Abstract
Most gefitinib-treated patients with non-small cell lung cancer (NSCLC) would eventually develop resistance. Lysimachia capillipes (LC) capilliposide extracts from LC Hemsl. show both in vitro and in vivo anti-cancer effects. In this study we investigated whether LC capilliposide in combination with gefitinib could overcome the resistance of NSCLC cells to gefitinib and identified the signaling pathways involved. Treatment with LC capilliposide alone inhibited the growth of a panel of NSCLC cell lines (PC-9, H460, H1975, H1299 and PC-9-GR) sensitive or resistant to gefitinib with IC50 values in the range of μg/mL. In the gefitinib-resistant PC-9-GR cells (which have a T790M EGFR mutation), LC capilliposide (at the IC30, i.e.1.2 μg/mL) markedly enhanced the inhibitory effects of gefitinib with its IC50 value being decreased from 6.80±1.00 to 0.77±0.12 μmol/L. By using the median effect analysis we showed that combination treatment of LC capilliposide and gefitinib could restore gefitinib sensitivity in PC-9-GR cells. Furthermore, LC capilliposide (1.2 μg/mL) significantly increased the apoptotic responses to gefitinib (0.77 μmol/L) in PC-9-GR cells, but did not affect gefitinib-induced G0/G1 arrest. Moreover, LC capilliposide (1.2 μg/mL) in combination with gefitinib (0.77, 1.0 μmol/L) markedly decreased the phosphorylation of the EGFR downstream signaling molecule AKT, which neither LC capilliposide nor gefitinib alone affected. In PC-9-GR cells with siRNA knockdown of AKT, addition of LC capilliposide was unable to increase gefitinib sensitivity. In a PC-9-GR xenograft mouse model, combination treatment with LC capilliposide (15 mg·kg−1·d−1, ip) and gefitinib (50 mg·kg−1·d−1, ip) dramatically enhanced tumor growth suppression (with a TGI of 109.3%), compared with TGIs of 22.6% and 56.6%, respectively, in mice were treated with LC capilliposide or gefitinib alone. LC capilliposide can restore the cells' sensitivity to gefitinib through modulation of pAKT levels, suggesting that a combination of LC capilliposide and gefitinib may be a promising therapeutic strategy to overcome gefitinib resistance in NSCLCs with a T790M mutation.
Similar content being viewed by others
Introduction
Lung cancer is the most common cancer and the leading cause of cancer-related mortality worldwide1. The majority of lung cancers comprise NSCLC (non-small-cell lung cancer), and one-third of these patients are diagnosed with stage III disease when surgical excision is not an option and curative treatment is extremely limited2. For these patients, the combined treatment of radiotherapy and chemotherapy are extensively used3. Despite the tremendous efforts and progress in lung cancer research and the use of aggressive multimodal chemo- and radiotherapies, the overall treatment outcome for these NSCLC patients remains disappointing, with a 5-year survival rate of approximately 15%4,5; however, the discovery of activating epidermal growth factor receptor (EGFR) mutations in NSCLC has led to a paradigm shift in cancer treatment for NSCLC patients. The EGFR family of receptor tyrosine kinases (TKs) consists of four members (EGFR (HER1/ErbB1), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4)) that regulate many developmental, metabolic and physiological processes. The intracellular TK activity of EGFR is increased as a consequence of the binding of various cognate ligands, which include EGF, transforming growth factor-α, amphiregulin and others, leading to either the homodimerization of two EGFRs or the heterodimerization of EGFR with other family members6. The activation of receptor TKs results in the autophosphorylation of the intracellular domain of EGFR, and the resulting phosphotyrosine residues act as a docking site for various adapter molecules, leading to the activation of the Ras/mitogen-activated protein kinase pathway, the PI3K/AKT pathway and signal transducers and activators of transcription signaling pathways7,8,9. Mutations in the region of the EGFR gene that encode the TK domain of the receptor alter the enzymatic TK pocket of the receptor, resulting in constitutive activation; furthermore, these mutations are found in approximately 10% of NSCLCs in Caucasians and 30% of NSCLCs in East Asians10. A number of retrospective reviews and prospective trials have established that treatment with gefitinib or erlotinib (first-generation reversible EGFR TKIs) leads to radiographic responses in 75%–80% of patients with NSCLCs with EGFR mutations11,12,13,14,15,16. However, the initial response to first-generation TKIs is often limited with duration of 10–16 months due to acquired resistance, and almost 50% of cases are caused by an acquired or de novo T790M mutation17,18,19,20. Second-generation EGFR TKIs, including the drug afatinib, showed promising results in overcoming T790M drug resistance in preclinical studies and in clinical trials21,22,23,24. However, the nonspecific reactivity and potential for off-target activity that may cause tissue injury and drug-related toxicities were major concerns for the second-generation covalent TKI drugs25,26.
The third-generation EGFR-TKIs, which include AZD9291, CO-1886 and HM61713, were specifically designed to inhibit both activating/sensitizing mutations (EGFRm) and the resistant mutation T790M27. AZD9291 has been recently approved by the FDA with an objective response rate of 59% and a response duration of 12.4 months, which provides important new option for patients positive for the T790M mutation28. However, the high cost of the drug and its limited availability in a handful of countries is currently the great hurdle in clinical practice. Thus, exploring effective and feasible treatment strategies with few side effects to overcome the resistance to first generation EGFR-TKIs is still of significance for improving the prognosis of patients with NSCLC.
Traditional Chinese medicine (TCM) has a long history of being widely used for treating human diseases, including cancer. Lysimachia capillipes Hemsl grows in southeastern China and has been used extensively as a traditional medicine for treating cough, menstrual symptoms, rheumatalgia disorder and carcinomas. Recently, LC capilliposide extracted from Lysimachia capillipes Hemsl has been tested for its anti-cancer properties29,30, and the results revealed both in vitro and in vivo anti-cancer effects of LC capilliposide in prostate, gastric and breast cancer cells31,32,33. Our preclinical study has also demonstrated the potential therapeutic effects of LC capilliposide on human lung cancer cells34. In this study, we examined the combined effect of LC capilliposide and gefitinib in NSCLC cells, and our results showed that LC capilliposide not only synergistically enhances the killing effect of gefitinib on NSCLC cells but also restores gefitinib sensitivity to NSCLC cells with acquired gefitinib resistance.
Materials and methods
Cell culture and reagents
The human NSCLC cell lines PC-9, H460, H1975, and H1299 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The human NSCLC cell line PC-9-GR was developed by chronic exposure to gefitinib as we previously reported35. All of the cell lines were maintained in RPMI-1640 (Gibco, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Waltham, Massachusetts, USA). Gefitinib (Cayman, Ann Arbor, MI, USA) was dissolved in dimethyl sulfoxide (DMSO). LC capilliposide was obtained from the Department of Chinese Medicine Sciences & Engineering at Zhejiang University (Hangzhou, Zhejiang, China). All of the drugs were diluted with fresh media before each experiment.
Cell growth inhibition assay
Cell proliferation analysis was performed using the MTS assay (tetrazolium-based CellTiter 96 Aqueous One Solution Proliferation assay), as per the manufacturer's instructions (Promega, Fitchburg, WI, USA). Briefly, cells were plated in a 96-well plate (3000 cells/well). Approximately 24 h after plating, cells were treated with various concentrations of gefitinib and LC capilliposide, and cell viability was determined 72 h later. The IC50 value (defined as the concentration necessary for a 50% reduction in the absorbance) was calculated based on the nonlinear regression fit method by GraphPad Prism 5.0 software (San Diego, CA, USA). For the combination treatment, cells were seeded in a 96-well plate at the density of 3×103 per well and incubated for 24 h, after which LC capilliposide and gefitinib were added concurrently to the medium and incubated for 72 h before measurement. The half-maximal inhibitory concentration (IC50) was determined with the corresponding dose response data for each cell line.
Median effect analysis
Median effect analysis was performed as described previously36. Briefly, cells were treated with increasing total doses of gefitinib and LC capilliposide with a constant dose ratio based on the corresponding IC50 values, and cell viability was determined by the cell proliferation assay (tetrazolium-based CellTiter 96 Aqueous One Solution Proliferation assay, Promega, Fitchburg, WI, USA). A plot of the log of the total dose versus the log of the reciprocal of the cell fraction affected minus 1 yielded a linear plot. The slope and y-intercept from these plots were used to calculate the CI (combination index) by using CalcuSyn Version 2.0 software (BioSoft, Great Shelford, Cambridge, UK). The CI values were interpreted as follows: <1.0=synergism; 1.0=additive; >1.0=antagonism.
siRNA transfection
Cells were seeded at a density of 3×105 cells/well in 6-well plates. The cells were transfected with siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. siRNA-control and siRNA-AKT (Santa Cruz Biotech, Dallas, TX, CA) were used. Western blot analysis was used to verify the gene silencing efficiency. After 48 h, cells were pretreated with gefitinib and LC for cell growth inhibition analysis.
Detection of cell apoptosis
Cells were treated with DMSO, LC capilliposide only (1.20 μg/mL, IC30), gefitinib only (0.77 μmol/L, IC50), or gefitinib (0.77 μmol/L) combined with LC (1.20 μg/mL). Cells were then collected 48 h later and stained with annexin V-FITC (fluorescein isothiocyanate) as per the manufacturer's instructions (Annexin V-FITC Apoptosis Detection kit; BD Biosciences, USA). Flow cytometric analysis with a Becton Dickinson FACScan cytofluorimeter (BD Bioscience, Franklin Lakes, NJ, USA) was used to determine the percentage of apoptotic cells. Up to 5×104 cells were counted for each sample, and the quantification of apoptotic cells was calculated by CellQuest software. Both early apoptotic (annexin V-positive and PI-negative) and late apoptotic (annexin V-positive and PI-positive) cells were included as part of the total apoptosis.
Cell cycle analysis
PC-9-GR cells were seeded into 6-well culture plates overnight, and cells were then treated with 1.20 μg/mL of LC capilliposide, 0.77 μmol/L of gefitinib, or a combination of gefitinib (0.77 μmol/L) and LC capilliposide (1.20 μg/mL) for 24 h. DMSO was included as a control. After the treatments, cells were collected and fixed with 70% ethanol, stained with propidium iodide and analyzed by flow cytometry with 5×104 events counted per run. The percentage of cells in the G1, S, and G2/M phases of the cell cycle were determined by using FlowJo software (FlowJo, Ashland, OR, USA).
Western blot analysis
Cell lysates were prepared in RIPA buffer with mild sonication and subjected to SDS-PAGE gel for immunoblot assays. Antibodies against phospho-EGFR, EGFR, phospho-AKT, AKT, phospho-ERK, ERK, and GAPDH were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). Densitometry using Image Lab 5.0 software (BioRad Laboratories, Hercules, CA, USA) was conducted to determine the intensity of the Western blot signal, and the intensity of the target protein was normalized to the corresponding GAPDH band.
Phospho-receptor tyrosine kinase array assay
The Human Phospho-Kinase Array Kit (ARY003B, R&D Systems, Minneapolis, MN, USA) was used to detect the relative levels of phosphorylation for 43 kinase targets as per the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). Briefly, cell lysates were diluted to 500 μg of protein per array set and incubated overnight with the array. The array was washed to remove unbound proteins and then incubated with a cocktail of biotinylated detection antibodies. Streptavidin-HRP and chemiluminescent detection reagents were applied, and the signal was captured corresponding to the amount of bound phosphorylated protein using Image Lab 5.0 software (BioRad Laboratories, Hercules, CA, USA)
Tumor growth assay
PC-9-GR cells (1×106 in 0.2 mL 1×HBSS+1% HSA) were inoculated subcutaneously into the right thigh of 4- to 6-week-old female Nu/Nu mice (Charles River, Beijing, China). When the average tumor volume reached 200 mm3, the mice were randomized into 4 groups to receive the following treatments: (a) methylcellulose/Tween 80 as vehicle for 10 days; (b) gefitinib (50 mg·kg−1·d−1) for 10 days; (c) LC (15 mg·kg−1·d−1) for 10 days; and (d) gefitinib (50 mg·kg−1·d−1)+LC (15 mg·kg−1·d−1) for 10 days. All of the chemicals were administered by ip injection. Tumors were measured twice weekly, and the tumor volumes were determined from caliper measurements of the tumor length (L) and width (W) according to the formula (L×W2)/2. The equation [%TGI=(1–change of tumor volume in treatment group/change of tumor volume in control group)×100] was used to determine the percentage of tumor growth inhibition and antitumor efficacy.
Immunohistochemistry analysis
PC-9-GR xenograft tumors were collected after 10 days of the aforementioned daily treatments and fixed in 4% formalin. Antigen retrieval was conducted on FFPE tissues sections for 5 min with retrieval buffer (DAKO, Glostrup, Denmark) followed by washing under running water for 5 min. Tissue samples were then rinsed in TBS containing 1% Tween (TBST) and incubated with endogenous peroxidase blocker on a LabVision autostainer for 10 min. Slides were washed twice in TBST, incubated with primary antibodies against pEGFR, pAKT, or CC3 (Cell Signaling Technology) for 60 min at room temperature and then washed twice in TBST. DAKO EnVision™+System-HRP was used as the secondary antibody for visualization, and staining was detected using diaminobenzidine (DAKO). For Ki67 immunohistochemical analysis, tumor sections were incubated with biotinylated primary antibody (DAKO) for 1 min at room temperature and then washed twice with TBST. Following a 15-min streptavidin-peroxidase treatment and washing with TBST, the slides were counterstained with DAPI and visualized by chemiluminescence. For analysis of the baseline expression or modulation, IHC scoring of phospho-EGFR and phospho-AKT was conducted using the following formula: scoring=0×[% cells with no staining (0)]+1×[% cells staining faint to barely visible (1+)]+2×[% cells staining weak to moderately (2+)]+3×[% cells staining strongly (3+)]. The samples were analyzed by 2 separate pathologists using microscopy. Quantification of Ki67- and CC3-positive signals was conducted using the ARIOL system (Genetix, San Jose, CA, USA).
Statistical analysis
Data were presented as the means±SD from at least three independent experiments. Student's t-test was used to determine the significance between groups. P<0.05 was defined as statistically significant.
Results
Effects of LC capilliposide and gefitinib on growth of NSCLC cells
We first determined the inhibitory effects of gefitinib and LC capilliposide on the growth of a panel of five NSCLC cell lines. Of these cell lines, H1299 expresses wild-type EGFR, PC-9 expresses mutant EGFR with a 15-bp deletion (EGFR-19Del), H1975 and PC-9-GR express mutant EGFRs with dual mutations, one of which includes T790M (EGFR-L858R/T790M for H1975 and EGFR-19Del/T790M for PC-9-GR)35, and H460 expresses mutant KRAS (KRAS-Q61H). With IC50 values determined by the MTS assay, we found that these cells responded to gefitinib treatment with a wide range of IC50 values, but only PC-9 cells showed an IC50 value at the nmol/L level (31.00±6.15 nmol/L). Of note, the MTS assay showed that the IC50 values of the other four cell lines ranged from near 7 to 16 μmol/L, indicating that these cells are relatively resistant to gefitinib treatment (Figure 1A and Table 1). However, no significant difference was observed for the IC50 values of LC capilliposide determined in these tested cells, as the IC50 values for LC capilliposide in all five cell lines were in the range of μg/mL (Figure 1B and Table 1).
Cytotoxic effects of gefitinib and LC capilliposide on NSCLC cells and the synergy of LC capilliposide and gefitinib to sensitize NSCLC cells to gefitinib treatment. Cells were cultured in 96-well plates and were treated with different doses of gefitinib (A) or LC capilliposide (B) for 72 h. (C) Effects of LC capilliposide on cell growth inhibition in NSCLC cells treated with different doses of gefitinib. Cells were treated with a combination of LC capilliposide (at doses of IC30) and different concentrations of gefitinib for 72 h. Error bars indicate the standard deviation of three independent measurements. (D) Median effect analysis. The combination index (CI) was determined in PC-9-GR cells treated with LC capilliposide and gefitinib. (E) The CI was determined in PC-9 cells treated with LC capilliposide and gefitinib.
Combination treatment of LC capilliposide and gefitinib inhibits cell growth of NSCLC cells with acquired gefitinib-resistance
We next examined the potential effects of LC capilliposide on the growth of four gefitinib-resistant cell lines and one sensitive cell line in response to gefitinib treatment. In this study, we used the IC30 values as the appropriate dose for LC capilliposide treatment, and we found no obvious inhibitions on cell growth for all of the tested cell lines when they were treated with LC capilliposide alone for 72 h. However, we noticed that the presence of LC capilliposide increased the inhibitory effects of gefitinib in these cells, with decreases of the IC50 values from 6.80±1.00 to 0.77±0.12 μmol/L for PC-9-GR, 12.01±2.80 to 2.80±0.30 μmol/L for H1975, 12.75±2.10 to 6.0±0.87 μmol/L for H1299, 15.63±2.53 to 12.5±1.02 μmol/L for H460, and 31.00±6.15 to 11.5±0.92 nmol/L for PC-9 (Figure 1C). Of interest, the concurrent treatment of LC capilliposide and gefitinib caused the most significant decrease of the IC50 value of gefitinib in PC-9-GR, a cell line with acquired resistance to gefitinib treatment that was established after long-term exposure to gefitinib35.
To test whether exposure to LC capilliposide could restore the sensitivity of gefitinib in PC-9-GR cells, we performed median effect analyses. Our results showed that the combination treatment with LC capilliposide and gefitinib, at a constant ratio of corresponding IC50 values for each drug, led to overall CI value of <1.0, indicating a synergistic sensitization effect for LC capilliposide on gefitinib-inhibited cell growth in PC-9-GR cells (Figure 1D). Relatively weak synergism of this sensitization effect was observed in PC-9 cells (Figure 1E).
Thus, our results not only suggested that LC capilliposide could enhance the inhibitory effect of gefitinib on the cell growth of gefitinib-resistant NSCLC cells but also that the potential of LC capilliposide to restore gefitinib sensitivity in NSCLC cells with acquired gefitinib resistance.
LC capilliposide enhances the apoptotic response to gefitinib treatment in PC-9-GR cells
To investigate the potential mechanisms of the enhancing effect of LC capilliposide on the cytotoxicity of gefitinib in PC-9-GR cells, we performed an apoptosis analysis in cells after treatment with 1.2 μg/mL LC capilliposide alone, 0.77 μmol/L gefitinib alone (ie, the IC50 value in the presence of LC capilliposide), or a combination of both drugs. As shown in Figure 2A, we detected a significantly higher percentage (34.6±2.0) of total apoptosis in cells treated with the combination of gefitinib and LC capilliposide for 24 h when compared to that in cells treated with either gefitinib (14.7±1.4) or LC capilliposide alone (14.3±1.3). The combination treatment with gefitinib and LC capilliposide also induced a higher percentage (26.6±1.7) of early apoptosis (versus 8.7±1.0 for gefitinib alone and 7.5±1.2 for LC capilliposide alone) (Figure 2B). These results indicate that LC capilliposide enhances the apoptotic response to gefitinib treatment in PC-9-GR cells.
Effects LC capilliposide on gefitinib-induced apoptosis and cell cycling in PC-9-GR cells. Cells were treated with 1.20 μg/mL of LC capilliposide, 0.77 μmol/L gefitinib, or a combination of the two drugs. (A) Apoptosis analysis. Apoptosis was determined 48 h after treatment using flow cytometry analysis. (B) Graphs showing the changes in apoptosis. *P<0.05. (C) Representative images showing the cell cycle changes in PC-9-GR cells after the indicated treatments. Error bars indicate the standard deviation of three independent measurements.
However, no obvious effect of LC capilliposide was observed on gefitinib-induced G0/G1 arrest in PC-9-GR cells (Figure 2C).
LC capilliposide modulates gefitinib-inhibited EGFR downstream signaling pathways in PC-9-GR cells
We next determined the effects of the combination treatment (gefitinib+LC capilliposide) on activation of the EGFR downstream signaling pathways in PC-9-GR cells. In this experiment, we also used 1 μmol/L of gefitinib, which is the maximum clinical dose, as a treatment option. Our results showed that treatment with gefitinib alone, at both doses of 0.77 and 1 μmol/L, reduced the phosphorylation of EGFR, AKT and ERK1/2 in PC-9-GR cells. The presence of LC capilliposide alone did not elicit any changes for the gefitinib-affected phosphorylation of ERK1/2; however, LC capilliposide further down-regulated the phosphorylation level of AKT in cells when combined with gefitinib (P<0.05, Figure 3A and 3B). The phospho-kinase array (Figure 3C and Supplementary Figure S1) also showed a significant decrease of AKT phosphorylation in PC-9-GR cells treated with the combination of gefitinib and LC capilliposide when compared to that of gefitinib treatment alone. In addition, the array results further revealed that the LC capilliposide treatment could lead to enhanced inhibitory effects of gefitinib on the phosphorylation of WNK1 and PRAS40.
Effects of gefitinib in combination with LC capilliposide on protein phosphorylation. (A) Western blot analysis. (B) Quantitative analysis for the changes in protein phosphorylation. Densitometry of the Western blot signals was conducted, and the intensity of the targeted protein/modification was normalized to the corresponding GAPDH band. Data represent the average results from three independent experiments. *P<0.05. (C) Representative images showing the changes of kinase phosphorylation in the Human Phospho-kinase Array. Select phosphorylated proteins on the kinase array membrane are indicated.
To further evaluate the role of pAKT on the restoration of LC-induced sensitivity to gefitinib in PC-9-GR cells, we decreased the phosphorylation of AKT by knocking down AKT with siRNA transfection. Compared with the PC-9-GR cells with siRNA knockdown of AKT and treated with gefitinib, the addition of LC was unable to increase the sensitivity to gefitinib (Figure 4). Thus, our results indicate that LC exposure can restore the cells' sensitivity to gefitinib through modulation of pAKT levels.
LC capilliposide restores the sensitivity to gefitinib in NSCLC cells with acquired gefitinib resistance through pAKT. (A) PC-9-GR cells were transiently transfected with either AKT siRNA or scramble-siRNA as a control. Western blot results show the inhibitory effect of siRNAs against AKT and pAKT protein in cells collected 72 h after transfection. (B) LC capilliposide was unable to increase the sensitivity to gefitinib when the AKT was knocked down by siRNA.
Effects of LC capilliposide on gefitinib-inhibited tumor growth of PC-9-GR xenografts in vivo
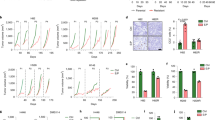
Studies were extended to an in vivo xenograft mouse model. We investigated the potential effect of LC capilliposide on tumor growth of PC-9-GR xenografts in response to gefitinib treatment. Our results showed that treatment with either LC capilliposide (15 mg/kg for 10 d) or gefitinib (50 mg/kg for 10 d) alone could inhibit in vivo PC-9-GR tumor growth with TGIs of 22.6% and 56.6%, respectively. The combination treatment, however, dramatically increased tumor growth suppression (with a TGI of 109.3%) when compared to the treatment with gefitinib or LC capilliposide alone (P <0.05) (Figure 5A). In this experiment, we also measured the mouse body weight to assess the tolerability of systemic therapies, and no obvious body weight changes were observed (Supplementary Figure S2), suggesting that cotreatment with gefitinib and LC capilliposide is well tolerated.
LC capilliposide enhances tumor growth inhibition in response to gefitinib treatment in a PC-9-GR xenograft model. (A) The growth curves represented the average values of 8 mice in each group. Mean±SD. (B) Representative IHC images for the indicated proteins in the xenograft tumors. The scale bar represents 100 μm, and all of the images are to the same scale.
We also performed immunohistochemistry (IHC) to detect the phosphorylation of EGFR and AKT, the cell proliferation marker Ki67 and the apoptotic marker CC3 (cleavage of caspase 3) in tumor specimens that were collected from PC-9-GR xenograft tumors with the indicated treatments. Our results showed that treatment with gefitinib alone suppressed phosphorylation levels of EGFR and AKT. Gefitinib treatment also decreased Ki67 expression in the PC-9-GR xenograft tumors. Treatment with LC capilliposide alone resulted in a significantly reduced phosphorylation level of AKT and also caused slightly decreases of EGFR phosphorylation and Ki67 expression. Of note, the combination treatment further decreased AKT phosphorylation and Ki67 expression; however, it did not cause an obvious change in EGFR phosphorylation when compared to each single treatment in the PC-9-GR xenograft tumors. In addition, we observed an increase of positive CC3 staining in the tumor specimens of the xenograft subjected to the combination treatment (Figure 5B and Supplemental Table 1).
Taken together, our data suggest that LC capilliposide can enhance gefitinib-inhibited AKT signaling and restore the gefitinib sensitivity to NSCLC cells with acquired gefitinib resistance.
Discussion
The empirical criteria for defining clinical subtypes of lung cancer are gradually transiting from histopathology to genetic variations in driver genes. Targeting these driver mutations, such as sensitizing EGFR mutations, has dramatically improved the prognosis of patients with advanced NSCLC. To date, clinical trials have confirmed that EGFR-TKI therapy with first-generation TKIs is superior to chemotherapy as a first-line treatment, with an increase in progression-free survival (PFS) and objective response rate (ORR) of approximately 25% in NSCLC patients with EGFR mutations37,38. However, nearly all patients who experienced a marked response to these agents eventually developed an acquired resistance to TKI therapies, resulting in disease progression. In addition, some patients are intrinsically resistant to EGFR-TKIs even though their tumors harbor activating mutations of EGFR39,40.
There is still much to learn about the molecular causes of resistance to first-generation EGFR-TKI therapy. EGFR activates several well-characterized signal transduction pathways known to be implicated in cell survival and proliferation. If an EGFR mutant cancer can maintain the activity of the downstream signaling pathways in the presence of either gefitinib or erlotinib, this may lead to resistance. Indeed, different “escape pathways” such as MET amplification, HER2, BRAF, AXL, MAPK1, or PIK3CA signaling via point mutations or up-regulation either alone or in conjunction with each other have been found in approximately 22% of tumor specimens from NSCLC patients with acquired resistance41,42,43.
Of note, several preclinical studies have shown that continued activation of AKT downstream signaling is sufficient to confer resistance against EGFR-TKIs. In addition, most, if not all, laboratory models of acquired resistance show continued activation of the AKT pathway despite TKI treatment. Thus, targeting AKT signaling may provide a rationale for novel therapeutic strategies to overcome EGFR-TKI resistance in NSCLC44,45,46,47,48. In this study, we present data showing that LC capilliposide can inhibit AKT activation and restore, at least partially, gefitinib sensitivity to NSCLC cells with acquired gefitinib resistance. In the presence of LC capilliposide, we observed a reduced IC50 for gefitinib in NSCLC cells expressing both wild-type EGFR and different EGFR mutations, indicating that LC capilliposide could sensitize a wide range of NSCLC cells to gefitinib treatment. However, we also noticed that the enhancing effect of LC capilliposide on gefitinib-induced cell death was limited in H460 cells, which contains wild-type EGFR and mutant KRAS. It should be indicated that a previous study revealed that EGFR-TKI acts on EGFR mainly through the downstream PI3K/AKT and MEK/ERK signaling in cells expressing wild-type KRAS, while EGFR TKIs may also act on other downstream pathways in addition to the PI3K/AKT and MEK/ERK pathways in cells harboring mutant KRAS47,49,50. Of note, our results also showed that exposure to LC capilliposide inhibits the activation of several other kinases, including WNK1, in gefitinib-treated PC-9-GR cells. WNK1 was reported to be required for EGF-dependent stimulation of ERK5 by protein-protein interactions to assemble an ERK5 activation complex and to act as an upstream regulator of the ERK5 pathway without affecting the activation of the ERK1/2, JNK or p38 MAP kinases51,52. The down-regulation of WNK1 activation may indicate that other AKT-independent pathways could also play roles in the observed synergistic effects of LC capilliposide on cell growth inhibition and cell apoptosis. Thus, these results suggested that the potential therapeutic effects of LC capilliposide when combined with gefitinib may depend on the genetic alterations of NSCLC cells.
Recently, Chinese herbal medicine has attracted increasing attention due to its effects on multidrug resistance for cancer therapy53,54. Our data presented here suggest a potential clinical impact of the therapeutic strategy with a regimen including LC capilliposide for NSCLC patients who failed in TKI therapy or have acquired TKI resistance. The combination treatment of gefitinib with LC capilliposide may also benefit NSCLC patients receiving TKI treatment as a first-line therapy. However, further studies are needed to reveal the detailed mechanisms and the epigenetics of NSCLC cells regarding the therapeutic effects of combining LC capilliposide with a first-generation TKI.
Author contribution
Shi-rong ZHANG, Fan-zhu LI, and Sheng-lin MA designed the research; Shi-rong ZHANG, Ya-si XU, and Er JIN performed the research; Lu-cheng ZHU and Bing XIA analyzed the data; and Shi-rong ZHANG and Xu-feng CHEN wrote the paper.
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30.
Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest 2005; 128: 452–62.
O'Rourke N, Macbeth F . Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer? A review of guidelines and evidence. Clin Oncol (R Coll Radiol) 2010; 22: 347–55.
Socinski MA, Evans T, Gettinger S, Hensing TA, Sequist LV, Ireland B, et al. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e341S–68S.
Bradley CJ, Given CW, Roberts C . Disparities in cancer diagnosis and survival. Cancer 2001; 91: 178–88.
Bazley LA, Gullick WJ . The epidermal growth factor receptor family. Endocr Relat Cancer 2005; 12 Suppl 1: S17–27.
Wells A . EGF receptor. Int J Biochem Cell Biol 1999; 31: 637–43.
Arteaga CL . Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol 2002; 29: 3–9.
Yarden Y . The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001; 37 Suppl 4: S3–8.
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba, II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97: 339–46.
Riely GJ, Politi KA, Miller VA, Pao W . Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res 2006; 12: 7232–41.
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 2003; 21: 2237–46.
Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 2004; 22: 3238–47.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353: 123–32.
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005; 366: 1527–37.
Gazdar AF . Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009; 28 Suppl 1: S24–31.
Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol 2012; 30: 433–40.
Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, Tsuboi M, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 2006; 12: 5764–9.
Shih JY, Gow CH, Yang PC . EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 2005; 353: 207–8.
Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2005; 23: 2493–501.
Hirsh V . Next-generation covalent irreversible kinase inhibitors in NSCLC: focus on afatinib. BioDrugs 2015; 29: 167–83.
Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012; 343: 342–50.
Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34.
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22.
Engel J, Lategahn J, Rauh D . Hope and disappointment: covalent inhibitors to overcome drug resistance in non-small cell lung cancer. ACS Med Chem Lett 2016; 7: 2–5.
Barf T, Kaptein A . Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem 2012; 55: 6243–62.
Tan CS, Gilligan D, Pacey S . Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol 2015; 16: e447–59.
Yver A . Osimertinib (AZD9291)–a science-driven, collaborative approach to rapid drug design and development. Ann Oncol 2016 2016; 27: 1165–70.
Liang B, Zhang L, Tian J, Xu L, Yang S . Isolation and characterization of two new saponins from Lysimachia capillipes. Carbohydr Res 2006; 341: 2444–8.
Tian JK, Xu LZ, Zou ZM, Yang SL . Two new triterpene saponins from Lysimachia capillipes. J Asian Nat Prod Res 2006; 8: 439–44.
Xu Y, Rong Y, Liu X, Ying HM . Experimental studies on anti-tumor effects of capilliposide. Chin Pharmacol Bull 2012; 28: 545–9.
Tian JK, Xu LZ, Zou ZM, Yang SL . Three novel triterpenoid saponins from Lysimachia capillipes and their cytotoxic activities. Chem Pharm Bull (Tokyo) 2006; 54: 567–9.
Ying H, Qi Z, Guo D, Yu T, Zhang L, Tian J . Quantitative determination of capilliposide B and capilliposide C in Lysimachia capillipes by HPLC-ELSD assay. Chin Pharm J 2011; 46: 704–6.
Fei ZH, Wu K, Chen YL, Wang B, Zhang SR, Ma SL . Capilliposide isolated from Lysimachia capillipes Hemsl induces ROS generation, cell cycle arrest, and apoptosis in human nonsmall cell lung cancer cell lines. Evid Based Complement Alternat Med 2014; 2014: 497456.
Zhang S, Zheng X, Huang H, Wu K, Wang B, Chen X, et al. Afatinib increases sensitivity to radiation in non-small cell lung cancer cells with acquired EGFR T790M mutation. Oncotarget 2015; 6: 5832–45.
Kahan BD, Kramer WG . Median effect analysis of efficacy versus adverse effects of immunosuppressants. Clin Pharmacol Ther 2001; 70: 74–81.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57.
Mitsudomi T, Yatabe Y . Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007; 98: 1817–24.
Takeda M, Okamoto I, Fujita Y, Arao T, Ito H, Fukuoka M, et al. De novo resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR mutation-positive patients with non-small cell lung cancer. J Thorac Oncol 2010; 5: 399–400.
Pallis A, Briasoulis E, Linardou H, Papadimitriou C, Bafaloukos D, Kosmidis P, et al. Mechanisms of resistance to epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small-cell lung cancer: clinical and molecular considerations. Curr Med Chem 2011; 18: 1613–28.
Yamada T, Matsumoto K, Wang W, Li Q, Nishioka Y, Sekido Y, et al. Hepatocyte growth factor reduces susceptibility to an irreversible epidermal growth factor receptor inhibitor in EGFR-T790M mutant lung cancer. Clin Cancer Res 2010; 16: 174–83.
Kono SA, Marshall ME, Ware KE, Heasley LE . The fibroblast growth factor receptor signaling pathway as a mediator of intrinsic resistance to EGFR-specific tyrosine kinase inhibitors in non-small cell lung cancer. Drug Resist Updat 2009; 12: 95–102.
Gadgeel SM, Wozniak A . Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer 2013; 14: 322–32.
Huang L, Fu L . Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015; 5: 390–401.
Engelman JA, Janne PA . Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008; 14: 2895–9.
Li H, Schmid-Bindert G, Wang D, Zhao Y, Yang X, Su B, et al. Blocking the PI3K/AKT and MEK/ERK signaling pathways can overcome gefitinib-resistance in non-small cell lung cancer cell lines. Adv Med Sci 2011; 56: 275–84.
Niu FY, Wu YL . Novel agents and strategies for overcoming EGFR TKIs resistance. Exp Hematol Oncol 2014; 3: 2.
Samatar AA, Poulikakos PI . Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov 2014; 13: 928–42.
Rosell R, Karachaliou N, Morales-Espinosa D, Costa C, Molina MA, Sansano I, et al. Adaptive resistance to targeted therapies in cancer. Transl Lung Cancer Res 2013; 2: 152–9.
Moniz S, Jordan P . Emerging roles for WNK kinases in cancer. Cell Mol Life Sci 2010; 67: 1265–76.
Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, et al. Properties of WNK1 and implications for other family members. J Biol Chem 2005; 280: 26653–8.
Hsiao WL, Liu L . The role of traditional Chinese herbal medicines in cancer therapy — from TCM theory to mechanistic insights. Planta Med 2010; 76: 1118–31.
Parekh HS, Liu G, Wei MQ . A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer 2009; 8: 21.
Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province (LY15H160010), the Medical Science Foundation of Zhejiang Province (2014KYA178), the Hangzhou Key Disease and Discipline Foundation (20140733Q15), and the Science and Technology Project of Zhejiang Provincial Bureau (2015ZA133).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
The phospho-kinase array also showed a significant decrease of AKT phosphorylation in PC-9-GR cells treated with the combination of gefitinib and LC capilliposide when compared to that of gefitinib treatment alone. (JPG 156 kb)
Supplementary Figure S2
Cotreatment with gefitinib and LC capilliposide had no obvious effects on the body weight of mice with PC-9-GR xenografts. (JPG 82 kb)
Supplementary Table S1
IHC analysis of tumor sections showing modulation of pEGFR, Ki67, CC3, and pAKT treated with LC capilliposide and gefitinib. (DOC 16 kb)
Rights and permissions
About this article
Cite this article
Zhang, Sr., Xu, Ys., Jin, E. et al. Capilliposide from Lysimachia capillipes inhibits AKT activation and restores gefitinib sensitivity in human non-small cell lung cancer cells with acquired gefitinib resistance. Acta Pharmacol Sin 38, 100–109 (2017). https://doi.org/10.1038/aps.2016.116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.116