Abstract
It is difficult to accurately evaluate the efficacy of traditional Chinese medicine (TCM), which leads to the uncertainty and complexity of dose-effect analysis. In this study we established the “Focus” mode of biomarkers to characterize the dose-effect relationship of Gegen Qinlian Decoction (GQD), a TCM formula for treating type 2 diabetes mellitus (2-DM). A rat model of 2-DM was established through high fat diet feeding combined with low-dose STZ injection. Rats with 2-DM were administered high, middle or low doses (6.785, 4.071, 1.357 mg·kg−1·d−1, respectively) of GQD extract for 60 d. Metformin (300 mg·kg−1·d−1) was taken as the positive control. Blood samples were collected to assess serum biochemical indexes and metabolic profiling. After “Focus” analysis, the biochemical index triglycerides (TG) and insulin sensitivity (ISI) were identified as focused integrated biomarkers (FIBs), while arachidonic acid and docosatetraenoic acid were the metabolic FIBs. Dose-effect relationship curves of GQD were built based on these types of FIBs. Furthermore, the two dose-effect relationship curves showed similar trends with the middle dosage displaying the greatest efficacy, suggesting that insulin function and arachidonic acid metabolism played important roles in 2-DM and the responses to GQD. The metabolic FIB docosatetraenoic should be further explored for understanding its involvement in the process of 2-DM occurrence and the treatment. This “Focus” mode provides a novel strategy to evaluate the dose-effect relationship of a TCM. The system and concepts established here may also be applicable for assessing the dose-effect relationships of Western medicines.
Similar content being viewed by others
Introduction
In past decades, substantial efforts have been made to understand the dose-effect relationship of traditional Chinese medicine (TCM). However, most of these efforts have been limited to theoretical studies. The main reason for this is that TCM has a mixture of the characteristics of its multiple components. Thus, it is difficult to accurately evaluate TCM drug efficacy, which leads to the uncertainty and complexity of dose-effect analysis. Furthermore, the TCM dose-effect relationship cannot be described as simply as with the dose-effect relationship of chemical drugs. To date, no specific methods for exploring the TCM dose-effect relationship have been developed1.
Gegen Qinlian Decoction (GQD), a well-known classical TCM for diarrhea related to damp-heat syndrome, originates from the “Treatise on Cold Pathogenic Diseases” compiled by Zhong-jing ZHANG. There are four herbs in this formula: Pueraria lobata (Ge-Gen), Scutellaria baicalensis Georgi (Huang-Qin), Coptis Chinensis Franch (Huang-Lian), and Glycyrrhiza uralensis Fisch (Gan-Cao)2. Prof Xiao-lin TONG used GQD to treat type 2 diabetes mellitus (2-DM) according to the “treating different diseases with the same therapy” concept of TCM and achieved good results3.
Metabolomics is a systemic approach for studying metabolic profiles in vivo, and it has been increasingly applied to discover biomarkers, identify perturbed pathways, explore therapeutic targets, evaluate drug efficacy, assess drug toxicology and develop new drugs4,5. Similar to genomic and proteomic experiments, metabolomic experiments usually generate thousands of data points. In processing such big data, there are two major limitations to be resolved: First, as metabolomics is a systemic approach without specific targets, it is difficult to reflect drug efficacy accurately and specifically. Second, without focus and validation, the obtained potential biomarkers are unfavorable to clinical application.
Recently, the concept of an “integrated biomarker system” (IBS) has been proposed by Prof Luo to overcome the issue of lack of focus in systems biology research6. Based on the establishment of an IBS, the “Focus” mode of big data analysis was proposed to solve the above mentioned limitations and was applied to research on a dose-effect relationship in this study. The detailed concept of the “Focus” mode is presented in Figure 1. Here, we characterized the dose-effect profile of GQD using the “Focus” mode for the first time.
Schematic diagram of the “Focus” mode of integrated biomarker identification.
Materials and methods
Animal treatments
SPF Sprague Dawley (SD) rats, half male and half female, with a body weight of 180–200 g were purchased from Beijing Vital River Laboratory Animal Technology Co, Ltd (Beijing, China, 0263262). The rats were housed in controlled environmental conditions (temperature of 22–25 °C; relative humidity of 50%±5%) and kept on a light/dark cycle of 12/12 h. Water and standard chow could be accessed freely. All experiments were performed in accordance with established animal protocols and guidelines approved by the Ethics Review Committee on Animal Experimentation of Tsinghua University.
After 7 d of acclimatization, 10 rats were randomly selected as the normal control group (NC), and the remaining rats were fed a high-fat diet for four consecutive weeks. Then, the rats were fasted for 12 h, and the high-fat diet-fed rats were subjected to an injection of streptozotocin (STZ) (Sigma-Aldrich, Shanghai, China) at a dose of 30 mg/kg via the caudal vein7. One week later, the blood glucose level was measured. If the blood glucose level was less than 16.7 mmol/L, the rats were injected with STZ at the same dosage again. If the blood glucose level was higher than 16.7 mmol/L, the 2-DM rat model was successfully established.
The GQD extract was prepared in our laboratory according to a previous report8. The high, middle and low doses of GQD raw materials used in the clinic were 247.5, 148.5, 49.5 g/kg, respectively, according to Prof Xiao-lin TONG from Guang'anmen Hospital. Based on the formula rat dose=human dose/70 kg*6.3, the high, middle and low doses of GQD raw materials for rats were 22.275, 13.365 and 4.455 g/kg, respectively. In accordance with the yield of GQD extract, the high, middle and low doses of GQD extract administered to rats were 6.785, 4.071 and 1.357 g/kg, respectively.
All 2-DM rats were randomly divided into five groups: the high-dose GQD group (GQD-H, 6.785 g·kg−1·d−1), the middle-dose GQD group (GQD-M, 4.071 g·kg−1·d−1), the low-dose GQD group (GQD-L, 1.357 g·kg−1·d−1), the positive control group [PC, 300 mg·kg−1·d−1 metformin (Bristol-Myers Squibb, Shanghai, China)]7, and the 2-DM model group (DM). In total, 12 rats were assigned to each group. All rats from the treatment groups were administered the corresponding drugs for 60 d. The NC group was administered an equivalent amount of CMC-Na.
Sample preparation
On d 0, 15, 30, 45 and 60 after the treatment, blood samples were harvested from the ocular vein of rats after 12 h of fasting for blood glucose measurement. Serum samples were collected at the 60th d after the treatment for measurement of fasting insulin, triglyceride (TG), total cholesterol (TC), superoxide dismutase (SOD) activity, methane dicarboxylic aldehyde (MDA) and hepatic glycogen levels. At the end of the experimental period, the rats were fasted overnight, and blood was collected from the hepatic vein after anesthetization with chloral hydrate (350 mg/kg, ip). Then, the rats were quickly dissected to remove the liver, pancreas and kidney. These tissues were flushed with ice-cold phosphate buffer solution and weighed, and then the pancreas and kidney were fixed in 10% formaldehyde solution for further hematoxylin and eosin (H&E) staining.
Biochemical index determination
Fasting insulin kits were provided by Mercodia (Sweden). TG, TC, SOD activity, MDA and hepatic glycogen and fasting blood glucose (FBG) levels were measured using assay kits (Nanjing Jiancheng, Nanjing, China) following the manufacturer's instructions. The insulin sensitivity index (ISI) and insulin resistance index (IRI) were calculated using the following formulas: ISI=Ln[1/(FBG×fasting insulin)]; IRI=(FBG×fasting insulin)/22.5.
H&E staining
The fresh pancreatic tissue samples were fixed in 10% formalin for 24 h. Then, the samples were embedded in paraffin and sliced for H&E staining. The tissue staining results were observed via microscopy.
UPLC-TOF MS analysis
Thawed serum samples (100 μL) were mixed with methanol (400 μL) by vortexing for 2 min and then centrifuged at 4 °C for 15 min at 12 000 rounds per minute. In total, 200 μL of supernatant was transferred to a 1.5-mL polypropylene tube, dried under nitrogen and reconstituted with 300 μL of ultrapure water for UPLC-TOF MS analysis.
Serum metabolic profiling was performed via UPLC-MS analysis. Chromatography was carried out with an ACQUITY BEH C18 chromatography column (2.1 mm×100 mm, 1.7 μm, Waters, MA, USA). The column temperature was maintained at 40 °C, and a binary mobile phase composed of phase A (acetonitrile) and phase B (water with 0.1% formic acid) was used.
The elution gradients for serum samples were as follows: 0–3 min, 5%–50% A; 3–7 min, 50%–60% A; 7–10 min, 60%–70% A; 10–14 min 70%–95% A; and 14–16.5 min, 95% A. The proportion of phase B was returned to 5% for 0.5 min, and the column was allowed to re-equilibrate for 2 min before the next injection. The flow rate was maintained at 0.4 mL/min, and 5 μL of sample was injected into the column.
For MS, a negative ion electrospray mode was used. The capillary voltage was set at 2500 V, and the cone voltage was set at 30 V. Nitrogen was used as the dry gas, the desolvation gas flow rate was set at 600 L/h, and the cone gas flow rate was maintained at 50 L/h. The desolvation temperature was set at 350 °C, and the source temperature was set at 110 °C. The scanning time and inter-scan delay were set to 0.1 s and 0.02 s, respectively. The TOF MS data were collected from 50 to 1000 m/z. All data were acquired using an independent reference lock mass via the LockSprayTM interface to ensure accuracy and reproducibility. Leucine enkaphalin was used as the reference compound ([M–H]–=554.2615) at a concentration of 50 pg/μL under a flow rate of 10 μL/min. The data were collected in the centroid mode, and the LockSpray frequency was set at 10 s, with averaging over 10 scans for correction.
Statistical analysis
All data were first tested for normality of the distribution, and then, parametric tests (Student's t test) or ANOVA was performed to evaluate the statistical significance of the differences in the abundance of metabolites between groups over time. The significance of differences between groups in biochemical indexes was assessed using SPSS 13.0 software (GraphPad Software Inc, USA). A difference was considered to be significant at a P value less than 0.05. All data were expressed as the mean±SEM.
The metabonomic data were first analyzed via principal component analysis (PCA) and supervised partial least-squares discriminant analysis (PLS-DA). Then, FormRules, INForm software and receiver-operating characteristic curve (ROC, MedCalc 11.4) analysis were used to screen and verify the diagnostic value of FIBs.
Results
Measurement of FBG
The FBG results are shown in Table 1. Compared with normal rats, the FBG level in 2-DM model rats was significantly increased. After treatment for 60 d, the FBG levels in the four treatment groups were much lower than those in the 2-DM group (P<0.05). Our data indicated that all tested dosages of GQD and metformin controlled the FBG levels in 2-DM model rats. Among the three dosages of GQD, the middle dosage showed the greatest efficacy.
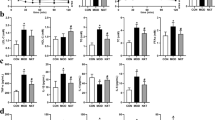
Additionally, the serum levels of hepatic glycogen, TG, TC, SOD activity, MDA, ISI, the left kidney weight index, the right kidney weight index and the liver weight index were measured. The results are summarized in Supplementary Figure S1.
Pathological changes in pancreatic tissue
The results of H&E staining are depicted in Figure 2. Compared with normal rats, the islet morphology of 2-DM model rats was irregular, the boundary of islet cells and the exocrine gland was blurry, and vacuolar degeneration was observed in the cytoplasm of some islet cells. The above phenomena improved in rats treated with GQD or metformin, especially in the group treated with the middle dosage of GQD. The results of FBG determination and pathological analysis confirmed that the 2-DM rat model was successful and that GQD showed good efficacy in the 2-DM model rats.
Results of H&E staining of pancreatic tissues (×400) in different groups, (A) 2-DM group, (B) NC group, (C) PC group, (D) GQD-L group, (E) GQD-M group, (F) GQD-H group.
Serum metabolic profiles
The rat serum metabolic data were obtained via UPLC coupled with time of flight mass spectrometry (UPLC-TOF-MS). To determine whether it was possible to distinguish the different experimental groups, PLS-DA was conducted. The PLS-DA scores for the first and second principal components (PC1 and PC2) showed that although there was overlap between the six groups, some clusters were evident (Figure 3A). In space, each treatment group was far from the 2-DM group and close to the normal group. Our findings indicate that the severity of metabolic disorder in the 2-DM model rats was alleviated by drug administration, especially in the rats that received the middle dose of GQD. These results were consistent with the result of the FBG assay. As shown in Figure 3B, GQD significantly changed the metabolism of the rats in each group, as the GQD-treated groups were far from the 2-DM model group and close to the normal group. Additionally, the metabolic changes among the groups treated with GQD revealed a clear dose-dependent trend.
Plots of PLS-DA scores obtained from UPLC-MS analysis of rat serum after treatment for 60 d. (A) All experimental groups; (B) The three groups treated with GQD at different dosages, 2-DM group, NC group.
Potential biomarkers and metabolic FIB identification
In this study, 1608 ion signals were detected in all groups using the MarkerLynx module (Waters). According to their variable importance in PLS-DA analysis and the results of T-tests comparing pairs of groups (P<0.05), 95 potential biomarkers were chosen as variables for further analysis. The focused analysis process is displayed in Figure 1. First, the candidate FIBs were screened using ANNs. FormRules software was used to obtain different reduced variable sets, of which specific sets (R2>0.95) were selected to conduct further research. If there were many variable sets with R2>0.95, we generally chose the variable set with R2 closest to 1, and the variable set containing the fewest elements or practical experience could be combined to select the optimal set of variables. The reduced variable sets were tested through the establishment of training, testing and evaluation data sets using INForm software, and an appropriate assessment of the tested results was made. Stability testing was used to ensure reproducibility through repetition of the above method three times. The metabolic FIBs included 2 metabolites, ×16 and ×21. The prediction accuracy of the FIBs was 96.34%, which was close to the 98.14% prediction accuracy of all 95 variables. The detailed parameters and prediction outcomes are shown in Supplementary Table S1.
Then, the two metabolic FIBs were formulated into a comprehensive variable (Xm) by linear equation fitting with discriminant analysis, as shown in the following equation:

Based on the above equation, ROC diagnostic analysis was carried out between different treatment groups. The prediction accuracy of Xm could be represented by the area of the ROC curve (AUC); when the AUC was >0.9, a higher prediction accuracy was indicated. The analysis results are shown in Figure 4. The prediction accuracy of Xm was 100% between the NC and 2-DM groups. This suggested that metabolic FIBs could fully distinguish between the disease and normal states. Between the 2-DM and GQD-M groups, the prediction accuracy of Xm was 97.0%. However, the prediction accuracy was only 68.5% between the NC and GQD-M groups, which indicated that the expression of FIBs in the group treated with the middle dose of GQD was similar to that in the NC group. According to the results of ROC analysis between the groups treated with different dosages of GQD, the efficacy of the high and middle doses of GQD was similar. These results also reflected the excellent treatment efficacy of GQD for 2-DM.
ROC curves of Xm between different experimental groups. (A) NC Group vs 2-DM Group; (B) NC Group vs GQD-M Group; (C) 2-DM Group vs GQD-M Group; (D) GQD-M Group vs GQD-H Group; (E) GQD-M Group vs GQD-L Group.
On the basis of the peak area of metabolic FIBs, a dose-effect curve was established, as shown in Figure 5A. The efficacy index Xm was increased in the GQD-treated groups compared to the 2-DM group, and the middle dosage of GQD showed the best efficacy, in accordance with the dose-effect results obtained from analysis of the biochemical indexes.
Dose-effect relationship reflected by FIBs from metabolites (A) and the serum contents of AA and DTA in all groups (B). *P<0.05, **P<0.01 vs NC group. #P<0.05, ##P<0.01 vs 2-DM group. Mean±SEM.
Two components were searched in the Human Metabolome Database (http://www.hmdb.ca/) to identify the molecular formula as well as other information: ×16 was arachidonic acid (AA), and ×21 was docosatetraenoic acid (DTA).
Biological functions of metabolic FIBs
The changes in serum contents of AA and DTA are shown in Figure 5B. The contents of AA and DTA exhibited a significant decrease in the 2-DM group; however, their contents showed an obvious increase in all treatment groups.
Both AA and DTA are important metabolites in the AA metabolism pathway, which could reflect the state of fatty acid metabolism in the body. Several studies have revealed that AA protects pancreatic β cells against alloxan-induced diabetes in experimental animals by attenuating oxidative stress9, and enhances the insulin secretion of beta-cells10. In addition, AA can stimulate glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane11. As shown in Figure 5B, significantly reduced AA levels implied a disordered state of AA metabolism. However, the obviously enhanced AA levels in the GQD-treated group, especially in the group treated with the middle dosage of GQD, were nearly equivalent to the AA levels in the NC group. This result indicated that AA was one of the important targets of GQD treatment in 2-DM.
DTA, a straight chain fatty acid, is also known to be a metabolite of AA12. Previous reports on DTA were focused on its role in diseases associated with neurodevelopmental disorders13,14,15. In the present study, the changes in DTA levels were consistent with previous reports. Therefore, DTA should be further explored for understanding its involvement in the process of 2-DM occurrence and treatment.
Focused analysis of biochemical indexes
In this experiment, in addition to FBG, 8 other biochemical indexes were also measured for the evaluation of GQD efficacy. These parameters were selected to examine the association of GQD treatment with metabolic profiles. This experiment provided direct evidence on whether the FBG assay was feasible as a biochemical indicator. The biochemical indexes were named from ×1 to ×9. The focused analysis was carried out, and the detailed information from the focused analysis process is shown in the Supplementary information. TG (×1) and ISI (×2) were the focused variables selected through the “Focus” mode and constituted the comprehensive variable (named Xb).
According to the concentrations of FIBs in serum, the dose-effect curve of Xb was established, as shown in Figure 6. Compared with the value for the 2-DM group, the efficacy index Xb was increased with increasing GQD dosage. Meanwhile, the ROC curve did not show a significant difference between the groups treated with GQD groups at the middle and high dosages. The curve revealed a parabola, and the middle dosage of GQD displayed the greatest treatment efficacy, consistent with the dose-effect relationship of GQD.
Dose-effect relationship reflected by FIBs from biochemical indexes.
Discussion
Correlation of TG levels and ISI with 2-DM
Recently, TG has been established to be an important factor in the occurrence and development of 2-DM and might be an independent risk factor for 2-DM16. The ratio between triglyceride and high-density lipoprotein cholesterol (TG/HDL-C) has been identified as an index of heart disease mortality and 2-DM incidence in men17,18. Moreover, the postprandial level of TG is associated with the occurrence of complications such as atherosclerosis in adults with 2-DM19.
ISI is commonly used to describe the degree of insulin resistance (IR), suggesting that lower insulin sensitivity can result in a smaller effect per unit of insulin. IR plays an important role in the pathogenesis and development of 2-DM20, and alleviating IR through strategies such as increasing magnesium intake can reduce the risk of 2-DM21. In previous studies, the main mechanisms of IR associated with 2-DM have been clearly illustrated, but the physiopathological mechanisms of IR in diabetes complications and other chronic diseases are still unclear22. According to recent results, IR is not only recognized as the initiator of 2-DM but also as a major cause of a variety of metabolic diseases.
In this study, a rat model of 2-DM was established by high-fat diet feeding combined with low-dose STZ injection. The mechanism underlying 2-DM development in this model is that the high-fat diet leads to IR in the rats and low-dose STZ injection damages pancreatic islet β cells23. After “Focus” analysis, TG and ISI were identified as FIBs. Interestingly, TG levels were closely correlated with fatty acid metabolism, and ISI was associated with insulin function. This showed that the FIBs can accurately reflect the characteristics of this animal model. However, it is worth noting that animal models do not exhibit all symptoms of clinical diseases. Whether these FIBs can be applied to the diagnosis of clinical disease and the evaluation of 2-DM treatment efficacy needs to be further explored.
“Focus” mode, a new tool for research on the dose-effect relationship of TCM
Recently, the development of systems biology and various quantitative analytical techniques has provided a strong platform for the diagnosis of major diseases, risk assessment, and new drug screening approaches, such as genomics, proteomics, metabolomics, and lipidomics24,25,26. Potential biomarkers have gradually provided unique advantages in disease diagnosis and efficacy evaluation6,27. The above mentioned results confirmed that the “Focus” mode of identifying potential biomarkers was applicable for analyzing metabolomic parameters and biochemical indexes.
The dose-effect curves of GQD generated from two kinds of data displayed consistent trends, as shown in Figure 5A and 6. However, considering the NC group as the control, the FIBs from metabolites were more sensitive for detecting the efficacy of GQD than the FIBs from biochemical indexes. As observed in Figure 7, the level of the comprehensive variable composed of FIBs from metabolites after GQD treatment was much closer to level in the normal group. This may be because the biochemical indexes are the ultimate performance characteristics of the body state and because hysteresis may occur in the change in biochemical indexes induced by drug administration. In contrast, the metabolites can more rapidly reflect a change in the body state. The FIBs from metabolites are expected to serve as targets for the prevention and treatment of disease with TCM.
The differences between sets of biochemical and metabolic FIBs. Note: *P<0.05, **P<0.01 vs NC group. #P<0.05, ##P<0.01 vs 2-DM group.
With the development of science and technology, Western medicine has highlighted the limitations of a single targeted therapy. Therefore, the research and development of compound medicine is becoming more and more widely accepted. For example, the “polypill”, a four-component combination pill, can achieve increased efficacy for cardiovascular diseases through drug combination28,29. With the increase in the number of drugs in Western combination therapies, research on the dose-effect relationships of individual compounds will face a great challenge. This “Focus” mode may provide a possible solution for analyzing the dose-effect relationships of Western compound medicines.
Our future work aims to explore the dose-effect relationship of TCM from the perspective of changes in gene or protein expression. Through correlation analysis of FIBs at different levels (gene, protein, metabolite, biochemical index, etc), the “fragmentation” phenomenon in systems biology is expected to be alleviated.
Conclusion
In conclusion, the “Focus” mode established in this study accurately focused on a key set of biomarkers — FIBs, thus avoiding a great deal of effort associated with the identification of all metabolites. This provides a new research method for the establishment of dose-effect relationships and for further research on the pharmacodynamic mechanisms of compound medicine. Based on the “Focus” mode, the dose-effect relationship of GQD was evaluated in this study, and finally, the dose-effect curves of GQD were established for biochemical FIBs and metabolic FIBs. Both sets of FIBs accurately reflected the dose-effect relationship of GQD. Our results thus highlight the importance of the “Focus” mode and FIBs for accurately representing the effectiveness of TCM. Our studies not only yield new insights into the evaluation of the dose-effect relationship of TCM but also provide the possibility of introducing FIBs to the analysis of Western compound medicines.
Author contribution
Zhi-qiang HUANG, Xue-mei FAN, Guo-an LUO, Xiao-lin TONG, and Chao CHEN conceived and designed the study; Zhi-qiang HUANG, Xue-mei FAN, Yi-ming WANG, Qiong-lin LIANG conducted the experiments; Zhi-qiang HUANG, Xue-mei FAN, and Ying-meng LI acquired animal experimental data; Zhi-qiang HUANG and Yu BAI performed analysis and interpretation of the data; Zhi-qiang HUANG and Xue-mei FAN wrote the manuscript; Guo-an LUO and Chao CHEN reviewed the manuscript. All authors read and approved the manuscript.
References
Xie WQ, Lian FM, Ji HY, Chen XY, Tong XL . Research progress on dose-effect relationship of TCM. J Tradit Chin Med 2011; 52: 1696–9.
Zhang Q, Xu G, Li J, Wang H, Li BT, Tu J, et al. Metabonomic study on the plasma of streptozotocin-induced diabetic rats treated with Ge Gen Qin Lian Decoction by ultra high performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal 2016; 120: 175–80.
Zhao LH, Lian FM, Ji HY, Zhou Q, Xia L, Tong XL . Clinial examples of treatment for type 2 diabetes by professor Tong Xiao-lin using Ge-Gen-Qin-Lian decoction. Chin J Exp Tradit Med Formul 2011; 17: 249–51.
Li TJ, wang S, Meng XS, Bao YR, Guan SS, Liu B, et al. Metabolomics coupled with multivariate data and pathway analysis on potential biomarkers in gastric ulcer and intervention effects of corydalis yanhusuoalkaloid. PLoS One 2014; 9: e82499.
Zhou YT, Liao QF, Lin MN, Deng XJ, Zhang PT, Yao M, et al. Combination of H-1 NMR- and GC-MS-based metabonomics to study on the toxicity of Coptidis Rhizome in rats. PLoS One 2014; 9: e88281.
Huang M, Liang QL, Li P, Xia JF, Wang YM, Luo GA, et al. Biomarkers for early diagnosis of type 2 diabetic nephropathy: a study based on an integrated biomarker system. Mol Biosyst 2013; 9: 2134–41.
Zhang CH, Xu GL, Liu YH, Rao Y, Yu RY, Zhang ZW, et al. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine 2013; 20: 221–9.
Li YM, Fan XM, Wang YM, Liang QL, Luo GA . Therapeutic effects of Gegen Qinlian decoction and its mechanism of action on type 2 diabetic rats. Yao Xue Xue Bao 2013; 48: 1415–21.
Suresh Y, Das UN . Differential effect of saturated, monounsaturated, and polyunsaturated fatty acids on alloxan-induced diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 2006; 74: 199–213 .
Keane D, Newsholme P . Saturated and unsaturated (including arachidonic acid) non-esterified fatty acid modulation of insulin secretion from pancreatic β-cells. Biochem Soc Trans 2008; 36: 955–8 .
Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VK, O'Rahilly S . Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. J Biol Chem 2001; 276: 9149–57.
Richardson AJ, Easton T, Puri BK . Red cell and plasma fatty acid changes accompanying symptom remission in a patient with schizophrenia treated with eicosapentaenoic acid. Eur Neuropsychopharmacol 2000; 10: 189–93.
Lim SN, Gladman SJ, Dyall SC, Patel U, Virani N, Kang JX, et al. Transgenic mice with high endogenous omega-3 fatty acids are protected from spinal cord injury. Neurobiol Dis 2013; 51: 104–12.
van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE . A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res 2012; 141: 153–61.
Hamazaki K, Hamazaki T, Inadera H . Fatty acid composition in the postmortem amygdala of patients with schizophrenia, bipolar disorder, and major depressive disorder. J Psychiatr Res 2012; 46: 1024–8.
Vega-López S, Calle MC, Fernandez ML, Kollannoor-Samuel G, Chhabra J, Todd M, et al. Triglyceride screening may improve cardiometabolic disease risk assessment in Latinos with poorly controlled type 2 diabetes. J Health Care Poor Underserved 2013; 24: 1739–55.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF . Triglyceride–to–high density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Invest Med 2014; 62: 345–9.
Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus A global case–control study in 13 countries. Circulation 2014; 129: 999–1008.
Idei M, Hirayama S, Miyake N, Kon M, Horiuchi Y, Ueno T, et al. Mean postprandial triglyceride concentration is an independent risk factor for carotid atherosclerosis in patients with type 2 diabetes. Clin Chim Acta 2014; 430: 134–9.
Okuno A, Kaji N, Takahashi A, Nagakubo D, Ohno-Ichiki K, Shirai M, et al. Role of insulin resistance in the pathogenesis and development of type 2 diabetes in WBN/Kob-Leprfa rats. J Vet Med Sci 2013; 75: 1557–61.
Hata A, Ninomiya T, Mukai N, Hirakawa Y, Hata J, Ozawa M, et al. Magnesium intake decreases type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama Study. Diabetic Med 2013; 30: 1487–94.
Ndisang JF, Rastogi S, Vannacci A . Insulin resistance, type 1 and type 2 diabetes, and related complications: current status and future perspective. J Diabetes Res 2014; 2014: 276475.
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P . Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005; 52: 313–20.
Zhao C, Mao J, Ai J, Shenwu M, Shi T, Zhang D, et al. Integrated lipidomics and transcriptomic analysis of peripheral blood reveals significantly enriched pathways in type 2 diabetes mellitus. BMC Med Genomics 2013; 6: S12.
Zhang QB, Fillmore TL, Schepmoes AA, Clauss TR, Gritsenko MA, Mueller PW, et al. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. J Exp Med 2013; 210: 191–203.
Wahl S, Krug S, Then C, Kirchhofer A, Kastenmüller G, Brand T, et al. Comparative analysis of plasma metabolomics response to metabolic challenge tests in healthy subjects and influence of the FTO obesity risk allele. Metabolomics 2014; 10: 386–401.
Wheelock CE, Goss VM, Balgoma D, Nicholas B, Brandsma J, Skipp PJ, et al. Application of 'omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J 2013; 42: 802–25.
Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S . The polypill in the prevention of cardiovascular diseases key concepts, current status, challenges, and future directions. Circulation 2010; 122: 2078–88.
PILL Collaborative Group, Rodgers A, Patel A, Berwanger O, Bots M, Grimm R, et al. An international randomised placebo-controlled trial of a four-component combination pill (“polypill”) in people with raised cardiovascular risk. PLoS One 2011; 6: e19857.
Acknowledgements
This work was supported by the Chinese National “973” Project (Grant No 2010CB530600) and the National Natural Science Foundation of China (Grant No 81130066 and 81302731). We are thankful to Dr Qun SHAO of the University of Bradford for providing us with the FormRules and INForm software used in these studies.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available at the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary information
Supplementary materials (DOC 356 kb)
Rights and permissions
About this article
Cite this article
Huang, Zq., Fan, Xm., Wang, Ym. et al. A new method to evaluate the dose-effect relationship of a TCM formula Gegen Qinlian Decoction: “Focus” mode of integrated biomarkers. Acta Pharmacol Sin 38, 1141–1149 (2017). https://doi.org/10.1038/aps.2016.165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.165
Keywords
This article is cited by
-
Metformin and Gegen Qinlian Decoction boost islet α-cell proliferation of the STZ induced diabetic rats
BMC Complementary Medicine and Therapies (2022)