mediated SH-SY5Y cell death.
MK-4 rescues rotenone-induced microglia-mediated cell death. The CM was collected from BV2 cells that were treated with rotenone and various doses of MK-4 or without MK-4 for 24 h. The death of SH-SY5Y cells was analyzed using propidium iodide (PI) staining (A) or the MTT assay (B). Scale bars, 20 μm. The data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01 vs the group in which the cells were treated with 1 μmol/L rotenone and 0 μmol/L MK-4, as analyzed by one-way ANOVA.
Discussion
Mitochondrial dysfunction and oxidative stress are tightly associated with the pathogenesis of PD6,49,50. Recently, many studies have indicated that neuroinflammation is involved in DA neuronal degeneration and that an inhibition of neuroinflammation can significantly delay PD progression7,51.
Rotenone induces the activation of NF-κB, which depends on its nuclear translocation, to transactivate many pro-inflammatory genes16. In the present study, we demonstrate that MK-4 suppresses rotenone-induced activation of NF-κB and the production of inflammatory factors, including TNF-α, IL-1β, iNOS and COX-2, by inhibiting the nuclear translocation of NF-κB in BV2 cells. The phosphorylation of IKK initiates the phosphorylation and degradation of IκB, leading to the nuclear translocation of NF-κB16. In our observations, MK-4 inhibits the rotenone-induced phosphorylation of IKK, the degradation of IκB and the nuclear translocation of the NF-κB p65 subunit. Furthermore, the rotenone-induced activation of caspase-1 indicates that the activated inflammasome was also decreased in the presence of MK-4. Thus, our results suggest that MK-4 can inhibit rotenone-induced NF-κB activation and the production of inflammatory factors in microglial cells.
Rotenone-induced NF-κB activation is dependent upon p38 MAPK to induce ROS production and p38 activation16. Furthermore, ROS was reported to be a second messenger that activates diverse redox-sensitive signaling transduction cascades, including p38 and its associated downstream transcription factors such as NF-κB and AP-1, thereby regulating the expression of many proinflammatory genes52,53. Rotenone, as a mitochondrial complex I inhibitor, is reported to decrease the mitochondrial membrane potential, leading to ROS production41,44. Our study provides evidence that MK-4 not only restores the rotenone-induced decrease in the mitochondrial membrane potential but also inhibits ROS production and p38 activation.
Vitamin K2 is a fat soluble vitamin that has been suggested to play important roles in maintaining healthy levels of bone and cardiovascular system54,55. Recently, MK-4, a form of vitamin K2, was reported to be associated with lower concentrations of inflammatory markers in vivo and in vitro28,33 and to rescue the deficiency of PD-related genes in the fruit fly (Drosophila melanogaster), indicating a protective role of MK-4 in inflammation and PD30. In our study, we reveal that MK-4 suppresses rotenone-induced activation of microglia, most likely via the ROS-p38-NF-κB pathway. Importantly, MK-4 maintains mitochondrial membrane potential to suppress ROS production, an upstream cellular response to the rotenone insult. The suppression of rotenone-induced microglial activation by MK-4 decreases the production of inflammatory factors, which attenuates inflammatory factor-induced SH-SY5Y cell death. Thus our study provides a mechanistic explanation of how MK-4 functions in anti-inflammation to protect neurons.
Vitamin K includes two natural forms: vitamin K1 and vitamin K2, which differ in the lengths of the carbon side chains made of isoprenoid groups of atoms27. Green leafy vegetables have high concentration of vitamin K1. The bacteria in the colon convert vitamin K1 into vitamin K2 to meet our daily needs. Natural vitamin K2 is also found in bacterially fermented foods such as mature cheeses and the traditional Japanese dish natto56. There is no known toxicity associated with a high dose of vitamin K2, as it is not stored in any significant quantity in the liver. The MK-4 form of vitamin K2 transfers electrons in the mitochondrial electron transport chain to establish a proton motive force across the membrane in bacteria. It is also sufficient to facilitate mitochondrial electron transport in the fruit fly to rescue impaired mitochondria, similar to ubiquinone (coenzyme Q) and idbenone30, two factors that are associated with many illnesses such as PD, inflammation and Friedreich ataxia57,58,59,60. Coenzyme Q and idbenone are similar in structure, including an aromatic ring that may be in either the oxidized (quinone) or reduced (quinol) form but a difference in the length of the lipophilic carbon tail. The completely oxidized form and the completely reduced form of the aromatic ring enable them to perform their functions in the electron transport chain to reduce ROS production. The structure of MK-4 is similar to that of coenzyme Q and idbenone, suggesting that MK-4 may possibly function in the electron transport chain to restore the decreased mitochondrial membrane potential and to suppress ROS production when microglia are exposed to rotenone.
In summary, we have identified that the MK-4 form of vitamin K2 directly inhibits rotenone-induced NF-κB activation in BV2 cells. The inhibition of the activated NF-κB signaling pathway that may be dependent upon p38 MAPK significantly suppresses inflammatory factor production. Furthermore, MK-4 obviously reduces rotenone-induced ROS production and restores the mitochondrial membrane potential. Thus, our study revealed a role for MK-4 in the regulation of microglial activation.
Author contribution
Dong CHEN and Guang-hui WANG designed the research; Yan-xia YU, Yi-pei LI, Feng GAO, and Dong CHEN performed the research; Qing-song HU and Yan ZHANG contributed new analytical tools and reagents; Dong CHEN and Guanghui WANG analyzed data; Yan-xia YU drafted the manuscript; Dong CHEN and Guang-hui WANG revised the manuscript
References
Huang Y, Cheung L, Rowe D, Halliday G . Genetic contributions to Parkinson's disease. Brain Res Brain Res Rev 2004; 46: 44–70.
Hou XO, Si JM, Ren HG, Chen D, Wang HF, Ying Z, et al. Parkin represses 6-hydroxydopamine-induced apoptosis via stabilizing scaffold protein p62 in PC12 cells. Acta Pharmacol Sin 2015; 36: 1300–7.
Ma Q, Hu QS, Xu RJ, Zhen XC, Wang GH . Protease Omi facilitates neurite outgrowth in mouse neuroblastoma N2a cells by cleaving transcription factor E2F1. Acta Pharmacol Sin 2015; 36: 966–75.
Chen L, Feany MB . Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci 2005; 8: 657–63.
Jenner P . Oxidative stress in Parkinson's disease. Ann Neurol 2003; 53 Suppl 3: S26–36; discussion S36–8.
Lin MT, Beal MF . Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006; 443: 787–95.
Hirsch EC, Hunot S . Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 2009; 8: 382–97.
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT . Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 2000; 3: 1301–6.
Rajput AH, Uitti RJ, Stern W, Laverty W, O'Donnell K, O'Donnell D, et al. Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson's disease. Can J Neurol Sci 1987; 14: 414–8.
Sherer TB, Betarbet R, Kim JH, Greenamyre JT . Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci Lett 2003; 341: 87–90.
Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J 2005; 19: 533–42.
Martinez TN, Greenamyre JT . Toxin models of mitochondrial dysfunction in Parkinson's disease. Antioxid Redox Signal 2012; 16: 920–34.
Miller RL, James-Kracke M, Sun GY, Sun AY . Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res 2009; 34: 55–65.
Radad K, Rausch WD, Gille G . Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int 2006; 49: 379–86.
Sanders LH, Greenamyre JT . Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med 2013; 62: 111–20.
Gao F, Chen D, Hu Q, Wang G . Rotenone directly induces BV2 cell activation via the p38 MAPK pathway. PLoS One 2013; 8: e 72046.
Lucin KM, Wyss-Coray T . Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 2009; 64: 110–22.
Zhou F, Wu JY, Sun XL, Yao HH, Ding JH, Hu G . Iptakalim alleviates rotenone-induced degeneration of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology 2007; 32: 2570–80.
Gehrmann J, Matsumoto Y, Kreutzberg GW . Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 1995; 20: 269–87.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH . Mechanisms underlying inflammation in neurodegeneration. Cell 2010; 140: 918–34.
Teismann P, Schulz JB . Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res 2004; 318: 149–61.
Li C, Chen X, Zhang N, Song Y, Mu Y . Gastrodin inhibits neuroinflammation in rotenone-induced Parkinson's disease model rats. Neural Regen Res 2012; 7: 325–31.
Etminan M, Suissa S . NSAID use and the risk of Parkinson's disease. Curr Drug Saf 2006; 1: 223–5.
Shearer MJ, Newman P . Metabolism and cell biology of vitamin K. Thromb Haemost 2008; 100: 530–47.
Wu SM, Stanley TB, Mutucumarana VP, Stafford DW . Characterization of the gamma-glutamyl carboxylase. Thromb Haemost 1997; 78: 599–604.
Thijssen HH, Drittij-Reijnders MJ . Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4. Br J Nutr 1994; 72: 415–25.
Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, et al. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 2008; 283: 11270–9.
Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB Sr, Dawson-Hughes B, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol 2008; 167: 313–20.
Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995; 7: 287–90.
Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 2012; 336: 1306–10.
Tian LP, Zhang S, Xu L, Li W, Wang Y, Chen SD, et al. Selenite benefits embryonic stem cells therapy in Parkinson's disease. Curr Mol Med 2012; 12: 1005–14.
Wu JY, Li M, Cao LJ, Sun ML, Chen D, Ren HG, et al. Protease Omi cleaving Hax-1 protein contributes to OGD/R-induced mitochondrial damage in neuroblastoma N2a cells and cerebral injury in MCAO mice. Acta Pharmacol Sin 2015; 36: 1043–52.
Ohsaki Y, Shirakawa H, Miura A, Giriwono PE, Sato S, Ohashi A, et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation. J Nutr Biochem 2010; 21: 1120–6.
Moriya M, Nakatsuji Y, Okuno T, Hamasaki T, Sawada M, Sakoda S . Vitamin K2 ameliorates experimental autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol 2005; 170: 11–20.
Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN . LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood 2005; 106: 3114–22.
Li Q, Verma IM . NF-kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–34.
Schroder K, Tschopp J . The inflammasomes. Cell 2010; 140: 821–32.
Tak PP, Firestein GS . NF-kappaB: a key role in inflammatory diseases. J Clin Invest 2001; 107: 7–11.
Hayden MS, Ghosh S . Shared principles in NF-kappaB signaling. Cell 2008; 132: 344–62.
Kuwahara I, Lillehoj EP, Lu W, Singh IS, Isohama Y, Miyata T, et al. Neutrophil elastase induces IL-8 gene transcription and protein release through p38/NF-{kappa}B activation via EGFR transactivation in a lung epithelial cell line. Am J Physiol Lung Cell Mol Physiol 2006; 291: L407–16.
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003; 278: 8516–25.
Zhou Q, Liu C, Liu W, Zhang H, Zhang R, Liu J, et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol Sci 2015; 143: 81–96.
Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, et al. Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proc Natl Acad Sci U S A 2011; 108: 18055–60.
Gottlieb E, Armour SM, Harris MH, Thompson CB . Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ 2003; 10: 709–17.
Isenberg JS, Klaunig JE . Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol Sci 2000; 53: 340–51.
Turrens JF . Superoxide production by the mitochondrial respiratory chain. Biosci Rep 1997; 17: 3–8.
Hu Q, Li B, Xu R, Chen D, Mu C, Fei E, et al. The protease Omi cleaves the mitogen-activated protein kinase kinase MEK1 to inhibit microglial activation. Sci Signal 2012; 5: ra61.
Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H, et al. Induction of COX-2-PGE2 synthesis by activation of the MAPK/ERK pathway contributes to neuronal death triggered by TDP-43-depleted microglia. Cell Death Dis 2015; 6: e1702.
Keating DJ . Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem 2008; 104: 298–305.
Henchcliffe C, Beal MF . Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008; 4: 600–9.
Berliocchi L, Corasaniti MT, Bagetta G, Lipton SA . Neuroinflammation in neuronal degeneration and repair. Cell Death Differ 2007; 14: 883–4.
Droge W . Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95.
Jia YT, Wei W, Ma B, Xu Y, Liu WJ, Wang Y, et al. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J Immunol 2007; 179: 7808–19.
Erkkila AT, Booth SL, Hu FB, Jacques PF, Lichtenstein AH . Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis 2007; 17: 58–62.
Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, et al. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab 2004; 89: 4904–9.
Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 2001; 17: 315–21.
Morris G, Anderson G, Berk M, Maes M . Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol Neurobiol 2013; 48: 883–903.
Delatycki MB, Camakaris J, Brooks H, Evans-Whipp T, Thorburn DR, Williamson R, et al. Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann Neurol 1999; 45: 673–5.
Schulz JB, Dehmer T, Schols L, Mende H, Hardt C, Vorgerd M, et al. Oxidative stress in patients with Friedreich ataxia. Neurology 2000; 55: 1719–21.
Lodi R, Cooper JM, Bradley JL, Manners D, Styles P, Taylor DJ, et al. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc Natl Acad Sci U S A 1999; 96: 11492–5.
Acknowledgements
This work was supported in part by the National Natural Sciences Foundation of China (No 31330030), the National High-tech Research and Development program of China 973-projects (No 2012CB947602), and the National Natural Sciences Foundation of China (Nos 81371393 and 31300887), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Suzhou Clinical Research Center of Neurological Disease (No Szzx201503) and Jiangsu Provincial Special Program of Medical Science (No BL2014042).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, Yx., Li, Yp., Gao, F. et al. Vitamin K2 suppresses rotenone-induced microglial activation in vitro. Acta Pharmacol Sin 37, 1178–1189 (2016). https://doi.org/10.1038/aps.2016.68
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.68
Keywords
This article is cited by
-
Pazopanib alleviates neuroinflammation and protects dopaminergic neurons in LPS-stimulated mouse model by inhibiting MEK4-JNK-AP-1 pathway
Acta Pharmacologica Sinica (2023)
-
The emerging relationship between vitamin K and neurodegenerative diseases: a review of current evidence
Molecular Biology Reports (2023)
-
Association of Glial Activation and α-Synuclein Pathology in Parkinson’s Disease
Neuroscience Bulletin (2023)
-
Vitamin K: a Potential Neuroprotective Agent
Revista Brasileira de Farmacognosia (2023)
-
Delivery of the reduced form of vitamin K2(20) to NIH/3T3 cells partially protects against rotenone induced cell death
Scientific Reports (2022)