Abstract
Aim:
Tubeimoside-1 (TBMS1), a triterpenoid saponin extracted from the Chinese herbal medicine Bolbostemma paniculatum (Maxim) Franquet (Cucurbitaceae), has shown anticancer activities in various cancer cell lines. The aim of this study was to investigate the anticancer activity and molecular targets of TBMS1 in human prostate cancer cells in vitro.
Methods:
DU145 and P3 human prostate cancer cells were treated with TBMS1. Cell viability and apoptosis were detected. ROS generation, mitochondrial membrane potential and cell cycle profile were examined. Western blotting was used to measure the expression of relevant proteins in the cells.
Results:
TBMS1 (5–100 μmol/L) significantly suppressed the viability of DU145 and P3 cells with IC50 values of approximately 10 and 20 μmol/L, respectively. Furthermore, TBMS1 dose-dependently induced apoptosis and cell cycle arrest at G0/G1 phase in DU145 and P3 cells. In DU145 cells, TBMS1 induced mitochondrial apoptosis, evidenced by ROS generation, mitochondrial dysfunction, endoplasmic reticulum stress, modulated Bcl-2 family protein and cleaved caspase-3, and activated ASK-1 and its downstream targets p38 and JNK. The G0/G1 phase arrest was linked to increased expression of p53 and p21 and decreased expression of cyclin E and cdk2. Co-treatment with Z-VAD-FMK (pan-caspase inhibitor) could attenuate TBMS1-induced apoptosis but did not prevent G0/G1 arrest. Moreover, co-treatment with NAC (ROS scavenger), SB203580 (p38 inhibitor), SP600125 (JNK inhibitor) or salubrinal (ER stress inhibitor) significantly attenuated TBMS1-induced apoptosis.
Conclusion:
TBMS1 induces oxidative stress-mediated apoptosis in DU145 human prostate cancer cells in vitro via the mitochondrial pathway.
Similar content being viewed by others
Introduction
Prostate cancer is one of the most commonly diagnosed cancers in men worldwide and is the second leading cause of cancer-related deaths in men1. Treatment options for prostate cancer principally include surgery, radiation therapy, hormone therapy and chemotherapy, depending on the stage of the disease and the characteristics of the patients2. Androgen deprivation therapy is still the primary treatment for prostate cancer patients. Although initially successful, this form of therapy frequently fails in the advanced stage of the disease, and patients develop castration-resistant prostate cancer, which is almost untreatable1. Surgery1 and radiation3 therapies are limited to localized prostate cancer. Thus, chemotherapy remains the only option for advanced-stage prostate cancer patients. Studies in recent years have shown that chemotherapeutic drugs, such as docetaxel and cabazitaxel, have improved the health status of prostate cancer patients; however, the prognosis of this disease is still very poor4,5.
Apoptosis is a highly synchronized process that plays a key role in tissue homoeostasis by selectively killing unwanted or cancerous cells. Inhibition of apoptosis results in tumorigenesis and chemo-resistance. Cancer cells achieve high proliferation rates by inhibiting apoptosis through various mechanisms6. Mitochondria are the major organelles involved in apoptosis and have been extensively studied in the last decade7. Reactive oxygen species (ROS) are produced in cells during normal oxidative metabolism, and cellular antioxidant systems neutralize them to maintain a redox balance. Over-production of ROS has been shown to induce mitochondrial apoptosis. It has been established that cancer cells possess higher oxidative stress compared to normal cells to fulfill the demands of uncontrolled growth1,7. Recent research has shown that natural bioactive compounds targeting ROS metabolism can selectively kill cancer cells by increasing the ROS level above a toxic threshold. As cancer cells contain higher levels of endogenous ROS, the toxic threshold can easily be achieved in cancer cells by targeting them with exogenous ROS-generating phytochemicals1,7,8. Prostate cancer cells have been reported to contain higher level of ROS9; therefore, targeting ROS metabolism in prostate cancer cells may be an effective treatment.
Traditional Chinese herbal medicine is a good source of cancer chemotherapeutic agents10,11,12. Tubeimoside-1 (TBMS1) is a naturally occurring triterpenoid saponin that was isolated from Bolbostemma paniculatum (Maxim.) Franquet (Cucurbitaceae). TBMS1 has been shown to have potent cytotoxicity against several cancer cell lines, including human glioma U8713, HepG2, esophageal squamous cell carcinoma EC10914, ovarian cancer SKOV-315, CDDP-resistant A278016, choriocarcinoma JEG-317, lung cancer A54918, cervical carcinoma HeLa19 and BGC823 gastric cancer cells20. However, its effect on human prostate cancer remains unexplored.
In the present study, we investigated the cytotoxic effect of TBMS1 on human prostate carcinoma cells. We found that TBMS1 could potently inhibit the proliferation of prostate cancer cells via oxidative stress, and the inhibitory activity was linked to induction of several important apoptosis-related signaling pathways.
Materials and methods
Chemicals and reagents
TBMS1 was purchased from the National Institutes for Food and Drug Control (Beijing, China), with >99% purity as confirmed by HPLC analysis. TBMS1 was dissolved in DMSO to generate a 20 mmol/L stock solution and stored at −80 °C. The stock solution was freshly diluted with culture medium before use, and the final concentration of DMSO was <1% in all experiments. The rabbit antihuman Bcl-2, Bax, p-p38, p53, CHOP, and p-JNK monoclonal antibodies were purchased from Beyotime (Shanghai, China); cyclin E, cdk2 and β-actin monoclonal antibodies were purchased from Boster (Wuhan, China); the p21 monoclonal antibody was purchased from ZSGB-Bio (Beijing, China); the p-ASK-1 monoclonal antibody was purchased from Santa Cruz (Santa Cruz, USA); the caspase inhibitor was purchased from Beyotime (Shanghai, China); a JNK inhibitor and p38 inhibitor were purchased from Sigma (Beijing, China). The thioredoxin antibody was purchased from Proteintech. Dulbecco's modified Eagle's medium (DMEM) and characterized grade fetal bovine serum (FBS) were purchased from HyClone (USA). Dimethyl sulfoxide (DMSO) was purchased from Sangon Biotech (Shanghai, China) Co Ltd. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], trypsin, Hoechst 33258, rhodamine 123, penicillin and streptomycin were purchased from Sigma (Beijing, China). The Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kit, Cell Cycle and Apoptosis Analysis Kit, Reactive Oxygen Species Assay Kit and BCA Protein Assay kit were purchased from Beyotime Institute of Biotechnology (Shanghai, China).
Cell culture and treatments
DU145 and PC3 human prostate cancer cell lines were purchased from the American Type Culture Collection (ATCC, China) and were routinely cultured in DMEM, supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5% CO2. Cells were treated with various concentrations of TBMS1 dissolved in dimethyl sulfoxide (DMSO) with a final DMSO concentration of 0.5%. DMSO-treated cells were used as a control.
Cell viability analysis
Cell viability was determined by the MTT assay as described previously21. Briefly, DU145 and PC3 cells were seeded in 96-well tissue culture plates and incubated in a CO2 incubator for 24 h, and the cells were then exposed to different concentrations of TBMS1 (1–100 μmol/L) for 24 h. Following treatment, 10 μL MTT reagent (5 mg/mL) was added to each well, and cells were further incubated at 37 °C for 4 h. Subsequently, 150 μL DMSO was added to dissolve the formazan crystals, and absorbance was measured at 570 nm in a microplate reader (Thermo Scientific, Varioskan Flash, USA). The percentage cell viability was calculated as follows:

The IC50 value was calculated using GraphPad Prism 5.
Observation of cell morphological changes
DU145 and PC3 cells were treated with the indicated concentrations of TBMS1 in the presence or absence of 3 mmol/L of the ROS scavenger NAC for 24 h, and cell morphological changes were observed and photographed by a phase contrast microscope (Olympus 1×71, Japan) equipped with a CCD camera (Olympus DP72, Japan).
Nuclear morphological changes by Hoechst 33258 staining
DU145 and PC3 cells were treated with different concentrations of TBMS1 for 24 h. Following treatment, cells were washed and fixed with 4% paraformaldehyde for 10 min at room temperature. After washing with PBS, cells were stained with Hoechst 33258 (50 μg/mL) and incubated at 37 °C for 20 min in the dark. Finally, the cells were washed and resuspended in PBS for the observation of nuclear morphology under a fluorescence microscope (Olympus 1×71, Japan) and photographed with a CCD camera (Olympus DP72, Japan). Apoptotic cells were defined as cells with nuclear shrinkage and chromatin condensation.
Flow cytometric analysis of apoptosis
DU145 and PC3 cells were treated with different concentrations of TBMS1 for varying times in the presence or absence of 20 μmol/L Z-VAD-FMK. Following treatment, the cells were collected and washed twice with ice-cold PBS. The cell pellets were then resuspended in 500 μL binding buffer. Five microliters of Annexin V-FITC and 10 μL PI were added, and cells were incubated in the dark for 15 min according to the manufacturer's instructions. The samples were then analyzed with flow cytometry (Beckman Coulter, Epics XL, USA).
Flow cytometric analysis of the cell cycle
DU145 and PC3 cells were treated with different concentrations of TBMS1 for varying times in the presence or absence of 20 μmol/L Z-VAD-FMK. After harvesting and fixing with 70% ice-cold ethanol overnight, the cells were washed twice with PBS. The cell pellets were then resuspended in a solution containing 50 μg/mL PI and 100 μg/mL RNase A for 30 min in the dark at room temperature as described previously22. The DNA contents were analyzed by flow cytometry (Beckman Coulter, Epics XL) using Cell Quest software.
Determination of intracellular reactive oxygen species (ROS) generation
TBMS1-induced ROS generation in DU145 cells was determined using the 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) staining method as described previously21. The DU145 cells were treated with different concentrations of TBMS1 for 24 h and then stained with DCFH-DA (10 μmol/L) at 37 °C for 30 min in the dark. The stained cells were then collected by centrifugation, washed 3 times with PBS, and resuspended in 200 μL PBS. The samples were analyzed for 2′,7′-dichlorofluorescein (DCF) fluorescence with flow cytometry (Beckman Coulter, Epics XL, USA).
Determination of mitochondrial transmembrane potential (MMP) depolarization
TBMS1-induced MMP depolarization in DU145 cells was assessed using rhodamine 123 (Rho123) as described previously23. The DU145 cells were treated with different concentrations of TBMS1 for 24 h. Following treatment, the cells were collected by centrifugation, washed with PBS twice and then stained with rhodamine 123 (10 μg/mL) at 37 °C for 30 min in dark. The stained cells were washed 3 times with PBS and resuspended in 200 μL PBS. Rhodamine 123 fluorescence was determined using flow cytometry (emission wavelength λex=488, excitation wavelength λem=530).
Western blotting
The DU145 cells were treated with different concentrations of TBMS1 for 24 h in the presence or absence of SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and salubrinal (a selective inhibitor of ER stress). The cells were collected, and the protein was extracted as described previously23. Protein concentrations of the supernatants were determined using a BCA Protein Assay Kit. Approximately 40 μg of total proteins was separated on SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc, USA). After blocking with 5% (w/v) nonfat milk and washing with Tris-buffered saline-Tween solution (TBST), membranes were incubated overnight at 4 °C with antibodies against thioredoxin (1:1000), p-ASK-1 (1:500), p38 (1:1000), p-JNK (1:1000), CHOP (1:1000), Bax (1:1000), Bcl-2, cleaved caspase-3 (1:1000), p53 (1:1000), p21 (1:100), cyclin E (1:200), cdk2 (1:400) and β-actin (1:400). After washing, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG secondary antibodies (1:5000) for 1 h at room temperature. After washing with TBST, signals were detected using an ECL Plus chemiluminescence kit with X-ray film (Millipore Corporation, Billerica, USA).
Statistical analysis
All data are expressed as the mean±SEM. One-way ANOVA was used to compare the control and test values. Student's t-test was used to determine significance when only two groups were compared. P values less than 0.05 were considered statistically significant.
Results
TBMS1 inhibits the proliferation of prostate cancer cells
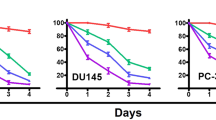
To initially investigate the antiproliferative effect of TBMS1 (Figure 1A) on prostate cancer cells, DU145 and PC3 cells were exposed to different concentrations of the test compound for 24 h. Cell viabilities were determined using the MTT assay. As shown in Figure 1B and 2A, TBMS1 inhibited the proliferation of DU145 and PC3 cells in a dose-dependent manner, with IC50 values of approximately 10 and 20 μmol/L, respectively. The cytotoxic effect of TBMS1 on cell morphology was also observed under a phase contrast microscope.
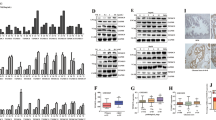
Antiproliferative effects of TBMS1 on growth and morphological characteristics of DU145 prostate cancer cells. (A) Chemical structure of TBMS1. (B) Dose-dependent effect of TBMS1 on the viability of DU145 cells. The cells were treated with the indicated concentrations of TBMS1 for 24 h. Cell viability was evaluated using the MTT assay. Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control. (C) DU145 cells were treated with 15 μmol/L TBMS1 in the presence or absence of 3 mmol/L NAC, cell viability was determined with a MTT assay. **P<0.01 vs control. ##P<0.01 vs TBMS1. (D) Cells were treated with the indicated concentrations of TBMS1 for 24 h, morphological changes were observed under a phase contrast microscope. (E) DU145 cells were treated with 15 μmol/L TBMS1 in the presence or absence of 3 mmol/L NAC, cell morphological changes were observed under a phase contrast microscope.
Antiproliferative effects of TBMS1 on growth and morphological characteristics of PC3 prostate cancer cells. (A) PC3 cells were treated with various concentrations of TBMS1, and cell viability was evaluated with a MTT assay. (B) PC3 cells were treated with 15 μmol/L TBMS1 in the presence or absence of 3 mmol/L NAC, cell viability was determined with a MTT assay. Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control. (C) PC3 cells were treated with various concentrations of TBMS1 for 24 h, and morphological changes were observed under a phase contrast microscope. **P<0.01 vs control. ##P<0.01 vs TBMS1. (D) PC3 cells were treated with 15 μmol/L TBMS1 in the presence or absence of 3 mmol/L NAC, cell morphological changes were observed under a phase contrast microscope.
As shown in Figure 1D and 2C, TBMS1-treated cells displayed severe morphological changes indicating cell death, including rounding and shrinkage accompanied by decreased cell number. Pretreatment of the cells with 3 mmol/L NAC significantly inhibited the cytotoxic effect of TBMS1 in DU145 (Figure 1E and 1C) and PC3 cells as shown in Figure 2D and 2B. These data indicate that TBMS1 induces oxidative stress-mediated cell death in both DU145 and PC3 cells.
TBMS1 induces apoptotic cell death in prostate cancer cells
Apoptosis and cell cycle arrest are considered the two major causes of cell growth inhibition. DNA fragmentation and loss of plasma membrane asymmetry are characteristic features of apoptotic cell death. First, we investigated the effects of TBMS1 on apoptosis of DU145 cells using an Annexin V-FITC/PI double staining kit and flow cytometry. The data showed that TBMS1 induced apoptosis in DU145 cells in a dose- and time-dependent manner as shown in Figure 3. Pretreatment of the cells with 20 μmol/L Z-VAD-FMK, a pan-caspase inhibitor, significantly inhibited the apoptotic effects of TBMS1, indicating that TBMS1 induces caspase-dependent apoptosis in DU145 cells (Figure 3F). The TBMS1-mediated apoptotic cell death was further confirmed using H33258 staining and fluorescence microscopy. As shown in Figure 3, TBMS1 significantly induced chromatin condensation and nuclear fragmentation in DU145 cells in a dose-dependent manner. The apoptotic effect of TBMS1 was also assessed in PC3 androgen-independent prostate cancer cells. As shown in Figure 4, TBMS1 induced dose-dependent apoptosis in PC3 cells in a similar fashion as observed in DU145 cells.
Induction of apoptosis in DU145 prostate cancer cells by TBMS1. (A) DU145 cells were incubated with (a) DMSO as a negative control, (b) 3 μmol/L, (c) 9 μmol/L, (d) and 15 μmol/L TBMS1 for 24 h. After staining with Hoechst 33258, the cells were examined under a fluorescence microscope. The condensed and fragmented nuclei are indicated with arrows, scale bar=20 μm. (B) The percentage of cleaved nuclei is expressed as the mean±SEM of three independent experiments. (C) Flow cytometry analysis of cell apoptosis. DU145 cells were incubated with (a) DMSO, (b) 3 μmol/L, (c) 9 μmol/L, (d) and 15 μmol/L TBMS1 for 24 h and then stained with FITC-conjugated Annexin V and PI. (D) Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control. (E) Time-dependent induction of apoptosis by TBMS1 in DU145 cells. The DU145 cells were treated with (a) DMSO, (b) 15 μmol/L TBMS1 for 12 h (c), 15 μmol/L TBMS1 for 24 h, or (d) 15 μmol/L TBMS1 plus a caspase inhibitor for 24 h. (F) Summary of the flow cytometry data of (E). Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control. #P<0.05 vs TBMS1 15 μmol/L for 24 h.
Effect of TBMS1 on apoptosis and the cell cycle of PC3 cells. (A) PC3 cells were treated with (a) DMSO as a negative control, (b) 3 μmol/L, (c) 9 μmol/L, (d) and 15 μmol/L TBMS1 for 24 h. After washing with PBS and fixing with 4% paraformaldehyde, the cells were stained with Hoechst 33258 and observed under a fluorescence microscope. The condensed and fragmented nuclei are indicated with arrows, scale bar=20 μm. (B) The percentage of cleaved and fragmented nuclei is expressed as the mean±SEM of three independent experiments. (C) PC3 cells were treated with (a) DMSO, (b) 3 μmol/L, (c) 9 μmol/L, (d) and 15 μmol/L TBMS1 for 24 h, and the rate of apoptosis was determined using flow cytometry. (D) Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control. (E) Cells were treated with various concentrations of TBMS1 for 24 h as indicated in (C). Cells were stained with PI, and the cell cycle profile was analyzed by flow cytometry. (F) Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control.
TBMS1 induces G0/G1 phase arrest in prostate cancer cells
To investigate the effects of TBMS1 on cell cycle distribution, the DU145 cells were treated with TBMS1, and the cell cycle profile was then assayed by flow cytometry using the PI staining method. The data demonstrated that TBMS1 induced cell cycle arrest at G0/G1 phase in a dose- and time-dependent manner as shown in Figure 5A and 5C, respectively. Z-VAD-FMK, a broad-spectrum caspase inhibitor, had no effect on cell cycle arrest, indicating that cell cycle arrest is an apoptosis-independent event as shown in Figure 5C and 5D. Next, we determined whether TBMS1 could also induce cell cycle arrest at G0/G1 phase in PC3 cells. For this, we treated PC3 cells with different concentrations of TBMS1 and analyzed the cell cycle profile. The data demonstrated that TBMS1 also caused G0/G1 phase cell cycle arrest in PC3 cells (Figure 4E and 4F). As DU145 cells were found to be more sensitive to TBMS1 treatment, further studies were conducted using DU145 cells.
Effect of TBMS1 on the cell cycle distribution in DU145 prostate cancer cells. (A) Flow cytometry analysis of cell cycle distribution. DU145 cells were incubated with (a) DMSO as a negative control, (b) 3 μmol/L, (c) 9 μmol/L, or (d) 15 μmol/L TBMS1 for 24 h. The cells were stained with PI and analyzed with flow cytometry. (B) Data are expressed as the mean±SEM of three independent experiments. *P<0.05 compared with the control. (C) Time-dependent cell cycle arrest was induced by TBMS1 in DU145 cells. The DU145 cells were treated with (a) DMSO, (b) 15 μmol/L TBMS1 for 12 h, (c) 24 h or (d) 15 μmol/L TBMS1 plus a caspase inhibitor Z-VAD-FMK for 24 h. (D) Data are expressed as the mean±SEM of three independent experiments. *P<0.05 vs the control.
TBMS1 induces ROS generation and disrupts the MMP
The intracellular ROS generation in DU145 cells was measured using DCFH-DA and flow cytometry. As shown in Figure 6A and 6B, the level of intracellular ROS in DU145 cells treated with 3, 9, and 15 μmol/L TBMS1 was 18.23%±1.26%, 26.88%±1.89% and 35.01%±1.07%, respectively. These values were significantly higher than the control group (2.97%±0.04%).
Effect of TBMS1 on the intracellular ROS generation (A, B) and mitochondrial membrane potential (C, D) of DU145 cells. (A) DU145 cells were incubated with (a) DMSO, (b) 3 μmol/L, (c) 9 μmol/L, (d) and 15 μmol/L TBMS1 for 24 h. ROS generation was determined using a reactive oxygen species assay kit. (B) Histograms show the average DCF fluorescence (mean±SEM) from three independent experiments. **P<0.01 vs the control. (C) DU145 cells were incubated with (a) DMSO, (b) 3 μmol/L, (c) 9 μmol/L, (d) and 15 μmol/L TBMS1 for 24 h. The MMP was determined using the Rho123 staining method. (D) Data are expressed as the mean±SEM of three independent experiments. **P<0.01 vs the control.
Mitochondria play key roles in the regulation of cell death and proliferation, and depolarization of the mitochondrial membrane potential (MMP) is a characteristic feature of apoptosis24. ROS generation has been reported to induce apoptosis by dissipating the MMP. We therefore investigated the effects of TBMS1 on MMP in DU145 cells. As shown in Figure 6C and 6D, treatment of the cells with 3, 9, and 15 μmol/L TBMS1 reduced the MMP from 95.03%±5.32% (control) to 94.98%±2.47%, 84.75%±7.01%, and 65.77%±4.22%, respectively.
TBMS1 activates ASK-1 and MAPK
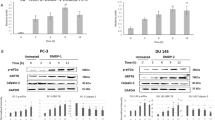
Apoptosis signal-regulating kinase 1 (ASK-1) is a member of the mitogen-activated protein kinase (MAPK) kinase kinase family, which can be activated by phosphorylation at Thr845 in response to oxidative stress, ER stress and chemotherapeutic agents25. Activated ASK-1 has been shown to activate the c-Jun N-terminal kinase (JNK) and p38 pathways by direct phosphorylation. Therefore, we determined the effect of TBMS1 on the expression of these stress-activated proteins. The data showed that TBMS1 activates ASK-1 by inducing phosphorylation at Thr845 in a dose-dependent manner. TBMS1 treatment also induced phosphorylation of the ASK-1 downstream target proteins JNK and p38 in a dose-dependent manner as shown in Figure 7. Because ASK-1 activation is associated with ER stress, we next asked if TBMS1 could induce ER stress in DU145 cells. Therefore, we assessed the expression of CCAAT/enhancer-binding protein-homologous protein (CHOP), a transcription factor that is induced during ER stress. The data demonstrated that TBMS1 treatment significantly increased the expression of CHOP in DU145 cells in a dose-dependent manner (Figure 7). Because thioredoxin is bound to ASK-1 and negatively regulates its activation25, we therefore measured the expression of thioredoxin in response to TBMS1 treatment. As expected, TBMS1 treatment decreased the expression of thioredoxin in a dose-dependent manner (Figure 7).
Effect of TBMS1 on oxidative stress-mediated protein expression in DU145 prostate cancer cells. (A) DU145 cells were treated with the indicated concentrations of TBMS1 for 24 h, and protein lysates were subjected to Western blotting to assess the expression of p-ASK-1, p-p38, p-JNK, and CHOP. β-Actin was used as a loading control. (B) Statistical data summarized from the Western blotting analysis. Data are expressed as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the control.
TBMS1 induces apoptosis in DU145 cells via the mitochondrial pathway
Intracellular ROS generation and dissipation of the mitochondrial membrane potential are the characteristic features of mitochondrial apoptosis. Therefore, we measured the expression of Bcl-2 family proteins, which are the main mediators of mitochondrial apoptosis. As shown in Figure 8A and 8B, the TBMS1 treatment substantially increased the expression of Bax and decreased the expression of Bcl-2 in a dose-dependent manner. Moreover, TBMS1 induced the cleavage of caspase-3 in a dose-dependent manner. The data clearly indicate that TBMS1 induces mitochondrial apoptosis in DU145 cells.
Effect of TBMS1 on the expression of mitochondrial apoptosis and cell cycle regulators in DU145 prostate cancer cells. (A) DU145 cells were treated with the indicated concentrations of TBMS1 for 24 h, and protein lysates were subjected to Western blotting for the expression of Bax, Bcl-2, and cleaved caspase-3. (B) Histograms show the average protein levels (mean±SEM) from three independent experiments with similar results. **P<0.01 compared with the control. (C) DU145 cells were treated with the indicated concentrations of TBMS1 for 24 h, and protein lysates were subjected to Western blotting for the expression of p53, p21, cyclin E, and cdk2. β-Actin was used as a loading control. (D) Histograms show the average protein levels from three independent experiments. **P<0.01 compared with the control.
TBMS1 up-regulates p53 and p21 expression and down-regulates cyclin E and cdk2 expression
TBMS1 induced G0/G1 phase arrest in DU145 cells. Therefore, we measured the expression of cell cycle regulators involved in the G1-S transition. p53, a tumor suppressor protein, has been shown to regulate cell cycle progression at G0/G1, as well as G2/M, phase by inducing the expression of its direct downstream target, p21. Thus, we assayed the expression of p53 and p21 in response to TBMS1 treatment. The data showed that TBMS1 dose-dependently increased the expression of p53 and p21. Next, we assessed the expression of cyclin E and cdk2, which play important roles in the transition of the cell cycle from G1-S phase. As shown in Figure 8C and 8D, TBMS1 attenuated the expressions of cyclin E and cdk2 in a dose-dependent fashion.
TBMS1 induces oxidative stress-mediated apoptosis in DU145 cells
As TBMS1 induced the expression of oxidative stress-mediated proteins (p38, JNK, and CHOP) in DU145 cells, we next determined whether these proteins were involved in TBMS1-mediated apoptosis. For this, we evaluated the expression of Bax, Bcl-2, and cleaved caspase-3 following inhibition of the expressions of these stress-mediated proteins using pharmacological inhibitors. As shown in Figure 9, pretreatment of the cells with SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and salubrinal (ER stress inhibitor) significantly reversed the TBMS1-mediated changes in the expression of Bax, Bcl-2, and caspase-3. These data indicate that TBMS1 induces oxidative stress-mediated apoptosis in DU145 cells.
TBMS1 induces the expression of mitochondrial apoptosis regulators through oxidative and ER stress. (A) DU145 cells were incubated with or without 15 μmol/L TBMS1 in the presence or absence of the indicated inhibitors for 24 h. Expression of Bax, Bcl-2, and cleaved caspase-3 in the DU145 cells was determined using a Western blot assay. β-Actin was used as loading control. (B) Histograms show the average protein levels from three independent experiments. **P<0.01 vs the control. ##P<0.01 vs TBS1 15 μmol/L.
Discussion
One of the major challenges in cancer chemotherapy is to identify new molecules that can eradicate cancer cells by inducing apoptosis. Apoptosis can be initiated either by activation and ligation of the death receptors to their respective ligands or by mitochondrial activation26. Cancer cells obtain their metabolic energy predominantly through glycolysis by altering the function of the mitochondria27. Mitochondria are a major component of the intrinsic apoptosis execution machinery and have been extensively studied26,28. Because the activation of mitochondria has been recognized as a “milestone” in the apoptotic process, the manipulation of mitochondrial activation to initiate apoptotic cell death in cancer cells has been considered a potential therapeutic approach.
In the present study, we found for the first time that TBMS1 could effectively inhibit the growth of and induce mitochondrial apoptosis in DU145 cells through oxidative stress. Accumulating research suggests that cancer cells exhibit high oxidative stress compared with normal cells, which plays an important role in cancer cell survival, proliferation, disruption of cell death signaling, metastasis, angiogenesis, and drug resistance29,30,31. Recent research has shown that in contrast to the tumor-promoting ability of higher ROS levels, this biochemical property of cancer cells may have therapeutic benefits. Targeting mitochondria to increase the ROS level above a toxic threshold by exogenous ROS-generating phytochemicals to selectively kill cancer cells has been shown to be feasible in various in vitro and in vivo experimental models29,30,31,32,33. In the present study, TBMS1 increased ROS generation, modulated Bax and Bcl-2 expression, dissipated mitochondrial membrane potential and ultimately induced apoptosis in DU145 cells by inducing DNA fragmentation and caspase-3 cleavage in a dose-dependent manner. The data clearly demonstrate that TBMS1 induces mitochondrial apoptosis in DU145 cells.
ROS are also known to act as second messengers to activate diverse redox-sensitive signaling cascades, including MAPK family members and their downstream transcription factors21. Next, we determined whether TBMS1 could activate the stress-activated MAPK family proteins in DU145 cells. ASK-1 is a member of MAPK kinase kinase family, which is activated by phosphorylation at Thr845 in response to oxidative stress, ER stress and chemotherapeutic agents25. ASK-1 acts as an upstream activator of the JNK and p38 MAPK pathways25. Under non-stressed conditions, thioredoxin binds to ASK-1 and inhibits its activity. Under stress, ASK-1 becomes activated via phosphorylation of the Thr845 residue and dissociates from thioredoxin25,34. Therefore, we determined the effect of TBMS1 on the expression of these stress-activated proteins. The data demonstrated that TBMS1 induced phosphorylation of ASK-1 and its downstream target proteins JNK and p38 and reduced the expression of thioredoxin in a dose-dependent manner. As ASK-1 is also associated with ER stress25, we therefore measured the expression of CHOP, a transcription factor that is induced during ER stress35. As expected, TBMS1 increased the expression of CHOP, indicating the involvement of ER stress in TBMS1-treated cells. These findings are further supported by a previous report showing that TBMS1 increased the expression of CHOP and phosphorylated p38 in SKOV-3 cells16.
The JNK and p38 MAPK pathways have been reported to play important roles in activation of the mitochondrial apoptotic pathway16,36. Next, we determined the functional link between stress-activated proteins and mitochondrial apoptosis regulators. To investigate the possible role of JNK, p38 and ER stress in TBMS1-induced apoptosis in DU145 cells, we measured the expression of Bcl-2, Bax, and cleaved caspase-3 in the presence or absence of specific pharmacological inhibitors. Pretreatment of the cells with SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and salubrinal (ER stress inhibitor) significantly reversed the TBMS1-mediated expression of Bax, Bcl-2, and caspase-3. The data show that TBMS1 induces oxidative stress-mediated apoptosis in DU145 cells.
Cell cycle arrest at a particular checkpoint is one of the major causes of cell death in cancer cells26. Many chemotherapeutic agents inhibit cell proliferation through activation of various intracellular signaling pathways to arrest the cell cycle at G0/G1, S, or G2/M phase22. p53, “the guardian of the genome,” has been shown to induce cell cycle arrest at both G0/G1 and G2/M phase by activating its direct downstream effector p2137,38. In the present study, TBMS1 arrested the cell cycle at G0/G1 phase in a dose-dependent manner by increasing the expression of p53 and p21. To confirm the G0/G1 phase arrest, we measured the expression of cyclin E and cdk2, which are involved in progression of the cell cycle from G1-S transition. Cells with suppressed cyclin E/cdk2 activity are arrested at G0/G1 phase39. Consistent with the flow cytometry data indicating cell cycle arrest, TBMS1 decreased the expression of cyclin E and cdk2 in a dose-dependent manner. In contrast to the study of Yin et al40, who showed that TBMS1 induces G2/M arrest in HepG2 cells, TBMS1 arrested the cell cycle at G0/G1 phase in DU145 cells. This incongruent data may be due to the different cancer cell types.
In conclusion, our data showed that TBMS1 effectively inhibited growth by inducing apoptosis and cell cycle arrest at G0/G1 phase. This TBMS1-induced mitochondrial apoptosis was found to be associated with the activation of several upstream oxidative stress-mediated activators, such as ASK-1, p38, JNK, and CHOP. Supplementation of the cells with a p38 inhibitor, JNK inhibitor and ER stress inhibitor could effectively reduce the TBMS1-induced mitochondrial apoptosis regulator expression. Taken together, the data showed that TBMS1 induces oxidative stress-mediated apoptosis in DU145 cells.
Author contribution
Muhammad KHAN, Hong-wen GAO, and Tong-hui MA designed the research. Jing-bo YANG, Yang-yang HE and Min YAO performed the laboratory work and analyzed the data. Yong-ming LI helped to conduct the experimental work. Muhammad KHAN and Jing-bo YANG wrote the manuscript.
References
Zhang X, Chen M, Zou P, Kanchana K, Weng Q, Chen W, et al. Curcumin analog WZ35 induced cell death via ROS-dependent ER stress and G2/M cell cycle arrest in human prostate cancer cells. BMC Cancer 2015 Nov 6; 15: 866. doi: 10.1186/s12885-015-1851-3.
Bayne CE, Williams SB, Cooperberg MR, Gleave Me, Graefen M, Montorsi F, et al. Treatment of the primary tumor in metastatic prostate cancer: current concepts and future perspectives. Eur Urol 2015; pii: S0302-2838(15)00378-4.
Kim HJ, Phak JH, Kim WC . Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy boost and whole pelvic radiotherapy for intermediate- and high-risk prostate cancer. Asia Pac J Clin Oncol 2016. doi: 10.1111/ajco.12472.
Tsao CK, Cutting E, Martin J, Oh WK . The role of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Urol 2014; 6: 97–104.
Schutz FA, Buzaid AC, Sartor O . Taxanes in the management of metastatic castration-resistant prostate cancer: efficacy and management of toxicity. Crit Rev Oncol Hematol 2014; 91: 248–56.
Khan M, Maryam A, Zhang H, Mehmood T, Ma T . Killing cancer with platycodin D through multiple mechanisms. J Cell Mol Med 2016; 20: 389–402.
Zong WX, Rabinowitz JD, White E . Mitochondria and cancer. Mol Cell 2016; 61: 667–76
Juan ME, Wenzel U, Daniel H, Planas JM . Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem 2008; 56: 4813–8.
Trachootham D, Alexandre J, Huang P . Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–91.
Alberti C . Taxane- and epothilone-based chemotherapy: from molecule cargo cytoskeletal logistics to management of castration-resistant prostate carcinoma. Eur Rev Med Pharmacol Sci 2013; 17: 1658–64.
Wang CY, Bai XY, Wang CH . Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. Am J Chin Med 2014; 42: 543–59.
Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS One 2013; 8: e 60338.
Ling CQ, Yue XQ, Ling C . Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med 2014; 12: 331–5.
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao N, et al. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/cytochrome c/caspase-3 pathway. Onco Targets Ther 2015; 30: 303–11.
Xu Y, Wang G, Chen Q, Lin T, Zeng Z, Luo Q, et al. Intrinsic apoptotic pathway and G2/M cell cycle arrest involved in tubeimoside I-induced EC109 cell death. Chin J Cancer Res 2013; 25: 312–21.
Chen WJ, Yu C, Yang Z, He JL, Yin J, Liu HZ, et al. Tubeimoside-1 induces G2/M phase arrest and apoptosis in SKOV-3 cells through increase of intracellular Ca2+ and caspase-dependent signaling pathways. Int J Oncol 2012; 40: 535–43.
Liu HZ, Yu C, Yang Z, He JL, Chen WJ, Yin J, et al. Tubeimoside I sensitizes cisplatin in cisplatin-resistant human ovarian cancer cells (A2780/DDP) through down-regulation of ERK and up-regulation of p38 signaling pathways. Mol Med Rep 2011; 4: 985–92.
Huang P, Yu C, Liu XQ, Ding YB, Wang YX, He JL . Cytotoxicity of tubeimoside I in human choriocarcinoma JEG-3 cells by induction of cytochrome c release and apoptosis via the mitochondrial-related signaling pathway. Int J Mol Med 2011; 28: 579–87.
Zhang Y, Xu X, He P . Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep 2011; 4: 25–9.
Xu Y, Ching YP, Zhou Y, Chiu JF, Chen F, He QY . Multiple pathways were involved in tubeimoside-1-induced cytotoxicity of HeLa cells. J Proteomics 2011; 75: 491–501.
Khan M, Ding C, Rasul A, Yi F, Li T, Gao H, et al. Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinoma PANC-1 cells. Int J Biol Sci 2012; 8: 533–47.
Khan M, Zheng B, Yi F, Rasul A, Gu Z, Li T, et al. Pseudolaric acid B induces caspase-dependent and caspase-independent apoptosis in u87 glioblastoma cells. Evid Based Complement Alternat Med 2012: 957568.
Khan M, Yi F, Rasul A, Li T, Wang N, Gao H, et al. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life 2012; 64: 783–94.
Abbas T, Dutta A . p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400–14.
Yu JS, Kim AK . Platycodin D induces reactive oxygen species-mediated apoptosis signal-regulating kinase 1 activation and endoplasmic reticulum stress response in human breast cancer cells. J Med Food 2012; 15: 691–9.
Khan M, Maryam A, Qazi JI, Ma T . Targeting apoptosis and multiple signaling pathways with Icariside II in cancer cells. Int J Biol Sci 2015; 11: 1100–12.
Hengartner MO . The biochemistry of apoptosis. Nature 2000; 407: 770–6.
Ferreira CG, Epping M, Kruyt FA, Giaccone G . Apoptosis: target of cancer therapy. Clin Cancer Res 2002; 8: 2024–34.
Trachootham D, Alexandre J, Huang P . Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–91.
Zhu L, Ren L, Chen Y, Fang J, Ge Z, Li X . Redox status of high-mobility group box 1 performs a dual role in angiogenesis of colorectal carcinoma. J Cell Mol Med 2015; 19: 2128–35.
Hong YH, Uddin MH, Jo U, Kim B, Song J, Suh DH, et al. ROS accumulation by PEITC selectively kills ovarian cancer cells via UPR-mediated apoptosis. Front Oncol 2015; 5: 167.
Wei C, Xiao Q, Kuang X, Zhang T, Yang Z, Wang L . Fucoidan inhibits proliferation of the SKM-1 acute myeloid leukaemia cell line via the activation of apoptotic pathways and production of reactive oxygen species. Mol Med Rep 2015; 12: 6649–55.
Seo KH, Ryu HW, Park MJ, Park KH, Kim JH, Lee MJ, et al. Mangosenone F, a furanoxanthone from Garciana mangostana, induces reactive oxygen species-mediated apoptosis in lung cancer cells and decreases Xenograft tumor growth. Phytother Res 2015; 29: 1753–60.
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998; 17: 2596–606.
Oyadomari S, Mori M . Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 2004; 11: 381–9.
Yu JS, Kim AK . Platycodin D induces apoptosis in MCF-7 human breast cancer cells. J Med Food 2010; 13: 298–305.
Khan M, Yu B, Rasul A, Al Shawi A, Yi F, Yang H, et al. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid Based Complement Alternat Med 2012: 703034.
Khan M, Rasul A, Yi F, Zhong L, Ma T . Jaceosidin induces p53-dependent G2/M phase arrest in U87 glioblastoma cells. Asian Pac J Cancer Prev 2011; 12: 3235–8.
Lunn CL, Chrivia JC, Baldassare JJ . Activation of Cdk2/cyclin E complexes is dependent on the origin of replication licensing factor Cdc6 in mammalian cells. Cell Cycle 2010; 9: 4533–41.
Yin Y, Chen W, Tang C, Ding H, Jang J, Weng M, et al. NF-kappaB, JNK and p53 pathways are involved in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative stress and G(2)/M cell cycle arrest. Food Chem Toxicol 2011; 49: 3046–54.
Acknowledgements
This work was supported by the Science and Technology Development Project of Jilin Province (No 3D515U393429), National Natural Science Fundation of China (No 81402330).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Jb., Khan, M., He, Yy. et al. Tubeimoside-1 induces oxidative stress-mediated apoptosis and G0/G1 phase arrest in human prostate carcinoma cells in vitro. Acta Pharmacol Sin 37, 950–962 (2016). https://doi.org/10.1038/aps.2016.34
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.34
Keywords
This article is cited by
-
Saponins as cytotoxic agents: an update (2010–2021). Part II—Triterpene saponins
Phytochemistry Reviews (2023)
-
The endoplasmic reticulum stress response in prostate cancer
Nature Reviews Urology (2022)
-
Tubeimoside-I sensitizes colorectal cancer cells to chemotherapy by inducing ROS-mediated impaired autophagolysosomes accumulation
Journal of Experimental & Clinical Cancer Research (2019)
-
Tubeimoside-1, a triterpenoid saponin, induces cytoprotective autophagy in human breast cancer cells in vitro via Akt-mediated pathway
Acta Pharmacologica Sinica (2019)
-
Tubeimoside I induces accumulation of impaired autophagolysosome against cervical cancer cells by both initiating autophagy and inhibiting lysosomal function
Cell Death & Disease (2018)