Abstract
Aim:
Liver failure is associated with dyshomeostasis of efflux transporters at the blood-brain barrier (BBB), which contributes to hepatic encephalopathy. In this study we examined whether breast cancer resistance protein (BCRP), a major efflux transporter at the BBB, was altered during liver failure in rats.
Methods:
Rats underwent bile duct ligation (BDL) surgery, and then were sacrificed after intravenous injection of prazosin on d3, d7 and d14. The brains and blood samples were collected. BCRP function at the BBB was assessed by the brain-to-plasma prazosin concentration ratio; Evans Blue extravasation in the brain tissues was used as an indicator of BBB integrity. The protein levels of BCRP in the brain tissues were detected. Human cerebral microvessel endothelial cells (HCMEC/D3) and Madin-Darby canine kidney cells expressing human BCRP (MDCK-BCRP) were tested in vitro. In addition, hyperbilirubinemia (HB) was induced in rats by intravenous injection of unconjugated bilirubin (UCB).
Results:
BDL rats exhibited progressive decline of liver function and HB from d3 to d14. In the brain tissues of BDL rats, both the function and protein levels of BCRP were progressively decreased, whereas the BBB integrity was intact. Furthermore, BDL rat serum significantly decreased BCRP function and protein levels in HCMEC/D3 cells. Among the abnormally altered components in BDL rat serum tested, UCB (10, 25 μmol/L) dose-dependently inhibit BCRP function and protein levels in HCMEC/D3 cells, whereas 3 bile acids (CDCA, UDCA and DCA) had no effect. Similar results were obtained in MDCK-BCRP cells and in the brains of HB rats. Correlation analysis revealed that UCB levels were negatively correlated with BCRP expression in the brain tissues of BDL rats and HB rats as well as in two types of cells tested in vitro.
Conclusion:
UCB elevation in BDL rats impairs the function and expression of BCRP at the BBB, thus contributing to hepatic encephalopathy.
Similar content being viewed by others
Introduction
Hepatic encephalopathy (HE) is a neuropsychiatric complication that occurs in both acute and chronic liver failure and results from the accumulation of neurotoxic and/or neuroactive substances in the brain1. Clinical manifestations of HE include personality changes, sleep disturbances, attention deficit, muscular incoordination and coma2. Under physiological condition, brain homoeostasis is precisely regulated by the blood–brain barrier (BBB)3. The BBB is further fortified by ATP-binding cassette (ABC) efflux transporters, thus preventing xenobiotics from entering the brain. P-glycoprotein (P-gp, ABCB1), the multidrug resistance-associated protein family (Mrps, ABCCs) and breast cancer resistance protein (BCRP, ABCG2) are major members of the ABC transporter superfamily that are highly expressed in the microvessel endothelial cells of the brain4,5. The efficacy and toxicity of drugs targeting the central nervous system are largely determined by the function and expression of these transporters and by their selectivity and affinity toward the substrates6,7. Several studies have verified that the functions of P-gp and Mrp2 at BBB are affected by various disease states, including diabetes mellitus8,9, acute myeloid leukemia10, cancer11 and liver failure12,13,14. Our previous studies have also demonstrated that both the function and expression of P-gp and Mrp2 are markedly altered in brain tissues in thioacetamide/hyperammonemia-induced liver dysfunction rodent models15,16.
In addition to the well-recognized role of P-gp and Mrp2, BCRP is an important efflux transporter that restricts the entrance of certain substances into brain17,18,19. Several studies have demonstrated that the function and expression of BCRP at the BBB are markedly altered under various disease states, including diabetes mellitus20, primary CNS lymphoma21 and glioblastoma22. However, little is known about the function and expression changes of BCRP at the BBB during liver failure. Bile-duct ligation (BDL) is a common rat model of chronic liver disease that reflects HE associated with cirrhosis and portal hypertension23 and mimics biliary liver disease in humans24. Significantly elevated unconjugated bilirubin (UCB) is a main feature of BDL and may contribute to the deterioration of liver function25,26,27. Several studies have demonstrated the modulation of Mrps and Pgp by UCB28,29, but information on how UCB regulates BCRP at the BBB is limited.
The aim of the study was first to investigate whether BDL altered the expression and function of BCRP in the brains of rats by using Western blotting and assessment of the concentration of prazosin, which is a typical substrate of BCRP30,31,32,33, respectively, and then to identify components in BDL rat serum that affected BCRP function and expression, by using human cerebral microvessel endothelial cells (HCMEC/D3) and Madin-Darby canine kidney cells expressing human BCRP (MDCK-BCRP) as in vitro models. Additionally, the contribution of UCB to the altered function and expression of BCRP in the brain by BDL was further investigated by both in vitro and in vivo studies.
Materials and methods
Reagents
Prazosin hydrochloride and terazosin hydrochloride were purchased from the National Institute of Control Pharmaceutical and Biological Products (Beijing, China). Bilirubin was supplied by Sigma-Aldrich (St Louis, MO, USA). Evans blue was purchased from Shanghai Reagent Co (Shanghai, China). The human cerebral microvessel endothelial cell line (HCMEC/D3) was purchased from JENNIO Biological Technology Ltd (Guangzhou, China). The Madin-Darby canine kidney expressing human BCRP cells (MDCK-BCRP) and Madin-Darby canine kidney wild type cells (MDCK-WT) were provided by Biowit Technologies Ltd (Shenzhen, China). Fetal bovine serum was purchased from ScienCell Research Laboratories (San Diego, CA, USA). Dulbecco's modified Eagle's medium (DMEM) and RPMI-1640 Medium were obtained from Invitrogen Co (Carlsbad, CA, USA). Rabbit polyclonal antibodies specific for ABCG2 (M-70), occludin (H-279) and claudin-5 (H-52) were purchased from Santa Cruz Technology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG was obtained from Cell Signaling Technologies (Danvers, MA, USA). The antibody specific to β-actin was purchased from Bioworld (Louis Park, MN, USA). Kits for bilirubin, bile acids, serum albumin and malondialdehyde (MDA) content detection, activity assays of superoxide dismutases (SOD), levels of ATP, NO, alanine amino transferase (ALT), aspartate amino transferase (AST) and alkaline phosphatase (ALP) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Radioimmunoprecipitation (RIPA) lysis buffer for protein extraction and bicinchoninic acid (BCA) kit for protein concentration detection were provided by Beyotime Institute of Biotechnology (Haimen, China). Deionized water was purified using a Milli-Q system (Millipore, Bedford, OH, USA). All other reagents used were of analytical grade and were commercially available.
Animals
Male Sprague-Dawley (SD) rats, weighing 220–250 g, were purchased from SIPPR/BK Experimental Animal Co Ltd (Shanghai, China). They were acclimated to the facilities for 7 d prior to experiments. Rats were housed under controlled environmental conditions (temperature, 23±1 °C; humidity, 55%±5%; 12-h light/dark cycle) and were given a commercial food diet and water ad libitum, except for an overnight fast before the surgery. All animals received humane care, and their use was approved by the Animal Ethics Committee of China Pharmaceutical University.
Development of BDL rats
BDL rats were induced according to a previously described method24,34,35. Briefly, rats were anesthetized via an injection of pentobarbitone (40 mg/kg, ip). The common bile duct was later exposed by midline abdominal incision and doubly ligated with 4–0 silk sutures, and this was followed by transecting between the ligatures. In sham-operated animals, laparotomy was performed to expose the common bile duct without ligation. The abdominal incision was closed in two layers, and all animals were allowed to recover. The BDL rats were randomly divided into 3 groups, group I (BDL-3d), group II (BDL-7d) and group III (BDL-14d), which were housed for 3, 7 and 14 d after BDL surgery, respectively. On d 3, 7 and 14 following surgical operation, 5 surviving animals from each group were selected for the following experiments.
Brain distribution of prazosin and Evans Blue in BDL rats
The brain distribution of prazosin was measured according to a previously described method20. Briefly, the rats were sacrificed under light ether anesthesia 40 min following the administration of prazosin (1 mg/kg, iv). Thereafter, the cerebral cortex, hippocampus and blood samples were immediately obtained. Plasma and serum samples were used for substrate analysis and biochemical parameter measurements, respectively. The brain tissues were obtained for substrate analysis, Western blot analysis and biochemical parameter measurements. The levels of bilirubin, bile acids, serum albumin and activities of ALT, AST and ALP in serum and levels of MDA, SOD, ATP and NO in brain were determined with commercial reagent kits according to the manufacturer's instructions15,36,37. Livers and spleens were also isolated and weighed. The concentrations of prazosin in the cerebral cortex, hippocampus and plasma were determined using high-performance liquid chromatography (HPLC). The BBB integrity of Sham and BDL rats was investigated by the extravasation of Evans Blue in the brains of Sham and BDL rats, according to a previously described method8. Briefly, Evans Blue (2% in saline, 4 mL/kg) was injected intravenously into the rats in groups II and III and in the Sham group. After 1 h, rats were sacrificed and perfused with physiological saline through the left ventricle until colorless perfusion fluid was seen to return to the right atrium. Then, the cerebral cortex and hippocampus were removed, and the levels of Evans Blue were measured by spectroflurophotometry (ex/em: 620 nm/680 nm).
Histopathological analysis of liver
The liver specimens from Sham and BDL rats were excised and fixed in 10% formalin solution and routinely processed, and the tissue blocks were embedded in paraffin. Thin sections (4 μm) were cut using a rotary ultra microtome, distributed onto glass slides and dried overnight. Then, the slides were stained with hematoxylin-eosin (H&E) and examined by light microscopy for tissue damage (200×).
Effects of BDL rat serum on the function and expression of BCRP in HCMEC/D3 and MDCK-BCRP cells
After incubation with serum from experimental rats, prazosin uptake and Western blotting were used to assess the function and expression of BCRP in HCMEC/D3 and MDCK-BCRP cells. The sera of Sham and BDL rats were inactivated by heating at 56 °C for 30 min following filtering through a 0.22 μm filter. Sub-confluent (approximately 80%) cells were incubated with RPMI-1640 medium or DMEM containing 10% serum from Sham or BDL rats for 24 h. Then, the uptake experiment was performed according to a previously described protocol with minor modifications38. Briefly, after treatment with the rat serum, cells were pre-incubated in 1 mL of Hanks' balanced salt solution (HBSS) at 37 °C for 15 min. Then, the solution was replaced by 1 mL of HBSS containing prazosin (0.5 μmol/L), and the cells were incubated for another 120 min. The uptake reaction was terminated by washing with 1 mL of ice-cold HBSS for three times; thereafter, 0.3 mL of purified water was added, frozen and melted repeatedly (three times) to break down the cells. The intracellular concentrations of prazosin were measured. The protein content was measured by using a BCA protein assay kit according to the manufacturer's instructions. Uptake, which was expressed as the cell-to-medium ratio (mL/mg protein), was obtained by dividing the substrate concentrations in incubation medium (μg/mL) by the retained amount of substrate in the cells (μg/mg protein). Another subset of cells cultured with experimental rat serum was obtained for Western blot analysis.
Effects of abnormally altered components in BDL rat serum on BCRP function and expression in HCMEC/D3 and MDCK-BCRP cells
The effects of UCB, chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA) and deoxycholic acid (DCA) on BCRP function and expression were examined in HCMEC/D3 and MDCK-BCRP cells. Briefly, sub-confluent (approximately 80%) cells were exposed to RPMI-1640 medium or DMEM containing different concentrations of UCB (0, 10, or 25 μmol/L), CDCA (0, 10, or 100 μmol/L), UDCA (0, 10, or 100 μmol/L) and DCA (0, 10, or 100 μmol/L) for 24 h. Data from the MTT assay showed that these agents did not damage cell viability at the concentrations tested. The function and expression of BCRP in HCMEC/D3 and MDCK-BCRP cells were evaluated by prazosin uptake and Western blotting, according to the method described above.
Effects of UCB treatment on the function and expression of BCRP in rat brains
To further confirm the contribution of UCB to the altered function and expression of BCRP by BDL, hyperbilirubinemia (HB) rats were developed by administering an intravenous dose of UCB (85.5 μmol/kg per day) to normal rats according to a previously described method, performed with minor modifications29,39. The rats used for experiments after 3, 7 and 14 d of consecutive UCB administration were defined as HB-3d, HB-7d and HB-14d rats, respectively. The control group was treated with vehicle. On the experimental day, 10 min after UCB administration, prazosin (1 mg/kg) was administered intravenously. Then, 40 min after prazosin administration, the rats were sacrificed to isolate the blood and brain tissues. The concentrations of bilirubin in the serum were measured. Then, substrate and Western blot analyses were carried out as described above.
Western blot analysis
The protein levels of BCRP, occludin and claudin-5 in rat brain and the protein levels of BCRP in HCMEC/D3 and MDCK-BCRP cells were measured by Western blot analysis. Briefly, the homogenized brain samples of rats and collected cell samples were lysed in RIPA lysis buffer in the appropriate proportions. The protein concentrations of tissues and cell lysates were measured by using a BCA protein assay kit. Equal amounts of proteins were separated by electrophoresis on SDS-polyacrylamide gels and were electrophoretically transferred to polyvinylidene fluoride membranes (Millipore Corporation, Billerica, MA, USA). Blots were blocked in 5% nonfat dry milk-TBS-0.1% Tween 20 at room temperature for 2 h and then washed. Thereafter, the membranes were incubated with primary antibodies overnight at 4 °C, and this was followed by incubation with an HRP-conjugated secondary anti-rabbit antibody (1:5000 dilution) for 2 h. Detection was performed using SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific Inc, Rockford, IL, USA) and a gel imaging system (Tanon 5200 multi, Tanon Science Instruments Co, Ltd, Shanghai, China). The primary antibodies used were against BCRP (1:200 dilution), occludin (1:250 dilution), claudin-5 (1:250 dilution) and β-actin (1:2500 dilution). Intensity values were normalized to the quantity of β-actin for each protein and analyzed with Image Gel software.
Correlation between UCB level and BCRP expression
We performed correlation analysis to further study the relationship between the UCB level and BCRP expression. The connection between the serum UCB levels and the BCRP protein levels in the brains of BDL or HB rats were studied. Because we used experimental rat serum to treat HCMEC/D3 and MDCK-BCRP cells, the relations of UCB levels with BCRP protein levels in cells were also investigated.
Statistical analysis
Values are expressed as the mean±standard deviation (SD). Significant differences among groups were analyzed by using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Significant differences between two groups was determined by Student's t-test. The relationship between two variables was analyzed by use of linear regression analysis. A P value less than 0.05 indicated a significant difference.
Results
Alterations in physiological and biochemical parameters in BDL rats
Liver failure in rats induced by BDL was confirmed by assessment of physiological and biochemical parameters (Table 1). The liver weight, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bile acids, total bilirubin and conjugated bilirubin levels in BDL rat serum were significantly higher than those in Sham rats. The serum albumin levels of BDL rats were significantly lower than those of Sham rats. The levels of ATP and NO in the cerebral cortexes of BDL rats showed significant decreases compared with those of Sham rats. However, neither the levels of malondialdehyde (MDA) nor the activities of superoxide dismutases (SOD), which are indicators of oxidative stress40, were altered by BDL in either the cerebral cortex or the hippocampus.
Effect of BDL on histopathological alterations in rat livers
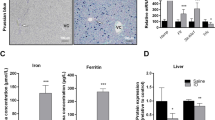
Histological assessment was used to verify liver injury induced by BDL. Histopathological changes were observed in the livers of all BDL groups compared with those of the Sham group. The livers in BDL rats showed clear hydropic degeneration, inflammatory infiltration and hepatocellular damage (Figure 1). The histopathological studies and the results of physiological and biochemical parameter examinations suggested that the liver function of the rats was damaged, exhibiting progressive necrosis in a time-dependent manner after BDL.
Effects of BDL on histological features of liver sections stained with H&E (200×). The Sham group showed normal hepatocytes without any inflammatory infiltration. BDL groups showed clear hepatocyte necrosis, inflammatory infiltration and bile ductular proliferation. Arrows indicate necrotic hepatocyte and inflammatory cell infiltration (bar=50 μm).
Effects of BDL on the brain distribution of prazosin and Evans Blue in rats
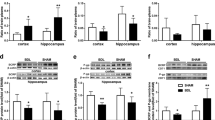
The results showed that BDL significantly increased prazosin concentrations in the cortexes and hippocampi of rats (Figure 2A). In contrast, plasma concentrations of prazosin in BDL rats showed a trend of decreasing over the time course of BDL (Figure 2B), significant changes were observed in rats on d 7 and 14 after BDL. Because the brain concentrations of prazosin were affected by the plasma concentrations, the brain-to-plasma concentration ratio of prazosin was calculated and served as the index of brain penetration15. Significant increases in the brain-to-plasma concentration ratios of prazosin were observed in the cortex and hippocampus. Brain-to-plasma concentration ratios of prazosin in BDL-14d rats displayed a recuperative trend but were still higher than those in Sham rats (Figure 2C).
Effects of BDL on the brain distribution of prazosin and Evans Blue in rats. Brain distribution of prazosin (A), plasma concentrations of prazosin (B) and brain-to-plasma concentration ratios of prazosin (C), as well as blood–brain barrier (BBB) integrity (D) in the cerebral cortexes and hippocampi of BDL rats are shown. BBB integrity was measured by Evans Blue extravasation. n=5. mean±SD. *P<0.05, **P<0.01 vs Sham.
To investigate whether the increased transportation of prazosin across the BBB resulted from the changes in BBB integrity, the concentrations of Evans Blue in the cerebral cortexes and hippocampi of BDL-7d and BDL-14d rats were measured at 1 h after the iv administration of Evans Blue. No significant differences in the Evans Blue distribution between Sham and BDL-treated rats were found (Figure 2D), indicating that BDL did not impair the integrity of BBB. These results all indicated that the increased transport of prazosin across the BBB might be due to the impairment of BCRP function at the BBB of BDL rats.
Effects of BDL on the protein levels of BCRP, occludin and claudin-5
BBB integrity is largely determined by the tight junctions (TJs) of the cerebral endothelial cells. Both occludin and claudin-5 are considered to be major TJ proteins because they are essential for maintaining the functional status of BBB41. The protein levels of BCRP, occludin and claudin-5 in rat brain were measured using Western blotting. The BCRP levels in the cerebral cortexes and hippocampi in BDL rats were significantly decreased, and the greatest changes in the BCRP levels were observed in BDL-7d rats. However, BDL did not affect the expression of occludin and claudin-5 in rat brains (Figure 3).
Effects of BDL on the protein expression of BCRP, occludin and claudin-5 in the cerebral cortexes and hippocampi of rats. Western blot of BCRP, occludin and claudin-5 in the cerebral cortex (A) and hippocampus (B) are represented. Quantification of Western blotting of BCRP (C), occludin (D) and claudin-5 (E) in the cerebral cortex and hippocampus are shown. n=3. Mean±SD. *P<0.05, **P<0.01 vs Sham.
Effects of BDL rat serum on the function and expression of BCRP in HCMEC/D3 cells
The function and expression of BCRP were determined using uptake of prazosin and Western blotting in HCMEC/D3 cells after 24 h of incubation with medium containing 10% serum from experimental rats. The results showed that BDL rat serum significantly increased the uptake of prazosin and decreased the protein levels of BCRP (Figure 4A and 4B), consistently with the in vivo data, thus indicating that some abnormal components in BDL rat serum may impair BCRP function at BBB.
Effects of abnormally altered components in BDL rat serum on the function and expression of BCRP in HCMEC/D3 cells. BCRP function (A) and expression (B) were determined in HCMEC/D3 cells after treatment with rat serum for 24 h. Functional alteration of BCRP (C), Western blot of BCRP (D) and quantification of Western blotting of BCRP levels (E) in HCMEC/D3 cells after incubation with abnormally altered components are shown. n=4 for A and C; n=3 for B, D and E. Mean±SD. *P<0.05, **P<0.01 vs Sham/control group.
Effects of abnormally altered components in BDL rat serum on the function and expression of BCRP in HCMEC/D3 cells
The present results showed that BDL rats exhibited significant elevations in the serum levels of UCB and bile acids. The effects of these abnormally altered components on BCRP function and expression in HCMEC/D3 cells were examined. The results clearly demonstrated that incubation with 25 μmol/L UCB significantly increased the uptake of prazosin and down-regulated the expression of BCRP protein in HCMEC/D3 cells. Moreover, the data showed that all of the tested bile acids (CDCA, UDCA, and DCA) increased the uptake of prazosin in HCMEC/D3 cells in a concentration-dependent manner but did not affect the expression of BCRP protein (Figure 4C–4E).
Effects of abnormally altered components in BDL rat serum on the function and expression of BCRP in MDCK-BCRP cells
MDCK cells transfected with the human ABCG2 gene (MDCK-BCRP) were used to further investigate the effects of abnormally altered components in BDL rat serum on the function and expression of BCRP. The overexpression of BCRP protein in MDCK-BCRP cells was confirmed by Western blotting (Figure 5A), in line with the results in HCMEC/D3 cells showing that incubation with BDL rat serum markedly down-regulated the function and expression of BCRP in MDCK-BCRP cells (Figure 5B and 5C). Moreover, incubation with 25 μmol/L UCB significantly increased the uptake of prazosin in MDCK-BCRP cells, and this was accompanied by marked downregulation of BCRP expression. However, neither the function nor expression of BCRP in MDCK-BCRP cells was affected by the tested bile acids (CDCA, UDCA and DCA) (Figure 5D–5F).
Effects of abnormally altered components in BDL rat serum on the function and expression of BCRP in MDCK-BCRP cells. Overexpression of BCRP (A) in MDCK-BCRP cells was confirmed. BCRP function (B) and expression (C) were determined in MDCK-BCRP cells after treatment with serum from experimental rats. Alteration of BCRP function (D), Western blot of BCRP (E) and quantification of Western blotting of BCRP levels (F) in MDCK-BCRP cells after incubation with abnormally altered components are shown. n=3 for A, C, E and F; n=4 for B and D. Mean±SD. *P<0.05, **P<0.01 vs Sham/control group.
Effects of UCB on the function and expression of BCRP in rat brains
The role of UCB in the altered function and expression of BCRP in rat brains was further verified in hyperbilirubinemia (HB) rats. The elevated UCB concentrations suggested the development of HB rats (Figure 6A). Consistently with our expectations, UCB treatment significantly increased the brain levels of prazosin, thus leading to significantly elevated brain-to-plasma concentration ratios of prazosin in the cortexes and hippocampi of HB rats (Figure 6B–6D). Moreover, UCB treatment reduced the BCRP levels in the cerebral cortexes and hippocampi of HB rats in a time-dependent manner (Figure 6E and 6F).
Effects of UCB on the function and expression of BCRP in rats. Serum total bilirubin concentrations and conjugated bilirubin (A), as well as brain (B) and plasma (C) prazosin concentrations were measured. BCRP function (D) was determined after treatment with UCB in rats for 3, 7 and 14 d. Western blot of BCRP (E) and quantification of Western blotting of BCRP levels (F) in rat brains are represented. n=5 for A–D; n=3 for E and F. Mean±SD. *P<0.05, **P<0.01 vs control group.
Correlations between UCB levels and BCRP expression
To further study the relationship between UCB concentrations and BCRP protein levels, correlation analysis was performed. Strongly negative correlations between UCB levels and BCRP expressions in the cortexes and hippocampi of BDL rats (Figure 7A and 7B) and HB rats (Figure 7C and 7D) were observed. Markedly negative relations of UCB levels with BCRP protein levels in HCMEC/D3 cells (Figure 7E) and MDCK-BCRP cells (Figure 7F) cultured with experimental rat serum were also found, thus suggesting a central role of elevated UCB levels in the BDL-induced downregulation of BCRP function and expression in rats.
Correlations between UCB levels and BCRP expressions. Correlations between serum UCB concentrations and BCRP protein levels in the cortexes (A) and hippocampi (B) of BDL rats are described. The relationships between serum UCB concentrations and BCRP protein levels in the cortexes (C) and hippocampi (D) of HB rats are shown. Links between serum UCB concentrations and BCRP expression in HCMEC/D3 (E) and MDCK-BCRP cells (F) are shown. n=3. *P<0.05, **P<0.01 vs Sham/control group.
Discussion
Liver failure in rats induced by BDL is a frequently used animal model that mimics low-grade encephalopathy, according to the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHENM)23. The aim of this study was to investigate the function and expression of BCRP at the BBB in BDL rats. Liver failure was confirmed by alterations in the physiological and biochemical parameters and by histopathological examinations. The main finding in this study was that BDL significantly increased the brain-to-plasma ratios of prazosin in both the cortexes and hippocampi of rats. This result was corroborated by the simultaneous decrease in expression of the BCRP protein. The key role of UCB in the altered function and expression of BCRP at BBB was verified with in vivo and in vitro studies.
It is generally accepted that the impairment of BBB integrity increases the passage of molecules from the circulation into the CNS. Herein, the brain concentrations of Evans Blue in the Sham and BDL-treated groups were similar, and neither occludin nor claudin-5 expression was altered by BDL in the brains of rats, thus indicating that the BBB remained intact. All of the data support the conclusion that the increased brain penetration of prazosin in the cortexes and hippocampi of BDL rats was at least partly due to a decrease in the function and expression of BCRP. It should also be noted that reports on BBB integrity in BDL rats often conflict. Several reports have shown that BDL affects neither BBB integrity42 nor the expression of occludin and claudin-5 in the brains of rats43, which is consistent with our findings. However, Faropoulos et al have reported that BDL time-dependently down-regulates occludin expression in the brains of rats35. Another study has demonstrated that BDL impairs the BBB integrity of rats, as evidenced by increased brain extravasation of Evans Blue44. The reason for this discrepancy is unclear, but it might be related to the duration of HE or animal strains and remains to be further explored.
Serum from experimental animals has been used to investigate the effects of pathological factors in in vitro cell models, such as rat brain microvessel endothelial cells44 and human endothelial cells45. In this study, the effects of serum from BDL rats on the function and expression of BCRP were also investigated by using HCMEC/D3 cells, a human cerebral microvascular endothelial cell line. The results clearly demonstrated that co-incubation with 10% serum from BDL rats significantly depressed the BCRP function and expression, indicating that some abnormally altered components in BDL rat serum might contribute to the impaired BCRP function and expression. Notably, BDL has been reported to be accompanied by hyperbilirubinemia and elevated bile acid concentrations29, which was confirmed by our work. Therefore, we demonstrated the effects of UCB and bile acids (CDCA, UDCA and DCA) on BCRP function and expression in HCMEC/D3 cells. Interestingly, our data indicated that UCB down-regulated BCRP function and expression in a concentration-dependent manner, consistently with our expectations. However, the tested bile acids reduced BCRP function without affecting its expression. These findings were further verified using MDCK-BCRP cells. The importance of UCB to the impaired function and expression of BCRP in the brains of BDL rats was further supported by the results in HB rats. Strongly negative correlations were observed between the UCB concentrations and BCRP protein levels in BDL rats, HB rats and cells cultured with experimental rat serum. All of the results suggested that UCB may be the key serum component that impairs the BCRP function and expression at the BBB in BDL rats. In addition, concentrations of prazosin in the plasma of BDL rats and hyperbilirubinemia rats were lower than those in the plasma of control rats, indicating that the decreased concentrations of prazosin may be partly attributed to the elevated levels of UCB. This needs to be investigated further.
In conclusion, the present study provided experimental evidence that BDL down-regulates the function and expression of BCRP at the BBB both in vivo and in vitro. The reduced BCRP function and expression were at least partially due to the elevated UCB content. Given that obstructive jaundice leads to portal hypertension, and prazosin is commonly used to reduce hepatic venous pressure gradient46, alterations of BCRP at the BBB in obstructive jaundice may affect the brain penetration of prazosin, resulting in increased distribution of prazosin in the brain. Similar risks may also exist when administrating antivirals such as abacavir or antibiotics such as ciprofloxacin to patients with liver disease, because such drugs are substrates of BCRP47,48.
Abbreviations
ABC, ATP-binding cassette; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BBB, blood-brain barrier; BCRP, breast cancer resistance protein; BDL, bile duct ligation; CDCA, chenodeoxycholic acid; CNS, central nervous system; DCA, deoxycholic acid; HB, hyperbilirubinemia; HBSS, Hanks' balanced salt solution; HCMEC/D3, human cerebral microvessel endothelial cell line; HE, Hepatic encephalopathy; MDA, malondialdehyde; MDCK-BCRP, Madin-Darby canine kidney expressing human BCRP cells; MDCK-WT, Madin-Darby canine kidney wild type cells; SOD, superoxide dismutase; TJ, tight junction; UCB, unconjugated bilirubin, free bilirubin; UDCA, ursodeoxycholic acid.
Author contribution
Ping XU and Xiao-dong LIU designed the experiments and analyzed the data; Ping XU wrote the paper; Xiao-dong LIU and Li LIU revised the paper; Ping XU, Zhao-li LING, Ji ZHANG, Ying LI, Nan SHU, Ze-yu ZHONG, Yang CHEN, Xin-yu DI, and Zhong-jian WANG performed the research.
References
Nguyen JH . Blood-brain barrier in acute liver failure. Neurochem Int 2012; 60: 676–83.
Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM . Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol 2009; 51: 528–34.
Bernacki J, Dobrowolska A, Nierwinska K, Malecki A . Physiology and pharmacological role of the blood-brain barrier. Pharmacol Rep 2008; 60: 600–22.
Miller DS . Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 2010; 31: 246–54.
Sun H, Dai H, Shaik N, Elmquist WF . Drug efflux transporters in the CNS. Adv Drug Deliv Rev 2003; 55: 83–105.
Rubin LL, Staddon JM . The cell biology of the blood-brain barrier. Annu Rev Neurosci 1999; 22: 11–28.
Lee G, Dallas S, Hong M, Bendayan R . Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev 2001; 53: 569–96.
Liu H, Xu X, Yang Z, Deng Y, Liu X, Xie L . Impaired function and expression of P-glycoprotein in blood-brain barrier of streptozotocin-induced diabetic rats. Brain Res 2006; 1123: 245–52.
Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, et al. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 2011; 32: 956–66.
van den Heuvel-Eibrink MM, Wiemer EA, Prins A, Meijerink JP, Vossebeld PJ, van der Holt B, et al. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 2002; 16: 833–9.
Gupta N, Martin PM, Miyauchi S, Ananth S, Herdman AV, Martindale RG, et al. Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem Biophys Res Commun 2006; 343: 571–7.
Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken MJ, Jakowski AB, et al. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos 2007; 35: 1963–9.
Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, et al. Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 2007; 35: 1970–8.
Dean M, Annilo T . Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet 2005; 6: 123–42.
Jin S, Wang XT, Liu L, Yao D, Liu C, Zhang M, et al. P-glycoprotein and multidrug resistance-associated protein 2 are oppositely altered in brain of rats with thioacetamide-induced acute liver failure. Liver Int 2013; 33: 274–82.
Zhang J, Zhang M, Sun B, Li Y, Xu P, Liu C, et al. Hyperammonemia enhances the function and expression of P-glycoprotein and Mrp2 at the blood-brain barrier through NF-kappaB. J Neurochem 2014; 131: 791–802.
Eisenblatter T, Galla HJ . A new multidrug resistance protein at the blood-brain barrier. Biochem Biophys Res Commun 2002; 293: 1273–8.
Eisenblatter T, Huwel S, Galla HJ . Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res 2003; 971: 221–31.
Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM . Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res 2004; 64: 3296–301.
Liu YC, Liu HY, Yang HW, Wen T, Shang Y, Liu XD, et al. Impaired expression and function of breast cancer resistance protein (Bcrp) in brain cortex of streptozocin-induced diabetic rats. Biochem Pharmacol 2007; 74: 1766–72.
Sakata S, Fujiwara M, Ohtsuka K, Kamma H, Nagane M, Sakamoto A, et al. ATP-binding cassette transporters in primary central nervous system lymphoma: decreased expression of MDR1 P-glycoprotein and breast cancer resistance protein in tumor capillary endothelial cells. Oncol Rep 2011; 25: 333–9.
Bleau AM, Huse JT, Holland EC . The ABCG2 resistance network of glioblastoma. Cell Cycle 2009; 8: 2936–44.
Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT . Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 2009; 29: 783–8.
Kountouras J, Billing BH, Scheuer PJ . Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol 1984; 65: 305–11.
Huang LT, Chen CC, Sheen JM, Chen YJ, Hsieh CS, Tain YL . The interaction between high ammonia diet and bile duct ligation in developing rats: assessment by spatial memory and asymmetric dimethylarginine. Int J Dev Neurosci 2010; 28: 169–74.
Villanger O, Bjornbeth BA, Lyberg T, Raeder MG . Bile acids protect the liver against the cholestatic effect of large bilirubin loads. Scand J Gastroenterol 1995; 30: 1186–93.
Wang WW, Smith DL, Zucker SD . Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004; 40: 424–33.
Gazzin S, Berengeno AL, Strazielle N, Fazzari F, Raseni A, Ostrow JD, et al. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by bilirubin at the blood-CSF and blood-brain barriers in the Gunn rat. PLoS One 2011; 6: e 16165.
Yokooji T, Mori N, Murakami T . Modulated function of tissue efflux transporters under hyperbilirubinemia in rats. Eur J Pharmacol 2010; 636: 166–72.
Chimezie C, Ewing A, Schexnayder C, Bratton M, Glotser E, Skripnikova E, et al. Glyceollin effects on MRP2 and BCRP in Caco-2 cells, and implications for metabolic and transport interactions. J Pharm Sci 2015. doi: 10.1002/jps.24605.
Schexnayder C, Stratford RE . Genistein and glyceollin effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 cells. Int J Environ Res Public Health 2015; 13: ijerph 13010017.
Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA . Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun 2004; 317: 269–75.
Xiao Y, Davidson R, Smith A, Pereira D, Zhao S, Soglia J, et al. A 96-well efflux assay to identify ABCG2 substrates using a stably transfected MDCK II cell line. Mol Pharm 2006; 3: 45–54.
Isaksson B, Rippe C, Simonoska R, Holm JE, Glaumann H, Segersvard R, et al. Obstructive jaundice results in increased liver expression of uncoupling protein 2 and intact skeletal muscle glucose metabolism in the rat. Scand J Gastroenterol 2002; 37: 104–11.
Faropoulos K, Chroni E, Assimakopoulos SF, Mavrakis A, Stamatopoulou V, Toumpeki C, et al. Altered occludin expression in brain capillaries induced by obstructive jaundice in rats. Brain Res 2010; 1325: 121–7.
Yu DK, Zhang CX, Zhao SS, Zhang SH, Zhang H, Cai SY, et al. The anti-fibrotic effects of epigallocatechin-3-gallate in bile duct-ligated cholestatic rats and human hepatic stellate LX-2 cells are mediated by the PI3K/Akt/Smad pathway. Acta Pharmacol Sin 2015; 36: 473–82.
Ren C, Bao YR, Meng XS, Diao YP, Kang TG . Comparison of the protective effects of ferulic acid and its drug-containing plasma on primary cultured neonatal rat cardiomyocytes with hypoxia/reoxygenation injury. Pharmacogn Mag 2013; 9: 202–9.
Liu X, Jing XY, Jin S, Li Y, Liu L, Yu YL, et al. Insulin suppresses the expression and function of breast cancer resistance protein in primary cultures of rat brain microvessel endothelial cells. Pharmacol Rep 2011; 63: 487–93.
Yokooji T, Murakami T, Ogawa K, Yumoto R, Nagai J, Takano M . Modulation of intestinal transport of 2,4-dinitrophenyl-S-glutathione, a multidrug resistance-associated protein 2 substrate, by bilirubin treatment in rats. J Pharm Pharmacol 2005; 57: 579–85.
Dhanda S, Kaur S, Sandhir R . Preventive effect of N-acetyl-L-cysteine on oxidative stress and cognitive impairment in hepatic encephalopathy following bile duct ligation. Free Radic Biol Med 2013; 56: 204–15.
Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD . Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006; 1: 223–36.
Bosoi CR, Yang X, Huynh J, Parent-Robitaille C, Jiang W, Tremblay M, et al. Systemic oxidative stress is implicated in the pathogenesis of brain edema in rats with chronic liver failure. Free Radic Biol Med 2012; 52: 1228–35.
Chen YC, Sheen JM, Tain YL, Chen CC, Tiao MM, Huang YH, et al. Alterations in NADPH oxidase expression and blood-brain barrier in bile duct ligation-treated young rats: effects of melatonin. Neurochem Int 2012; 60: 751–8.
Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S . Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis 2014; 46: 527–34.
Munzel D, Lehle K, Haubner F, Schmid C, Birnbaum DE, Preuner JG . Impact of diabetic serum on endothelial cells: an in-vitro-analysis of endothelial dysfunction in diabetes mellitus type 2. Biochem Biophys Res Commun 2007; 362: 238–44.
Bosch J, Garcia-Pagan JC . Complications of cirrhosis. I. Portal hypertension. J Hepatol 2000; 32: 141–56.
Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, et al. ABCG2: a perspective. Adv Drug Deliv Rev 2009; 61: 3–13.
Krishnamurthy P, Schuetz JD . Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 2006; 46: 381–410.
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (No 81373482, 81573490 and 81473273), the Graduate Student Research and Innovation Program of Jiangsu Province (No KYLX_0636) and the Fundamental Research Funds for the Central Universities (2015PT042 and 2016PT076).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, P., Ling, Zl., Zhang, J. et al. Unconjugated bilirubin elevation impairs the function and expression of breast cancer resistance protein (BCRP) at the blood-brain barrier in bile duct-ligated rats. Acta Pharmacol Sin 37, 1129–1140 (2016). https://doi.org/10.1038/aps.2016.25
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.25
Keywords
This article is cited by
-
Effects of oxidative stress on hepatic encephalopathy pathogenesis in mice
Nature Communications (2023)
-
Bile duct ligation causes opposite impacts on the expression and function of BCRP and P-gp in rat brain partly via affecting membrane expression of ezrin/radixin/moesin proteins
Acta Pharmacologica Sinica (2021)
-
Bile duct ligation enhances AZT CNS toxicity partly by impairing the expression and function of BCRP in rat brain
Acta Pharmacologica Sinica (2020)
-
Thermal maturation as revealed by micro-Raman spectroscopy of mineral-organic aggregation (MOA) in marine shales with high and over maturities
Science China Earth Sciences (2020)