Abstract
Aim:
IL-37b has shown anti-cancer activities in addition to its anti-inflammatory properties. In this study, we investigated the effects of IL-37b on breast carcinoma growth in mice and to determine the involvement of T cell activation in the effects.
Methods:
IL-37b gene was transferred into mouse breast carcinoma cell line 4T1 (4T1-IL37b cells), the expression of secretory IL-37b by the cells was detected, and the effects of IL-37b expression on the cell proliferation in vitro was evaluated. After injection of 4T1 cells or 4T1-IL37b cells into immunocompetent BALB/c mice, immunodeficient BALB/c nude mice and NOD-SCID mice, the tumor growth and survival rate were measured. The proliferation of T cells in vitro was also detected.
Results:
IL-37b was detected in the supernatants of 4T1-IL37b cells with a concentration of 12.02±0.875 ng/mL. IL-37b expression did not affect 4T1 cell proliferation in vitro. BALB/c mice inoculated with 4T1-IL37b cells showed significant retardation of tumor growth. BALB/c mice inoculated with both 4T1 cells and mitomycin C-treated 4T1-IL37b cells also showed significant retardation of tumor growth. But the anti-cancer activity of IL-37b was abrogated in BALB/c nude mice and NOD-SCID mice inoculated with 4T1-IL37b cells. Recombinant IL-37b slightly promoted CD4+ T cell proliferation without affecting CD8+ T cell proliferation.
Conclusion:
IL-37b exerts anti-4T1 breast carcinoma effects in vivo by modulating the tumor microenvironment and influencing T cell activation.
Similar content being viewed by others
Introduction
IL-37 (formerly IL-1F7), a cytokine in the IL-1 family, is expressed in a variety of normal tissues and tumors in humans, but a mouse homolog has not been identified. Among the various splicing forms of IL-37, IL-37b has been extensively studied1. Although the receptor and signaling pathway of IL-37 have not been clearly defined, it is known to function as a non-specific inhibitor of inflammation. Transgenic expression of IL-37b suppresses the production of pro-inflammatory mediators, such as IL-6 and IL-1β, in RAW macrophages, peripheral blood mononuclear cells (PBMCs), and dendritic cells (DCs)2. The use of siRNA to reduce the synthesis of the IL-37 protein in PBMCs leads to the increased production of several pro-inflammatory mediators, including IL-1α, IL-1β, IL-6, IL-12, G-CSF, GM-CSF, and TNF-α3. LPS challenge of IL-37b transgenic mice has not been found to induce significant circulating or organ levels of inflammatory cytokines (IL-1β, IL-6, IL-17, IFN-γ, etc), and the activation of DCs and macrophages are also suppressed3.
In a dextran sodium sulfate (DSS)-induced colitis model, the severity of intestinal inflammation has been shown to be significantly lower in IL-37b transgenic mice compared with wild-type controls4. The effects of IL-37b are at least partially mediated by Smad3 because two-dimensional gel electrophoresis and mass spectrometry have shown that IL-37 bound to Smad3 and anti-Smad3 siRNAs can reverse the suppression of inflammatory responses in transgenic mice3,5.
In addition to its anti-inflammatory properties, IL-37b demonstrates anti-tumor activities. Gao et al have found that intratumoral injection of an IL-37b-expressing adenovirus results in the dramatic growth suppression of MCA205 mouse fibrosarcoma. The anti-tumor activity of IL-37b has been shown to be abrogated in nude and SCID mice and in IL-12-, IFN-γ-, and Fas ligand-deficient mice6. These results suggest that IL-37 could play an important role in boosting anti-tumor adaptive immunity. An additional study has demonstrated that the high expression of IL-37 in primary hepatocellular carcinoma (HCC) tissues, as shown by immunohistochemistry, is associated with better overall survival and is positively associated with the density of tumor-infiltrating CD57+ natural killer (NK) cells7.
Despite the two aforementioned reports, the mechanisms by which IL-37b exerts anti-tumor effects have not been completely resolved, and in particular, the role of T cells has not been clearly defined. Thus, we evaluated the anti-tumor effects of IL-37b against breast carcinoma and explored the involvement of T cells in these effects by in vitro and in vivo experiments.
Materials and methods
Animal and tumor cell lines
Immunocompetent BALB/c mice that were 6–8 weeks old were purchased from the Institute of Hematology, Chinese Academy of Medical Sciences (Tianjin, China). Six- to eight-week-old BALB/c nude mice and NOD-SCID mice were purchased from Vital River Co, Ltd (Beijing, China). All mice were maintained in specific pathogen-free barrier facilities at the Institute of Hematology, Chinese Academy of Medical Sciences. All animal experiments were conducted according to the guidelines of the Animal Care and Use Committee of the Institute of Hematology, Chinese Academy of Medical Sciences. 4T1 is a murine breast carcinoma cell line, and it was maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 50 mmol/L β-mercaptoethanol (β-ME), 100 IU/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2/air.
Preparation of recombinant adenovirus
For the construction of a recombinant adenoviral vector expressing IL-37b (Ad-IL-37b), IL-37b cDNA, including the Kozak sequence (CCACC) and ATG at the 5′ end, which was cloned by PCR, was inserted into a pDC315-eGFP vector under the control of the mouse cytomegalovirus (CMV) promoter. pDC315-X and pBHGlox E1, 3Cre8,9 were co-transfected into HEK293 cells using LipoFiterTM transfection reagent purchased from Hanbio Co, Ltd (Shanghai, China) to generate recombinant adenoviruses. Ad-IL37b and Ad-eGFP (control virus) were propagated in HEK293 cells, and virus titers were measured with plaque assays by Hanbio Co, Ltd. The stock solutions of Ad-IL37b and Ad-eGFP were both 1×1010 plaque formation units (PFUs)/mL and were stored at −80 °C.
Transduction of tumor cells
4T1 cells cultured to 80% confluence were incubated with Ad-IL37b or Ad-eGFP at a multiplicity of infection (MOI) of 100 in RPMI-1640 medium (without FCS) for 2 h. Then, the media containing viruses was replaced by complete RPMI-1640 (with 10% FCS), and the 4T1 cells continued to be cultured for 48 to 72 h. Transfection efficiency was determined by fluorescence microscopy and flow cytometry. The 4T1 cells transduced with Ad-IL37b or Ad-eGFP were termed 4T1-IL37b and 4T1-eGFP, respectively.
IL-37b expression in 4T1 cells
After infection of Ad-IL37b, RPMI-1640 medium with 10% FCS was used for the continued culturing of the 4T1 cells in 1 mL media in each well of 12-well plates. The cells and supernatants were harvested at 48 h and 72 h later, respectively. Total RNA was extracted with TRIzol reagent, and real-time PCR was performed to determine the mRNA expression of IL-37b. The primers used to detect IL-37b were as follows: forward 5′-GGGAGTTTTGTCTCTACTGTGAC-3′ and reverse 5′-CCCACCTGAGCCCTATAAAAG-3′. IL-37b activity in the supernatants was measured using an sandwich enzyme-linked immunosorbent assay (ELISA) kit developed by R&D Systems (Minneapolis, MN, USA).
Treatment of 4T1-IL37b or 4T1-eGFP with mitomycin-C
The treatment of 4T1 cells with mitomycin-C has been described elsewhere10. Briefly, at 48 h after adenovirus transduction, 4T1-IL37b or 4T1-eGFP cells were treated with 10 μg/mL mitomycin-C (Sigma, London, UK) for 2 h at 37 °C. Then, the media containing mitomycin-C was replaced with complete media. The amounts of IL-37b in the supernatants were detected by ELISA at 24 h after the mitomycin-C treatment. In addition, 1×105 mitomycin-C-treated 4T1-eGFP or 4T1-IL37b (M-4T1-eGFP or M-4T1-IL37b) cells were injected into the intramammary gland fat pad in the right flank of the BALB/c mice to confirm the complete arrest of cell growth by mitomycin-C.
Cell proliferation assay
To compare the in vitro proliferation of 4T1 sublines transfected with or without adenovirus, 1×104 cells from each cell line were seeded in each well of 24-well plates in 500 μL of culture medium, and the cells were enumerated in triplicate.
The T cell proliferation assay was similar to the procedure previously described by our research group11. Briefly, CD4+ or CD8+ T cells were isolated from lymph node cells with a Dynabeads FlowComp™ Mouse CD4 or CD8 Kit (Invitrogen, Carlsbad, CA, USA). CD4+ or CD8+ T cells labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) were stimulated by an anti-CD3 antibody (BD PharMingen, San Jose, CA, USA), without or with addition of 200 ng/mL of recombinant IL-37b (R&D Systems). Cell size, which was examined by FSC, T cell proliferation, which was evaluated by CFSE, and expression of CD25 and CD69, which was detected with anti-CD25 and anti-CD69 antibodies, respectively, were assessed by flow cytometric analysis (LSRII, BD PharMingen) at 72 h. The data were analyzed by FlowJo software (Treestar).
Tumourigenesis studies
A total of 1×105 4T1, 4T1-eGFP, or 4T1-IL37b cells were injected into the intramammary gland fat pad of BALB/c or NOD-SCID mice and simultaneously into the right flank of BALB/c nude mice. In some experiments, 1×105 4T1 cells were co-injected with 1×105 mitomycin-C-treated 4T1-eGFP or 4T1-IL37b into the intramammary gland fat pad in the right flank of BALB/c mice. Tumor sizes were measured in millimeters with a caliper at various time points. The longest surface length (a) and its perpendicular width (b) were measured, and tumor volume was reported as 0.5×a×b2.
Statistical analysis
All statistical tests were performed using GraphPad Prism software. Unpaired Student's t-tests were used to statistically evaluate differences in sample mean values between two groups. For comparisons of more than two groups, we used one-way ANOVA with Tukey's post hoc test. The survival rates were analyzed using the log-rank (Mantel-Cox) test. Differences were considered significant at a P<0.05. The data are presented as the mean±SEM.
Results
IL-37b production by 4T1 cells transduced with Ad-IL37b
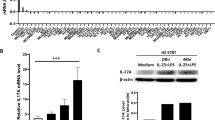
Ad-IL37b infections of 4T1 cells with an MOI of 100 showed the 78% positive expression of eGFP without apparent cytotoxicity (data not shown). IL-37b mRNA expression in the 4T1-IL37b cells was confirmed by real-time PCR (data not shown). The 4T1-IL37b cells secreted detectable amounts of IL-37b (approximately 12.02 ±0.875 ng/mL) into the supernatant (Figure 1A). Similar amounts of IL-37b (approximately 12.08 ±0.965 ng/mL) were detected in the supernatant of the mitomycin-C treated 4T1-IL37b cells (data not shown).
Expression of IL-37b did not affect 4T1 breast carcinoma cell growth in vitro. (A) A total of 2×105 4T1 cells were transduced with Ad-IL37b in each well of 12-well plates. At 48 and 72 h after virus transduction, the amounts of IL-37b in the culture supernatants were detected by ELISA. (B) Cells (1×104) from each subline were seeded in 48-well plates, and cell numbers were counted in triplicate at 24, 36, 48, 60, and 72 h. Mean±SEM. n=3.
To examine whether there was a difference in the proliferation of the three 4T1 sublines (4T1, 4T1-IL37b, and 4T1-eGFP), we performed in vitro cell proliferation assays. There were no significant differences in cell proliferation among 4T1, 4T1-IL37b, and 4T1-eGFP (Figure 1B).
Tumor growth of 4T1-IL37b cells in BALB/c mice
At 48 h after adenovirus transduction, 1×105 4T1, 4T1-eGFP, and 4T1-IL37b cells were injected into the mammary fat pads in the right flank of each mouse. 4T1 and 4T1-eGFP showed similar degrees of tumor growth. 4T1-IL37b showed a significant retardation of tumor growth compared with 4T1 or 4T1-eGFP (Figure 2A). The tumor volume and weight of the 4T1-IL37b group were significantly smaller than those of the 4T1-eGFP group (Figure 2C–2E). However, the survival rate was not significantly different among the three groups (data not shown).
4T1-IL37b showed retarded tumor growth in immunocompetent BALB/c mice. (A) Groups of five BALB/c mice were challenged with 4T1, 4T1-eGFP and 4T1-IL37b. Mice were injected with 1×105 cells into the intramammary gland fat pad in the right flank on d 0. Tumor size was measured every seven days starting on d 7 after tumor cell injection. (B–D) Groups of four BALB/c tumor models of 4T1-eGFP and 4T1-IL37b were euthanized on d 14, and tumors were excised. Then, the tumor volumes and weights were measured. Mean±SEM. n=3. cP<0.01.
Effect of mitomycin-C-treated 4T1-IL37b cells on tumor growth of 4T1 cells in BALB/c mice
To further demonstrate whether IL-37b suppresses tumor growth via affecting the tumor microenvironment or directly by affecting the tumor cells, we co-injected 1×105 mitomycin-C-treated 4T1-IL37b cells (M-4T1-IL37b) with 1×105 4T1 cells into the mammary fat pads of BALB/c mice and observed tumor growth. The mice that were injected with M-4T1-eGFP or M-4T1-IL37b did not develop tumors, indicating that the growth of the cells was completely arrested by mitomycin-C (data not shown). Co-injection with M-4T1-IL37b significantly suppressed 4T1 tumor growth (Figure 2B), but it did not significantly improve survival (data not shown).
Effect of recombinant IL-37b on T cell proliferation in vitro
To evaluate whether the direct activation of T cells is involved in the anti-tumor effect of IL-37b, an in vitro T cell proliferation assay was conducted by adding recombinant IL-37b to a culture of T cells stimulated with anti-CD3. After treatment with recombinant IL-37b for 72 h, the proliferation of CD4+ T cells was found to be promoted, as shown by FSC and CFSE. Slight increases in the expression levels of CD25 and CD69 on the CD4+ T cells were also observed following the treatment with recombinant IL-37b (Figure 4). However, the efficacy of the recombinant IL-37b in promoting isolated CD4+ T cell proliferation varied under the different experimental conditions (it was sometimes a little weaker, data not shown). The recombinant IL-37b did not notably impact the proliferation of the CD8+ T cells (Figure 4).
Recombinant IL-37b promoted CD4+ but not CD8+ T cell proliferation in vitro. Isolated CD4+ or CD8+ T cells from lymphocytes were stimulated with an anti-CD3 antibody for 72 h, with or without the addition of recombinant IL-37b. Cell size measured by FSC, the CFSE proliferation profile, and the expression of CD25 and CD69 were analyzed by flow cytometry. The data are representative of four independent experiments.
Tumor growth of 4T1-IL37b in BALB/c nude and NOD/SCID mice
To confirm the involvement of T cells in vivo, T cell-deficient BALB/c nude mice were used to test the tumor growth of 4T1, 4T1-eGFP and 4T1-IL37b cells. The tumor growth of these three 4T1 sublines showed no significant difference (Figure 5A). Moreover, tumor growth was observed in the NOD/SCID mice, which were deficient not only in functional T and B cells but also in innate immunity, exhibiting the damaged functioning of NK cells, macrophages and antigen-presenting cells (APCs). Soon after the growth of tumors to a measureable size on approximately d 12, the mice died rapidly. Therefore, we compared the tumor size at one time point on d 12. The tumor sizes of the three 4T1 sublines showed no significant difference (Figure 5B).
4T1-IL37b did not show retarded tumor growth in BALB/c nude and NOD/SCID mice. (A) Because BALB/c nude mice do not have mammary glands, they (seven in each group) were injected subcutaneously with tumor cells (1×105) into the right flank on d 0. (B) Groups of seven NOD/SCID mice were injected with tumor cells as described previously. Tumor volume was measured on d 12. Mean±SEM. n=2.
Discussion
IL-37 is expressed in a variety of normal tissues, inflammatory diseases and tumors3,12,13,14,15. Interestingly, IL-37 protein expression is upregulated in human breast carcinoma tissues16. This finding suggests that it is involved in both inflammatory responses and tumor progression. In the IL-1 family, IL-18 is a potent pro-inflammatory cytokine that induces the production of IFN-γ. A series of studies have demonstrated that IL-18 exhibits anti-tumor activities17,18,19,20, which is logical because of its pro-inflammatory properties. Although IL-37 shows anti-inflammatory properties, it also exhibits anti-tumor activity, of which the underlying mechanism is seemingly more complex. To date, only two published studies have demonstrated the anti-tumor activity of IL-37 in fibrosarcoma and hepatocellular carcinoma, but the underlying mechanism remains to be fully elucidated6,7.
In the present study, we used mouse 4T1 breast carcinoma models to study the anti-breast cancer effect of IL-37b and to explore whether T cells are involved in its anti-tumor mechanisms. First, an IL-37b-expressing adenoviral vector was constructed and transduced into the 4T1 cell line. IL-37b was successfully expressed inside of the 4T1 cells and secreted into the culture supernatants. Because IL-37b also functions inside of cells as a signaling molecule, it is possible that it directly affects 4T1 cell proliferation. However, this possibility was ruled out because we found that transduced IL-37b expression did not affect cell proliferation. IL-37b-expressing 4T1 cells were injected into the intramammary gland fat pads of immunocompetent BALB/c mice, resulting in significantly slower tumor growth compared with the 4T1 or 4T1-eGFP cells, confirming the anti-tumor activity of IL-37b.
To further clarify the anti-tumor effects of IL-37b in the tumor microenvironment, we co-injected mitomycin-C-treated 4T1-IL37b cells with 4T1 cells into immunocompetent BALB/c mice and observed tumor growth. Because the growth of mitomycin-C-treated 4T1-IL37b cells was completely arrested, these cells did not develop tumors, but they secreted IL-37b into the tumor microenvironment. Co-injection with mitomycin-C-treated 4T1-IL37b cells significantly suppressed 4T1 tumor growth in the BALB/c mice. Considering the abovementioned findings that IL-37b did not affect 4T1 cell proliferation, these results collectively indicate that IL-37b exerts anti-tumor effects through influencing the tumor microenvironment rather than directly affecting 4T1 cells.
Although IL-37b slowed 4T1 tumor growth in the tumor microenvironment, the survival rate was not significantly improved in the mouse models of either 4T1-IL37b or of the co-injection of 4T1 with mitomycin-C-treated 4T1-IL37b (data not shown). 4T1 mammary tumors are highly metastatic. Because the survival of mouse models is directly related to tumor metastasis, we postulate that IL-37b suppresses tumor growth but has no or little effect on tumor metastasis. However, in this study, we did not evaluate metastasis in some important organs, such as the lung, heart, liver and kidney, following the deaths of the mice.
The observation of the anti-tumor efficacy of IL-37b in the BALB/c mice prompted us to test whether it could directly affect the function of T cells, which have a central role in anti-tumor activity. In fact, IL-37b binds to the IL-18Rα chain and belongs to the IL-18 subfamily21; thus, we postulated that the function of IL-37b could be similar to that of IL-18 and involve the stimulation of the proliferation of T cells22. Our results showed that IL-37b directly stimulated CD4+ T cell activation and proliferation in vitro. However, the efficacy of IL-37b stimulation was fairly weak and varied occasionally, which may have been related to the number of prepared isolated CD4+T cells and concentration of stimulating anti-CD3 antibody (the titer decreased as storage time was extended). Overall, when the CD4+T cell concentration was lower and anti-CD3 stimulation was weaker, the stimulatory effect of IL-37b was stronger, suggesting that the strong activation of T cells with IL-2 (a major T cell growth factor produced by T cells themselves upon anti-CD3 stimulation) in cultures may have overridden the effects of IL-37b. Strikingly, IL-37b did not have an obvious direct effect on CD8+T cells.
Increasing evidence has demonstrated that CD4+ T cells contribute to tumor eradication, even in the absence of CD8+ T cells23. Because the direct effect of IL-37b on CD4+ T cells was weak, the direct activation of T cells might play a minor role in its suppression of tumor growth in the tumor microenvironment. However, given that its anti-tumor effect has been shown to be T cell-dependent in ours and others' study6, the indirect activation of T cells might play a major role in its anti-tumor effect in the tumor microenvironment. It has been demonstrated that the anti-tumor activity of IL-37b is also IL-12, IFN-γ, and Fas ligand-dependent6. IL-12 shows potent anti-tumor activity by acting as a major orchestrator of the Th1-type immune response against cancer24. IFN-γ is mainly produced by activated Th1 and CD8+ T cells and exerts anti-tumor effects by affecting the STAT1 signaling pathway25. Through inducing cell death, the Fas ligand helps to remove tumor cells. It has been reported that the T-cell receptor complex is essential for Fas signal transduction26. Therefore, IL-37b might indirectly activate T cells as mediated by IL-12, IFN-γ, and the Fas ligand to suppress tumor growth.
Because no effect of IL-37b on tumor growth was noted in the nude mice which preserve normal NK cell function, it implied that NK cells were not (or were not directly) involved in the anti-tumor activity of IL-37b, which is not consistent with a previous study describing its anti-tumor effect against hepatocellular carcinoma by Zhao et al7. We suggest that it may be a consequence of the different type of cancer studied by this group.
Finally, we observed the tumor growth of 4T1-IL37b in NOD-SCID mice, which were deficient in not only functional T and B cells but also innate immune components, such as NK cells, macrophages and APCs. The tumor growth of IL-37b-expressing 4T1 cells showed no difference compared with that of the 4T1 and 4T1-eGFP cells. Most of the NOD-SCID mice died after day 14 post-tumor cell inoculation, and the survival rate was apparently lower than that of the BALB/c nude mice. This finding indicates that innate immunity plays a key role in controlling tumor growth and metastasis, despite the fact that IL-37b seems to mainly exert anti-tumor functions via T cells.
In conclusion, our results indicate that IL-37b is capable of exerting anti-breast tumor activity by modulating the tumor microenvironment and that T cells are essential to its anti-tumor mechanisms. However, the indirect, but not the direct activation of T cells might play a major role in the suppression of tumor growth by IL-37b in the tumor environment. The molecular details of its effects on T cells need further investigation. This research highlights the potential usage of IL-37b gene/protein therapy in the future treatment of breast cancer in the clinic and provides insights for further research in this field.
Author contribution
Bang-mao WANG, Jing-xian YANG, and Xiao-ming FENG designed the study; Wei-qiang WANG, Dan ZHAO, Yu-shan ZHOU, Xiao-yu HU, Zhi-na SUN, Gang YU, Wan-tong WU, Song CHEN, Jiu-long KUANG, and Guo-gang XU conducted the experiment; Wei-qiang WANG and Dan ZHAO analyzed the data; and Wei-qiang WANG, Dan ZHAO, Zhong-chao HAN, and Xiao-ming FENG wrote the paper.
M-4T1-IL37b suppressed 4T1 tumor growth in immunocompetent BALB/c mice. Groups of five BALB/c mice were challenged with 1×105 4T1, 1×105 4T1+1×105 M-4T1-eGFP, or 1×105 4T1+1×105 M-4T1-IL37b. Tumor cell injections and tumor volume measurements were conducted as described previously. Mean±SEM. n=3. bP<0.05, cP<0.01.
References
Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw 2011; 22: 127–47.
Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, Mansell A, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci U S A 2014; 111: 2650–5.
Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA . IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010; 11: 1014–22.
McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 2011; 108: 16711–6.
Grimsby S, Jaensson H, Dubrovska A, Lomnytska M, Hellman U, Souchelnytskyi S . Proteomics-based identification of proteins interacting with Smad3: SREBP-2 forms a complex with Smad3 and inhibits its transcriptional activity. FEBS Lett 2004; 577: 93–100.
Gao W, Kumar S, Lotze MT, Hanning C, Robbins PD, Gambotto A . Innate immunity mediated by the cytokine IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive and profound antitumor immunity. J immunol 2003; 170: 107–13.
Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep 2014; 4: 5177.
Ng P, Parks RJ, Cummings DT, Evelegh CM, Sankar U, Graham FL . A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther 1999; 10: 2667–72.
Ng P, Parks RJ, Cummings DT, Evelegh CM, Graham FL . An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther 2000; 11: 693–9.
Abdul Hafid SR, Chakravarthi S, Nesaretnam K, Radhakrishnan AK . Tocotrienol-adjuvanted dendritic cells inhibit tumor growth and metastasis: a murine model of breast cancer. PloS One 2013; 8: e74753.
Hu X, Zhou Y, Dong K, Sun Z, Zhao D, Wang W, et al. Programming of the development of tumor-promoting neutrophils by mesenchymal stromal cells. Cell Physiol Biochem 2014; 33: 1802–14.
Pan G, Risser P, Mao W, Baldwin DT, Zhong AW, Filvaroff E, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 2001; 13: 1–7.
Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem 2000; 275: 10308–14.
Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA . Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J 2004; 381: 503–10.
Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol 2013; 172: 410–6.
Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 2002; 18: 61–71.
Chen ZF, Zhou R, Xia B, Deng CS . Interleukin-18 and -12 synergistically enhance cytotoxic functions of tumor-infiltrating lymphocytes. Chin Med J (Engl) 2012; 125: 4245–8.
Du G, Ye L, Zhang G, Dong Q, Liu K, Tian J . Human IL18-IL2 fusion protein as a potential antitumor reagent by enhancing NK cell cytotoxicity and IFN-gamma production. J Cancer Res Clin Oncol 2012; 138: 1727–36.
Tse BW, Russell PJ, Lochner M, Forster I, Power CA . IL-18 inhibits growth of murine orthotopic prostate carcinomas via both adaptive and innate immune mechanisms. PloS One 2011; 6: e24241.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71: 5393–9.
Dinarello CA . Overview of the interleukin-1 family of ligands and receptors. Semin Immunol 2013; 25: 389–93.
Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995; 378: 88–91.
Haabeth OA, Tveita AA, Fauskanger M, Schjesvold F, Lorvik KB, Hofgaard PO, et al. How Do CD4(+) T cells detect and eliminate tumor cells that either lack or express MHC class II molecules? Front Immunol 2014; 5: 174.
Lasek W, Zagozdzon R, Jakobisiak M . Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother 2014; 63: 419–35.
Bekisz J, Sato Y, Johnson C, Husain SR, Puri RK, Zoon KC . Immunomodulatory effects of interferons in malignancies. J Interferon Cytokine Res 2013; 33: 154–61.
Akimzhanov AM, Wang X, Sun J, Boehning D . T-cell receptor complex is essential for Fas signal transduction. Proc Natl Acad Sci U S A 2010; 107: 15105–10.
Acknowledgements
This work was supported by the National Basic Research Program of China (2013CB966904, 2015CB964402), the National Natural Science Foundation of China (81421002, 81273217, 81322007, 81170007), the Tianjin Research Program of Application Foundation and Advanced Technology (12JCYBJC32800), the Technology Foundation for Selected Overseas Chinese Scholars, the Fok Ying-Tong Education Foundation of China (131039), and the Recruitment Program of Global Youth Experts.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Wq., Zhao, D., Zhou, Ys. et al. Transfer of the IL-37b gene elicits anti-tumor responses in mice bearing 4T1 breast cancer. Acta Pharmacol Sin 36, 528–534 (2015). https://doi.org/10.1038/aps.2015.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2015.3
Keywords
This article is cited by
-
The novel role of IL-37 in prostate cancer: evidence as a promising radiosensitizer
Medical Oncology (2018)
-
The role of IL-37 in cancer
Medical Oncology (2016)
-
IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice
Acta Pharmacologica Sinica (2015)