Abstract
Aim:
To discover neuroprotective compounds and to characterize the discovered active compound YQ138 as a novel GSK-3β inhibitor.
Methods:
Primary rat cerebellar granule cells (CGCs) were treated with glutamate, and cell viability was analyzed with MTT assay, which was used as in vitro model for screening neuroprotective compounds. Active compound was further tested in OGD- or serum deprivation-induced neuronal injury models. The expression levels of GSK-3β downstream proteins (Nrf2, HO-1, NQO1, Tau and β-catenin) were detected with Western blotting. For evaluating the neuroprotective effects in vivo, adult male rats were subjected to transient middle cerebral artery occlusion (tMCAO), then treated with YQ138 (10 mg/kg, iv) at 2, 4 and 6 h after ischemia onset.
Results:
From a compound library consisting of about 2000 potential kinase inhibitors, YQ138 was found to exert neuroprotective effects: pretreatment with YQ138 (0.1–40 μmol/L) dose-dependently inhibited glutamate-induced neuronal death. Furthermore, pretreatment with YQ138 (10 μmol/L) significantly inhibited OGD- or serum deprivation-induced neuronal death. Among a panel of seven kinases tested, YQ138 selectively inhibited the activity of GSK-3β (IC50=0.52 nmol/L). Furthermore, YQ138 dose-dependently increased the expression of β-catenin, and decreased the phosphorylation of Tau in CGCs. Moreover, YQ138 significantly increased the expression of GSK-3β downstream antioxidative proteins Nrf2, HO-1, NQO1, GSH and SOD in CGCs. In rats with tMCAO, administration of YQ138 significantly decreased infarct volume, improved the neurological deficit, and increased the expression of Nrf2 and HO-1 and the activities of SOD and GSH in the cerebral cortex.
Conclusion:
A novel GSK-3β inhibitor YQ138 effectively suppresses brain ischemic injury in vitro and in vivo.
Similar content being viewed by others
Introduction
Glutamate plays important roles as the predominant excitatory neurotransmitter in the mammalian brain1. However, excessive release of glutamate results in excitotoxicity and is a major factor in neuronal injury associated with many acute and chronic brain disorders, including brain ischemia1,2,3. Ischemic stroke is one of the major causes of mortality and disability in adults, and it has become a burden worldwide4. Currently, there are no pharmacological treatments to ameliorate glutamate excitotoxicity and provide neuroprotection for brain ischemic stroke3. This indicates an urgent need to search for novel compounds with neuroprotective effects for the treatment of brain ischemic stroke.
Several lines of evidence have suggested that glycogen synthase kinase-3β (GSK-3β) is involved in ischemic brain injury5, and glutamate incubation in neurons increases GSK-3β activation6. GSK-3β is a serine/threonine protein kinase, which is abundant in the central nervous system (CNS), particularly in neurons7. GSK-3β phosphorylates and regulates many important metabolic and signaling proteins, structural proteins and transcription factors, and as a result, it plays a key role in glycogen metabolism, embryogenesis, mitotic regulation, inflammation and neuroplasticity8. One of the important substrates of GSK-3β is the microtubule binding protein Tau, which plays a critical role in microtubule function in specific neurons9. Tau phosphorylation is increased in transgenic mice conditionally overexpressing GSK-3β10. Recent studies have shown that the inhibition of GSK-3β attenuates apoptotic signals and prevents neuronal death11,12. GSK-3β phosphorylation at tyrosine 216 residue is increased after middle cerebral artery occlusion (MCAO), indicating the involvement of active GSK-3β13. GSK-3β inhibition by lithium has been shown to be protective against cerebral ischemia and to prevent neurons from glutamate-induced apoptosis, suggesting that GSK-3β inhibition improves brain neurons survival14,15,16.
The redox-sensitive transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a member of the cap 'n' collar family of transcription, is the downstream substrate of GSK-3β, which interacts with Kelch-like ECH-associated protein 1 (Keap1) under normal conditions17. When cells are exposed to oxidative, nitrosative or electrophilic stress, the Keap1-Nrf2 complex is dissociated; Nrf2 accumulates in the nucleus and, together with small Maf proteins, binds to antioxidant response element (ARE) regions, thus promoting the generation of antioxidant proteins, such as heme oxygenasea (HO-1)18. HO-1 is a ubiquitous and redox-sensitive enzyme, which has some favorable effects, such as anti-inflammatory, antioxidant and anti-proliferative effects. It also participates in the conversion of heme into biliverdin19. This antioxidant protein HO-1 may play an important role in suppressing neuronal damage or death.
In this study, using a well-characterized and reliable model, glutamate-induced neuronal injury in rat cerebellar granule cells (CGCs), we screened our in-house compound library consisting of 2000 small-molecule inhibitors against potential kinases and discovered the novel neuroprotectant small molecule YQ138. We determined the neuroprotective effects of YQ138 and the relevant underlying mechanisms. Our results indicated that the novel GSK-3β inhibitor YQ138 shows neuroprotective effects through activation of the antioxidative Nrf2 signaling pathway, further increasing glutathione (GSH) and superoxide dismutase (SOD) levels and thereby suppressing ischemia-induced brain injury.
Materials and methods
Materials
Cell-culture media and supplements were purchased from Invitrogen (Carlsbad, CA, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,3,5-triphenyltetrazolium chloride (TTC), trypsin, poly-L-lysine were purchased from Sigma-Aldrich (St Louis, MO, USA). Primary antibodies used for Western blotting analysis were mouse anti-β-actin (1:2000) and rabbit anti-Nrf2 (1:1000), purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit anti-HO-1 (1:1000), purchased from Abcam (Cambridge, UK); and rabbit anti-NAD(P)H:quinone oxidoreductase 1 (NQO1, 1:1000), rabbit anti-phospho-GSK-3βSer9 (1:1000), rabbit anti-phospho-Tau (1:1000), and rabbit anti-β-catenin (1:1000), purchased from Cell Signaling Technology (Danvers, MA, USA). The GSH and SOD assay kits and RIPA buffer were purchased from Beyotime Institute of Biotechnology (Nanjing, China). Protease inhibitor cocktail was purchased from Roche (Indianapolis, IN, USA). All other reagents were obtained from Sigma-Aldrich.
Primary CGC cultures
Primary CGCs were isolated from 8-d old Sprague-Dawley rat pups, through previously described procedures20. Cerebella were collected and placed into ice-cold Hanks' balanced salt solution (HBSS). The cerebella were dispersed into the same buffer containing 0.025% trypsin-EDTA after removal of the meninges and then digested for 15 min at 37 °C. Trypsin digestion was stopped by the addition of two volumes of Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) medium and 0.1 mg/mL DNase I. Digested tissues were centrifuged at 1500× r/min for 5 min at 4–10 °C after gentle trituration. The tissue was resuspended in a complete Neurobasal culture medium supplemented with 2% B27 and 0.5 mmol/L GlutaMax and filtered through a 70-μm nylon cell restrainer. The cells were diluted to 1×106 cells/mL in poly-L-lysine coated plates. Cultures were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Primary fetal cortical neuron cultures
Day E16 to E18 embryos were prepared for primary cultures of cortical neurons, as previously described21. Pregnant Sprague-Dawley rats were sacrificed by cervical dislocation. To isolate the cortical neurons, the fetuses were removed from the abdomens of the E16 to E18 rats. Cerebral cortices were placed into ice-cold HBSS. After the scalp and skull were peeled away, the cortex was removed and placed into ice-cold HBSS. Next, the tissue was incubated with 0.05% trypsin-EDTA for 10 min at 37 °C. The digestion was stopped by the addition of Neurobasal medium with 0.1 mg/mL DNase I. The following steps were performed in the same manner as the primary rat CGCs culture protocol.
Cell viability assay
CGCs were pretreated with compounds for 24 h and then were incubated with 200 μmol/L glutamate or without nutrient B27 for an additional 24 h, or were subjected to OGD conditions. The primary fetal cortical neurons were treated with glutamate at concentrations of 100 μmol/L for 10 min in an experimental buffer consisting of 120 mmol/L NaCl, 3.5 mmol/L KCl, 0.4 mmol/L KH2PO4, 5 mmol/L NaHCO3, 20 mmol/L HEPES, 1.2 mmol/L Na2SO4 supplemented with 15 mmol/L glucose and 1.2 mmol/L CaCl2 at pH 7.4. Cultures were rinsed with a 1.2 mmol/L MgCl2-supplemented experimental buffer and returned to preconditioned medium. The cells were then incubated with a serum-free medium containing MTT at a final concentration of 0.5 mg/mL in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The medium was replaced with DMSO after 4 h, and the absorbance was measured at 570 nm with a BD plate-reader.
Oxygen and glucose deprivation (OGD)
To induce ischemia, cells were pretreated with YQ138 for 24 h, and the culture medium was replaced with pre-warmed HBSS (PH 7.4). Cell cultures were then placed in a hypoxic chamber containing a mixture of 95% N2 and 5% CO2 for 6 h. OGD was stopped by replacing HBSS with the Neurobasal medium, and cells were then incubated for an additional 24 h under normal conditions. Control cells without OGD were maintained under normal conditions.
Western blotting analyses
After treatment, cells were lysed in Tris-Glycine SDS lysis buffer, and the cell lysis was boiled for 10 min. Whole protein samples were separated on SDS-PAGE gels. After separation on SDS-PAGE gels, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes at 100 V for 3 h. The membranes were blocked for 2 h with 5% bovine serum albumin (BSA) in Tris-buffered saline plus Tween 20 (TBST, PH 7.4) and incubated with primary antibodies diluted (1:1000) in blocking buffer with BSA. After several washes with TBST, the membranes were incubated with the secondary antibodies for 1 h at room temperature. The membranes were washed again, and the transferred proteins were visualized through chemiluminescence with a Bio-Rad ChemiDoc XRS (Bio-Rad, Hercules, CA, USA).
GSH and SOD measurement
The cellular GSH and SOD levels were measured by using the GSH and SOD assay kit according to the manufacturer's instructions after treatment. For the brain tissues, the rats were sacrificed, and the brains were collected at 24 h after MCAO. Next, brain cortices were homogenized, and the GSH and SOD concentrations were also measured by using the GSH and SOD assay kit.
Kinase assay
The human kinome, consisting of 518 kinases, is classified into CMGC, AGC, TK, TKL, CAMK, STE and 7 other subfamilies, on the basis of DNA sequences and evolution. GSK-3β belongs to CMGC family. Here, other family members were used to evaluate the selectivity of GSK-3β inhibitors, including PKCε (AGC family), JAK2 (TK family), BRAF (TKL family), DRAK2 (CAMK family) and IKKβ (other family). The recombinant GST-GSK-3β protein was expressed in Escherichia coli strain BL21-Codon Plus (DE3), purified by GSTrap affinity chromatography, and cleaved by thrombin. The GSK-3β kinase assay was performed with a Z'-LYTE™ kinase assay kit Ser/Thr 9 Peptide substrate (Invitrogen, Grand, NY, USA) in a 10-μL reaction volume containing 50 nmol/L enzyme, 30 μmol/L ATP and 2 μmol/L substrate peptide. DRAK2 proteins were provided by Professor Jiang-ping Wu (Laboratory of Immunology, Research Centre, CHUM, Notre Dame Hospital, Pavilion DeSève). The DRAK2 kinase reaction was performed in a final assay volume of 3.4 μL using the ADP-GLO kinase assay kit (Promega, Madison, WI, USA), according the ADP-GLO protocol and was read on an EnVision plate reader (Perkin Elmer, Wellesley, MA, USA). The recombinant PKCε, IKKβ, and JAK2 with N-terminal His-tag were expressed using the baculovirus expression system and purified with Ni-Beads. BRAF protein was obtained from Carna Biosciences, Inc (Kobe Port Island, Japan). Related kinase reactions were performed in a final assay volume of 10 μL using the related HTRF assay kit (Cisbio, Codolet, France). Reactions were performed according the HTRF protocol and were read on an EnVision plate reader. The CDK2/CycA2 protein was obtained from Carna Biosciences, Inc. The CDK2/CycA2 kinase assay was performed with a Invitrogen Z'-LYTE™ kinase assay kit (Ser/Thr 12 peptide substrate), with a final enzyme concentration of 2 nmol/L. All reactions were performed in triplicate. IC50 values (concentration at which a 50% of enzyme inhibition is shown) were derived from a nonlinear regression model (curve fit) based on sigmoidal dose response curve (variable slope) and computed using Graphpad Prism version 5.02, Graphpad Software (San Diego, CA, USA). Data are expressed as the mean±SD.
Preparation of compound YQ138
The synthetic approach of compound YQ138 is outlined in Figure 1. Indole 1 was reacted with oxalyl chloride in Et2O, followed by sodium methoxide to obtain 2. N-alkylation of 2 with 1-(2-chloroethyl)-1H-imidazole resulted in the key intermediate 3. Condensation of glyoxilic esters 3 with 2-(benzo[d]isoxazol-3-yl)acetamide 4 in the presence of t-BuOK in tetrahydrofuran yielded the target compound YQ138.
Synthetic route to YQ138. Reagents and conditions: (a) i (COCl)2, Et2O; ii CH3ONa, CH3OH; (b) NaH, DMF, 1-(2-chloroethyl)-1H-imidazole; (c) i t-BuOK, THF; ii concentrated HCl.
Animals
Adult male Sprague-Dawley (SD) rats (260–280 g, Grade II) were purchased from Zhejiang Laboratory Animals Center (Hangzhou, China) and maintained under standard housing conditions at temperatures between 20 °C and 23 °C with a 12-h light/dark cycle and a relative humidity of 50%. All procedures were performed according to the US National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals published by the US National Academy of Sciences (http://oacu.od.nih.gov/regs/index.htm). All animal tests and experimental procedures were approved by the Administration Committee of Experimental Animals in Jiangsu Province and the Ethics Committee of China Pharmaceutical University. The animal experiments were performed according to the National Research Council's guidelines.
Drug treatment
Animals were randomly divided into four groups (n=8–12 per group): sham, vehicle, YQ138 (10 mg/kg), and edaravone (3 mg/kg). Rats were intravenously treated with YQ138 and edaravone at 2 h, 4 h and 6 h after ischemia onset.
Animal surgery
Transient middle cerebral artery occlusion (tMCAO) was performed as previously described22. The right common carotid artery (CCA), internal carotid artery (ICA) and external carotid artery (ECA) of individual rats were exposed. The right MCA was occluded by inserting a monofilament nylon suture (diameter of approximately 0.26 mm) with a round tip into the internal carotid artery, and it was advanced further until it closed the origin of the MCA. After 2 h of MCAO, the filament was withdrawn to restore blood flow (reperfusion). Sham-operated control mice received the same surgical procedure without insertion of a filament. The body temperature was kept at 37 °C with a temperature control system. All animals had free access to food and water.
Measurement of neurological deficit, infarct size and brain-water content
The neurological deficits of individual rats were measured with Longa's method with minor modifications in a blinded manner at 24 h after MCAO23. The neurological deficits were scored on a five-point scale: 0, normal function; 1, flexion of the torso and contralateral forelimb after lifting the animal by the tail; 2, circling to the contralateral side but normal posture at rest; 3, recline to the contralateral side at rest; and 4, absence of spontaneous motor activity. After assessment of neurological deficit, the rats were euthanized at 24 h after tMCAO, and the brains were collected and dissected on ice and sectioned into 2 mm coronal sections. Sections were soaked in 2% TTC phosphate buffer for 20 min at 37 °C in the dark. Whereas infarct tissues stained white, the normal brain tissues stained red. The ratio percentages of the infarct areas to the total brain areas were calculated by morphometric analysis (image-pro plus). With regard to the brain-water content, some brain samples were weighed immediately to obtain the wet weight, subsequently dried for 8 h in an oven at 120 °C, and then reweighed to obtain the dry weight. The brain-water content was calculated with the following formula: [(wet weight−dry weight)/(wet weight)]×100.
Statistical analysis
All values are expressed as the mean±SD from at least three independent experiments. The results were analyzed using one-way ANOVA followed by Bonferroni's post hoc test. P<0.05 was considered statistically significant.
Results
Discovery of YQ138 using a glutamate-induced injury model in cerebellar granule neurons (CGCs)
Using our established glutamate-induced neuronal cell damage model to randomly screen an in-house compound library consisting of approximately 2000 potential kinase inhibitors, we found the small-molecule YQ138, which exhibited a potential neuroprotective effect by preventing CGCs from glutamate-induced injury (Figure 2). YQ138 pretreatment at 24 h before glutamate incubation inhibited glutamate-induced cell death in a dose-dependent manner in CGCs (Figure 2A). Pretreatment of YQ138 at 2 h before glutamate addition also significantly prevented neuronal injury induced by glutamate, but YQ138 incubation concurrently with or after glutamate incubation did not prevent glutamate-induced neuronal death (Figure 2B).
Neuroprotective effects of YQ138 on glutamate-induced neuronal injury. (A) Cerebellar granule neuronal cells (CGCs) were preincubated with various concentrations of YQ138 for 24 h and then treated with 200 μmol/L glutamate (Glu) for an additional 24 h. (B) CGCs were treated with YQ138 (10 μmol/L) at different time points before, during, or after 24-h Glu incubation. (C) CGCs were pretreated with YQ138 (10 μmol/L) for 24 h, and then exposed to oxygen-glucose deprivation (OGD) for 6 h, followed by 24 h of reoxygenation, or were maintained under normal conditions. (D) CGCs were preincubated with YQ138 for 24 h, followed by B27 deprivation (–B27) for an additional 24 h. (E) Morphology of CGCs pretreated with YQ138 (10 μmol/L) for 24 h followed by Glu incubation for an additional 24 h. Photomicrographs were obtained using an inverted light microscope. Cell viability was measured by using the MTT assay. The results are expressed as the mean±SD of at least three independent experiments. **P<0.01 vs control; #P<0.05, ##P<0.01 vs Glu, OGD, or –B27.
Neuroprotection of YQ138 in OGD- or B27 deprivation-induced neuronal injury model in CGCs
OGD insult, followed by reoxygenation and nutrient recovery, is thought to mimic the process of ischemia/reperfusion. YQ138 significantly inhibited CGCs injury induced by OGD conditions (Figure 2C). If the nutrient B27, a serum substitute, is removed, the majority of CGCs die via a cell apoptotic process24. As shown in Figure 2D, Y138 also significantly inhibited CGC injury caused by serum deprivation (B27 deprivation, -B27).
YQ138 induces GSH and SOD in CGCs
Glutamate-induced cell apoptosis usually occurs via reactive oxygen species (ROS) production25,26. GSH and SOD play important roles in the antioxidant system27. GSH, an endogenously synthesized tripeptide thiol, is involved in scavenging free radicals and protecting against oxidative stress28. SOD, an endogenous mitochondrial anti-oxidant enzyme, exhibits the effect of scavenging free radical and prevents the accumulation of superoxide29. We investigated the content of GSH and SOD after incubation with YQ138 and glutamate in CGCs and found that YQ138 reversed glutamate-caused decreases in GSH and SOD content in CGCs, thus protecting neurons against cellular injury caused by glutamate (Figure 3). However, YQ138 alone did not increase the GSH and SOD production in CGCs (Figure 3).
Effects of YQ138 on GSH concentration and SOD activity in CGCs. CGCs were preincubated with YQ138 for 24 h and then exposed to 200 μmol/L glutamate (Glu) for an additional 24 h to determined GSH concentration (A) and SOD activity (B). The results are expressed as the mean±SD of at least three independent experiments. **P<0.01 vs control; ##P<0.01 vs Glu.
YQ138 induces Nrf2, HO-1 and NQO1 expression in CGCs
Nrf2 is a member of the cap 'n' collar family, and plays a crucial role in regulating cellular antioxidant systems30. Exposure to glutamate resulted in a decrease in the level of Nrf2 in a time-dependent manner in CGCs, whereas YQ138 (10 μmol/L) completely prevented this effect (Figure 4A). Moreover, YQ138 increased Nrf2 expression in glutamate-incubated CGCs in a dose-dependent manner (Figure 4B). YQ138 also enhanced the protein expression levels of HO-1 and β-catenin and decreased the GSK-3β substrate Tau phosphorylation levels in CGCs (Figure 4C).
YQ138 induces Nrf2 protein expression in CGCs. (A) CGCs pretreated with YQ138 (10 μmol/L) were treated with 200 μmol/L glutamate (Glu) for different times before preparation of protein extracts. (B) Dose-response of YQ138 on the induction of Nrf2 protein levels in Glu-incubated CGCs for 24 h. (C) YQ138 treatment reversed the Glu-induced decrease of Nrf2 downstream target HO-1 protein levels via inhibition of cellular GSK-3β activity indicated by a decrease in Tau phosphorylation (Ser396) and increase in β-catenin expression in CGCs. Protein expression was measured using Western blotting analysis. β-Actin was used an internal control. One representative blot is shown. The results are expressed as the mean±SD of at least three independent experiments. *P<0.05, **P<0.01 vs control; #P<0.05, ##P<0.01 vs Glu.
Under stress, Nrf2 translocates into nuclei and binds AREs of phase II and antioxidant defense enzymes, such as HO-1 and NQO1, thus inhibiting oxidative stress18. HO-1 is a ubiquitous and redox-sensitive inducible stress protein, and it degrades heme to CO, iron, and biliverdin. NQO1, a detoxifying enzyme, reduces reactive quinones and quinone imines to less reactive and less toxic hydroquinones. We investigated the expression of Nrf2, HO-1 and NQO1 after incubation with YQ138 in CGCs. YQ138 increased the expression of Nrf2 and its downstream substrate, such as HO-1 and NQO1, in CGCs, in a dose-dependent manner (Figure 5A). We also found that YQ138 promoted the accumulation of Nrf2 in the nucleus (Figure 5B).
YQ138 induces Nrf2, HO-1 and NQO1 expression in CGCs. (A) CGCs were treated with various concentrations of YQ138 for 24 h to detect the expression of Nrf2, HO-1 and NQO1 using Western blotting analysis. (B) CGCs were treated with YQ138 (10 μmol/L) for 24 h and nuclear extracts were prepared. The nuclear protein levels of Nrf2 were determined using Western blotting analysis. H4 and β-actin were used as internal controls for nuclear extracts and whole-cell lysates, respectively. The results are expressed as the mean±SD of at least three independent experiments. One representative blot is shown. *P<0.05, **P<0.01 vs control.
YQ138 inhibits GSK-3β activity with high selectivity
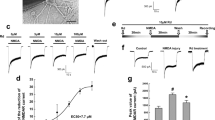
Given that GSK-3β down-regulated the transcriptional factor Nrf2 and antioxidant cell defense31,32, we speculated that YQ138 may interact with a GSK-3β protein and inhibit its activity to manifest its neuroprotective effects. The reported GSK-3β inhibitors lithium chloride (5 mmol/L)16 and AR-A014418 (10 μmol/L)33 exhibited similar neuroprotective effects to those of YQ138 (Figure 6A). YQ138 directly inhibited GSK-3β activity at a molecular level with an IC50 value of 0.52 nmol/L (Figure 6B). In addition, YQ138 displayed high selectivity for GSK-3β over other tested kinases (Table 1).
YQ138 inhibits GSK-3β activity. (A) Neuroprotective effects of GSK-3β inhibitors, lithium chloride (LiCl) and AR-A014418 (AR), on glutamate (Glu)-induced neuronal injury. CGCs were preincubated with LiCl (5 mmol/L), AR (10 μmol/L) or YQ138 (10 μmol/L) for 24 h and then treated with 200 μmol/L Glu for an additional 24 h. Cell viability was measured by using the MTT assay. (B) IC50 curve of YQ138 against GSK-3β activity. (C) YQ138 increases GSK-3β substrate β-catenin expression but decreases Tau phosphorylation (pTau) in CGCs. CGCs were treated with various concentrations of YQ138 for 24 h to measure the expression of pTau and β-catenin using Western blotting analysis. β-Actin was used as an internal control. One representative blot is shown here. The results are expressed as the mean±SD of at least three independent experiments. **P<0.01 vs control, ##P<0.01 vs Glu.
Effects of YQ138 on downstream substrates of GSK-3β in primary neurons
Tau protein is a microtubule-associated protein (MAP) and is abundantly expressed in CNS. Previous reports have demonstrated that hyperphosphorylated Tau causes cognitive impairment34. Phosphorylation modification of Tau is directly regulated by protein kinases and the protein phosphatase system. GSK-3β is the most important kinase involving Tau phosphorylation9,10. As a GSK-3β inhibitor, YQ138 decreased the phosphorylation of Tau protein dose-dependently in CGCs (Figure 6C). In addition, inactivating GSK-3β activity causes the stabilization and activation of β-catenin35. YQ138 increased the stability and expression of β-catenin by inactivating GSK-3β (Figure 6C).
Molecular modeling study
The binding mode between GSK-3β (1Q3D) and YQ138 was proposed using the Discovery studio 2.5/LigandFit, as shown in Figure 7. Compound YQ138 tightly occupies the ATP binding site of GSK-3β, resulting in the docking result, which is consistent with its potent GSK-3β inhibitory activity. The NH and carbonyl group in a maleimide ring of YQ138 can strongly interact with Asp133 and Val135 in the hinge domain of GSK-3β via two hydrogen bonds, respectively. In addition, the 3-position nitrogen atom of the imidazole ring of YQ138 can form another hydrogen bond with Asp200.
Molecular docking of YQ138 into the GSK-3β crystal structure. (A) The ribbon shows the compound YQ138 bound to GSK-3β. (B) The surface shows the compound YQ138 docking into ATP-binding pocket of GSK-3β.
Neuroprotection of YQ138 in the OGD-induced neuronal injury model in primary rat cortical neurons
OGD conditions induce neuronal damage in the cortex and striatum during ischemic stroke. OGD insult, followed by reoxygenation, is thought to mimic the pathological conditions of ischemia. YQ138 significantly inhibited cortical neuronal injury induced by OGD (Figure 8A). In addition, OGD conditions decreased the protein expression levels of Nrf2 and β-catenin, but YQ138 treatment reversed the OGD-induced decrease of Nrf2 expression in primary rat cortical neurons (Figure 8B).
YQ138 protects cortical neurons from OGD-induced cell death. Primary rat cortical neurons were treated with YQ138 (10 μmol/L) for 24 h and then exposed to OGD for 6 h followed by 24 h of reoxygenation or maintained under normal conditions (normoxia). (A) Cell viability was measured by using the MTT assay. (B) YQ138 treatment reversed the OGD-induced decrease of Nrf2 expression levels in cortical neurons. Nrf2 protein expression was measured using Western blotting analysis. β-Actin was used as an internal control. The results are expressed as the mean±SD of at least three independent experiments. One representative blot is shown. **P<0.01 vs control; ##P<0.01 vs OGD.
YQ138 decreases infarct volume and improves neurological deficit in tMCAO rats
Rats were intravenously treated with YQ138 (10 mg/kg) and Edaravone (3 mg/kg) at 2 h, 4 h and 6 h after ischemia onset. These results showed that YQ138, compared with vehicle, decreased infarct volume and water content (Figure 9A–9C). Moreover, YQ138 administration improved the neurological deficit scores in cerebral ischemic rats (Figure 9D). These findings indirectly suggested that YQ138 may protect the blood-brain barrier and exhibit neuroprotective effects.
The effect of YQ138 on acute cerebral ischemia in rats at 24 h after transient middle cerebral artery occlusion (tMCAO). YQ138 and Edaravone decreased ischemia-induced infarct volume (A, B) and brain edema (C). YQ138 administration also improved the neurological deficit score in cerebral ischemic rats (D). Data are expressed as the mean±SD of individual groups of rats (n=8−12). **P<0.01 vs vehicle (tMCAO group).
YQ138 increased the levels of Nrf2, HO-1, GSH and SOD in tMCAO rats
We investigated the antioxidative proteins Nrf2, HO-1, GSH and SOD levels in the cerebral cortex of rats with tMCAO. A significant decrease in the levels of GSH and SOD was revealed in rats 24 h after MCAO when compared with the sham treatment, whereas YQ138 treatment significantly increased the levels of GSH and SOD (Figure 10A, 10B). In addition, YQ138 treatment significantly enhanced the MCAO-caused decrease in the protein expression levels of Nrf2, HO-1, β-catenin and phosphorylated GSK-3β (Ser9) in the cerebral cortex of ischemic rats (Figure 10C).
Effects of YQ138 on the antioxidative protein expression of Nrf2 and HO-1, GSH concentration and SOD activity in the cerebral cortex of rats with tMCAO. GSH concentration (A), SOD activity (B), the protein expression of Nrf2, HO-1, β-catenin, and phosphorylated GSK-3βSer9 (C), determined at 24 h after surgery. Data are expressed as the mean±SD of individual groups of rats (n=3−5). *P<0.05, **P<0.01 vs sham group; #P<0.05, ##P<0.01 vs vehicle (MCAO group).
Discussion
In this study, a novel GSK-3β inhibitor YQ138 was found to exhibit neuroprotective effects in primary neuronal injury models or cerebral ischemic rat models. The underlying mechanisms for YQ138's neuroprotective role involved the inhibition of GSK-3β activity at the molecular and cellular levels, as well as the enhancement of GSK-3β downstream of the Nrf2 signaling pathway and related antioxidative systems.
Glutamate, one of the most important excitatory amino acids in the nervous system, plays a crucial role in many functions of the mammalian brain1. Excitotoxicity caused by glutamate receptor overactivation has been shown to result in neuronal injury and infarct growth after acute ischemic stroke36. The cerebellar granule cells in culture are more vulnerable to glutamate-induced neuronal excitotoxicity than are cultured cortical neurons. The in vitro model of glutamate excitotoxicity using primary culture of cerebellar granule cells and an in vivo model of cerebral ischemia have been used to determine potential neuroprotective compounds in previous studies6,15. Thus, we chose to study cerebellar granule cells in vitro, which is a well-characterized and reliable model to analyze mechanisms and excitotoxic neuronal damage. We found that YQ138 functioned as a GSK-3β inhibitor, and it suppressed the excitotoxicity induced by glutamate in rat primary cerebellar granule neuronal cells (Figure 2). Stroke is the neurological condition that develops when a portion of the brain is deprived of oxygen and glucose. Therefore, we further investigated the role of YQ138 in ischemic neuronal injury using primary cerebellar granule neuronal cells and primary cortical neurons exposed to OGD. Our data showed that YQ138 prevented neuronal death induced by OGD insult (Figure 2 and 8). The differentiated neurons in CNS require nutrients, such as serum, to survive against apoptosis and to exert their functions23. In our study, we found that pretreatment with YQ138 partially blocked B27 deprivation-induced neuronal cell death (Figure 2).
GSH is involved in numerous basic cellular processes, including maintaining redox status, conjugation/detoxification reactions, and scavenging free radicals and electrophilic intermediates26,27. SOD is a free radical scavenger that converts superoxide radicals formed by cerebral ischemia into the less reactive hydrogen peroxide form28. In this study, we found increases in the levels of GSH and SOD upon YQ138 treatment after cells were exposed to glutamate (Figure 3). In addition, we also investigated the levels of GSH and SOD in the cerebral cortex of rats with tMCAO, and we observed that YQ138 significantly increased the activity of GSH and SOD (Figure 10) compared with the vehicle group.
Previous research has established that Nrf2 activation is an effective strategy for the prevention of oxidative stress-induced cellular damage, and it delays the progression of inflammation29,30,31,32. We found that YQ138 increased the level of Nrf2 in a time-dependent and dose-dependent manner against the excitotoxicity caused by glutamate (Figure 4). We also found that nuclear Nrf2 accumulation was significantly enhanced after incubation with YQ138 (Figure 5).
Previous reports have shown that HO-1 may provide protection against excitotoxicity and cerebral ischemia19. Moreover, NQO1 is induced by electrophilic metabolites and oxidative stress, and it is transcriptionally regulated by ARE30. In this study, we found that HO-1, NQO1 and Nrf2 protein levels were increased after incubation with YQ138 in a dose-dependent manner (Figure 5).
The biological activity of Tau is regulated by its degree of phosphorylation. However, hyperphosphorylation of Tau not only converts it into a cytotoxic protein, which sequesters MAPs but also damages its biological activity37. Overexpression of GSK-3β in transgenic mice results in the hyperphosphorylation of Tau10. Moreover, β-catenin, which regulates the coordination of cell-cell adhesion and gene transcription, is also regulated by GSK-3β35. Thus, we investigated the effects of YQ138 on the expression of Tau and β-catenin. Our data indicated that YQ138 inhibited cellular GSK-3β activity, as indicated by the enhanced protein levels of Tau phosphorylation and β-catenin in neurons (Figure 6).
To determine whether administration of YQ138 would attenuate the injury of ischemia in vivo, we investigated the effect of YQ138 in MCAO rats. Previous studies have suggested that MCAO mimics clinical ischemia stroke in animal models22,38. Our data showed that YQ138 improved the infarct volume and brain edema, and it also improved the neurological deficit in rats after MCAO (Figure 9). Improvement of antioxidative systems, such as Nrf2, HO-1, GSH and SOD, may be responsible for the beneficial effects of YQ138 in cerebral ischemic rats (Figure 10). YQ138 potentially decreased water content in the infarct region via protection of the blood-brain barrier, which can be disrupted by cerebral ischemia, resulting in vasogenic brain edema. Glutamate excitotoxicity plays an important role in the pathogenesis of several brain disorders, including ischemic stroke, but there is currently no effective treatment for brain ischemic stroke. Together, these observations suggest that YQ138, a novel GSK-3β inhibitor, may be developed as a neuroprotective agent to protect against ischemic stroke via an up-regulation of the anti-oxidative system.
In conclusion, in the present study, we provide the first evidence that YQ138, a GSK-3β inhibitor, functions as a novel neuroprotective agent for ischemia stroke by increasing Nrf2 and antioxidative signaling.
Abbreviations
ARE, antioxidant response element; BSA, bovine serum albumin; CCA, common carotid artery; CGCs, cerebellar granule cells; CNS, central nervous system; DMEM, Dulbecco's modified Eagle's medium; DNase I, deoxyribonuclease I; EBSS, Earle's balanced salt solution; ECA, external carotid artery; Eda, edaravone; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSH, glutathione; GSK-3β, glycogen synthase kinase 3β; HBSS, Hank's balanced saline solution; HO-1, heme oxygenase-1; ICA, internal carotid artery; Keap1, Kelch-like ECH-associated protein 1; MCAO, middle cerebral artery occlusion; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NQO1, uinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor 2; OGD, oxygen and glucose deprivation; PVDF, polyvinylidene difluoride; ROS, reactive oxygen species; SD, Sprague-Dawley; SOD, superoxide dismutase; TBST, Tris-buffered saline plus Tween20; tMCAO, transient middle cerebral artery occlusion; TTC, 2,3,5-triphenyltetrazolium chloride.
Author contribution
Tao PANG, Hong LIAO, Lu-yong ZHANG, Jian-rong GAO, Qing YE, and Jia LI designed the research; Tao PANG, Yun-jie WANG, Yuan-xue GAO, Yuan XU, Yu-bo ZHOU, and Lei XU performed the research; Qiu LI and Qing YE synthesized the compound YQ138; Tao PANG, Zhang-jian HUANG, Qing YE, and Jia LI analyzed the data; and Tao PANG, Qing YE, and Jia LI wrote the paper.
References
Coyle JT, Puttfarcken P . Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993; 262: 689–95.
Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C . Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 2010; 113: 564–70.
Lau A, Tymianski M . Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 2010; 460: 525–42.
Donnan GA, Fisher M, Macleod M, Davis SM . Stroke. Lancet 2008; 371: 1612–23.
Woodgett JR . Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 1990; 9: 2431–8.
Wang J, Pang T, Hafko R, Benicky J, Sanchez-Lemus E, Saavedra JM . Telmisartan ameliorates glutamate-induced neurotoxicity: roles of AT(1) receptor blockade and PPARγ activation. Neuropharmacology 2014; 79: 249–61.
Leroy K, Brion JP . Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J Chem Neuroanat 1999; 16: 279–93.
Seira O, Del Rio JA . Glycogen synthase kinase 3 beta (GSK3β) at the tip of neuronal development and regeneration. Mol Neurobiol 2014; 49: 931–44.
Lovestone S, Reynolds CH, Latimer D, Davis DR, Anderton BH, Gallo JM, et al. Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol 1994; 4: 1077–86.
Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J . Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J 2001; 20: 27–39.
Castro A, Martinez A . A new therapeutical strategy for the treatment of Alzheimer's disease and other neurodegenerative disorders. Expert Opin Ther Pat 2000; 10: 1519–8.
Cross DAE, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD . Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurons from death. J Neurochem 2001; 77: 94–102.
Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A 2000; 97: 11074–9.
Nonaka S, Chuang DM . Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport 1998; 9: 2081–4.
Leng Y, Chuang DM . Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci 2006; 26: 7502–12.
Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM . Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 2008; 28: 2576–88.
Cuadrado A . Structural and functional characterization of NRF2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic Biol Med 2015; 88: 147–57.
Satoh T, McKercher SR, Lipton SA . Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 2013; 65: 645–57.
Chen J . Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci 2014; 25: 269–80.
Pang T, Sun LX, Wang T, Jiang ZZ, Liao H, Zhang LY . Telmisartan protects central neurons against nutrient deprivation-induced apoptosis in vitro through activation of PPARγ and the Akt/GSK-3β pathway. Acta Pharmacol Sin 2014; 35: 727–37.
Liu F, Wang Y, Yan M, Zhang L, Pang T, Liao H . Glimepiride attenuates Aβ production via suppressing BACE1 activity in cortical neurons. Neurosci Lett 2013; 557: 90–4.
Chen T, Wang J, Li C, Zhang W, Zhang L, An L, et al. Nafamostat mesilate attenuates neuronal damage in a rat model of transient focal cerebral ischemia through thrombin inhibition. Sci Rep 2014; 4: 5531.
Gao Y, Xu X, Chang S, Wang Y, Xu Y, Ran S, et al. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction. Toxicol Appl Pharmacol 2015; 289: 142–54.
Hetman M, Cavanaugh JE, Kimelman D, Xia Z . Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci 2000; 20: 2567–74.
Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE . NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem 1995; 65: 2016–21.
Savolainen KM, Loikkanen J, Eerikainen S, Naarala J . Glutamate-stimulated ROS production in neuronal cultures: interactions with lead and the cholinergic system. Neurotoxicology 1998; 19: 669–74.
Zhang Q, Ding M, Cao Z, Zhang J, Ding F, Ke K . Pyrroloquinoline quinine protects rat brain cortex against acute glutamate-induced neurotoxicity. Neurochem Res 2013; 38: 1661–71.
Yang YC, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, et al. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic Biol Med 2011; 51: 2073–81.
Valdivia A, Perez-Alvarez S, Aroca-Aguilar JD, Ikuta I, Jordan J . Superoxide dismutases: a physiopharmacological update. J Physiol Biochem 2009; 65: 195–208.
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 2000; 275: 16023–9.
Rojo AI, Sagarra MR, Cuadrado A . GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem 2008a; 105: 192–202.
Rojo AI, Rada P, Egea J, Rosa AO, López MG, Cuadrado A . Functional interference between glycogen synthase kinase-3 beta and the transcription afctor Nrf2 in protection against kainite-induced hippocampal cell death. Mol Cell Neurosci 2008; 39: 125–32.
Budni J, Lobato KR, Binfaré RW, Freitas AE, Costa AP, Martín-de-Saavedra MD, et al. Involvement of PI3K, GSK-3β and PPARγ in the antidepressant-like effect of folic acid in the forced swimming test in mice. J Psychopharmacol 2012; 26: 714–23.
Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci 2007; 27: 12211–20.
Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases pglycoprotein expression in brain endothelial cells. J Neurochem 2008; 106: 1855–65.
Castellanos M, Sobrino T, Pedraza S, Moldes O, Pumar JM, Silva Y, et al. High plasma glutamate concentrations are associated with infarct growth in acute ischemic stroke. Neurology 2008; 71: 1862–8.
Alonso AC, Grundke-Iqbal I, Iqbal K . Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 1996; 2: 783–7.
Wu J, Ling J, Wang X, Li T, Liu J, Lai Y, et al. Discovery of a potential anti-ischemic stroke agent: 3-pentylbenzo[c]thiophen-1(3H)-one. J Med Chem 2012; 55: 7173–81.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21402241), the Natural Science Foundation of Jiangsu Province (BK20130653), the Program for Jiangsu Province “Shuang Chuang” Team, the Natural Science Foundation of Zhejiang (LY14H300003), the Postdoctoral Science Foundation of China (2014M550256), the Open Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMKF201407), the Fundamental Research Funds for the Central Universities (JKZD2013006), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China (to Tao PANG).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pang, T., Wang, Yj., Gao, Yx. et al. A novel GSK-3β inhibitor YQ138 prevents neuronal injury induced by glutamate and brain ischemia through activation of the Nrf2 signaling pathway. Acta Pharmacol Sin 37, 741–752 (2016). https://doi.org/10.1038/aps.2016.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.3
Keywords
This article is cited by
-
Genetic and Pharmacological Modulation of P75 Neurotrophin Receptor Attenuate Brain Damage After Ischemic Stroke in Mice
Molecular Neurobiology (2024)
-
The PGC-1α Activator ZLN005 Ameliorates Ischemia-Induced Neuronal Injury In Vitro and In Vivo
Cellular and Molecular Neurobiology (2018)
-
The natural product 4,10-aromadendranediol induces neuritogenesis in neuronal cells in vitro through activation of the ERK pathway
Acta Pharmacologica Sinica (2017)