Abstract
Aim:
Considering the characterization of vitamin D deficiency as a risk factor of ectopic fat deposition, the association of serum 25-hydroxy vitamin D3 [25(OH)D3] levels with non-alcoholic fatty liver disease (NAFLD) was evaluated in Chinese men with normal body mass index (BMI) and enzyme markers of liver function.
Methods:
A total of 514 participants (22 to 79 years old) with normal BMI and liver enzymes were identified for analysis. Abdominal ultrasound was performed to diagnose NAFLD, and the fatty liver index (FLI) was calculated to quantify liver steatosis. Serum 25(OH)D3 levels were determined by an electrochemiluminescence immunoassay.
Results:
Among the entire study population, the mean levels of serum 25(OH)D3 were 15.32±5.77 ng/mL. However, when serum 25(OH)D3 levels were compared between non-NAFLD subjects (n=438) and NAFLD subjects (n=76), the latter showed significantly lower levels (15.65±5.89 ng/mL vs 13.46±4.65 ng/mL, P=0.002). In addition, serum 25(OH)D3 levels were found to be significantly correlated with FLI after adjustment for age and BMI (r=−0.108, P=0.014). Logistic regression showed that serum 25(OH)D3 levels were independently correlated with NAFLD (OR: 0.937, 95% CI: 0.884–0.993, P=0.028). Furthermore, stepwise regression analysis revealed that serum 25(OH)D3 levels were inversely associated with FLI (β=−0.055, P=0.040).
Conclusion:
The present study demonstrated that serum 25(OH)D3 levels were inversely associated with NAFLD, even in subjects with normal total body fat, suggesting a potential role of lower levels of vitamin D in the occurrence and development of NAFLD.
Similar content being viewed by others
Introduction
Vitamin D is an important lipid-soluble vitamin that contributes to the broad array of calcium-related molecular processes under normal physiological conditions, exerting its biological effects by triggering a signaling pathway upon binding to its cognate receptor, the vitamin D receptor (VDR). Although the most well-characterized function of vitamin D-VDR signaling involves the calcium-related effects on bone metabolism, more recent studies have suggested that this signaling cascade may play a protective role in cardiovascular disease, cancer, and autoimmune disease1,2,3. Moreover, studies of the potential pathological detriment of vitamin D deficiency, in both animal- and clinical-based settings, have uncovered a potential role in obesity and visceral obesity. In a clinical study of Chinese men with normal glucose tolerance, a marked decrease in serum vitamin D levels was observed in subjects with higher amounts of adipose tissue, especially those with increased visceral adipose4.
Although obesity defined by body mass index (BMI) presents a reliable relationship with adverse metabolic outcomes, it is not applicable for some specific subtypes of subjects, such as metabolically obese but normal weight (MONW) subjects, who are defined as subjects of normal weight with a cluster of obesity-related abnormalities (including insulin resistance, type 2 diabetes, dyslipidemia, visceral obesity and cardiovascular disease)5. Those with MONW are common in the general population and represent a high-risk population of metabolic syndrome (MetS)5. Non-alcoholic fatty liver disease (NAFLD), an obesity-related disease, is considered as the hepatic manifestation of MetS6. A study from Korea reported that the prevalence of NAFLD in the nonobese was 23.4%. Hence, identifying NAFLD in those with a normal BMI might have important clinical significance7.
From a clinical perspective, NAFLD is characterized by ectopic fat deposition. Considering the demonstrated (likely protective) role of vitamin D in adipose tissue, as described above, vitamin D may play a role in the pathological mechanism of NAFLD. Indeed, the serum vitamin D level was found to be lower in biopsy-diagnosed NAFLD patients and subjects with elevated alanine aminotransferase (ALT) levels, suggesting a possible correlation with liver function status8,9. However, in another study of Chinese subjects that used ultrasonography to diagnose NAFLD, no difference in serum vitamin D levels was found10.
Serum vitamin D levels are known to be influenced by a wide array of physiological, genetic and environmental factors, including but not limited to BMI, gender, ethnicity, and sunlight exposure11,12. However, the effects of serum vitamin D levels on NAFLD remain poorly understood after adjusting for the influencing factors mentioned above. Currently, the diagnosis of NAFLD relies on the findings from examinations of biopsied liver tissues and imaging analyses by ultrasonography, computed tomography, and magnetic resonance13. The ongoing search for other non-invasive diagnostic methods with high accuracy and low cost has provided promising results for the fatty liver index (FLI), which is an algorithm assessing serum markers related to liver function14.
To gain further insight into the role of serum vitamin D levels in NAFLD, we measured serum levels of 25-hydroxy vitamin D3 [25(OH)D3], the most stable form of vitamin D, in Chinese men with normal BMI and liver enzymes. In addition, the correlations of 25(OH)D3 with NAFLD and FLI were assessed by statistical analysis.
Materials and methods
Subjects
The Ethics Committee of Shanghai Jiaotong University Affiliated Sixth People's Hospital approved the study design and all procedures. The Chinese adult male subjects were selected for analysis from the database of participants in the Shanghai Obesity Study (SHOS)15. Upon initial enrollment in the SHOS, all subjects provided written informed consent for use of their study-related information and for participation in ongoing research.
The general background information of each SHOS participant was obtained by questionnaires and recorded in the database. The selection criteria for the current study included men who had been sampled between May and September in 2010–2011 and who had complete background information. Candidates were excluded according to the following additional criteria: (1) BMI<18.5 kg/m2 and BMI≥25 kg/m2; (2) abnormal liver enzymes [ALT, aspartate aminotransferase (AST), alkaline phosphatase (AKP), and gamma-glutamyl transpeptidase (GGT)]; (3) diagnosis of autoimmune liver disease; (4) positive test results for either hepatitis B surface antigen or hepatitis C antibody; (5) weekly alcohol consumption ≥140 g; (6) history of cardiovascular disease; (7) renal dysfunction; (8) hypo- or hyperthyroidism; (9) serum calcium level of ≥10.5 mg/dL; (10) current use of drugs known to influence 25(OH)D3 metabolism, including glucocorticoids and calcium/vitamin D supplements; (11) severe disability, bone fracture, or psychiatric disorder; (12) current infectious condition; (13) C-reactive protein (CRP) >10 mg/L; and (14) the presence of a tumor and severe anemia.
Anthropometric measurements
Each subject underwent a physical examination. Measurements of weight (to the nearest 0.1 kg) and height (to the nearest 0.1 cm) were used to calculate the BMI [=(kg/m2)]. Waist circumference (W) was measured on the midaxillary line between the lower border of the rib cage and the upper margin of the iliac crest. Average resting blood pressure (BP) was obtained from three measurements made with a standard mercury sphygmomanometer at 3-minute intervals.
Biochemical assessments
A 10-h fasting blood draw was taken and immediately followed by a 75-g oral glucose tolerance test (100-g carbohydrate test for subjects with a validated diabetes history). Fasting plasma glucose (FPG) and 2 h postprandial glucose (2hPG) were measured by the glucose oxidase method, and glycated hemoglobin A1c (HbA1c) levels were determined by high-pressure liquid chromatography (Variant II; Bio-Rad, Hercules, CA, USA). Total cholesterol (TC) and triglyceride (TG) levels were assessed using a standard enzymatic method, and low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c) concentrations were measured using a direct assay method. Levels of the liver function markers ALT, AST, AKP, and GGT were assessed by enzymatic methods. The concentration of serum fasting insulin (FINS) was quantified by an electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany), with intra- and inter-assay variation coefficients of 1.7% and 2.5%, respectively. Insulin resistance (IR) was assessed by calculating the homeostasis model assessment index (HOMA-IR)16: [FPG (mmol/L)×FINS (mU/L)/22.5]. Serum 25(OH)D3 levels were quantified by an electrochemiluminescence immunoassay method (Roche Diagnostics GmbH); the intra- and inter-assay variation coefficients were 5.6% and 8.0%, respectively. CRP concentration was measured using a particle-enhanced immunonephelometry analyzer (Siemens Healthcare Diagnostics Inc, Newark, NJ, USA).
NAFLD evaluation
Liver ultrasound was performed with a Voluson 730 Expert B-mode ultrasonogram (GE Healthcare, Waukesha, WI, USA) equipped with a 5-MHz probe. A single experienced sonographer who was blinded to the study subjects' clinical characteristics and who was unaware of the study design carried out all of the scans.
Because performing liver biopsies for the exclusive purpose of a study is inappropriate (ie, for study participants who have no clinical indications suggestive of disease or invasive testing), the diagnosis of NAFLD was made for all study participants according to the working definition of NAFLD in China as recommended by the 2010 Revised Guidelines for the Diagnosis and Management of NAFLD published by the Chinese Hepatology Association (2010)13. The following alternative etiologies of fatty liver were considered (and ruled out for the NAFLD diagnosis): alcohol-induced liver disease, viral hepatitis, autoimmune liver disease, drug-induced liver disease, and total parenteral nutrition-induced steatosis.
In addition, FLI (a quantitative estimate of liver steatosis) was also calculated14: (e0.953×loge(TG)+0.139×BMI+0.718×loge(GGT)+0.053×W–15.745)/(1+e0.953×loge(TG)+0.139×BMI+0.718×loge(GGT)+0.053×W–15.745)×100. In the present study, subjects were divided into two groups using an FLI of 30 as the cutoff point, as previously recommended14.
Definition of MetS
All study participants were assessed for MetS according to the 2007 Joint Committee for Developing Chinese Guidelines, which recommends that a diagnosis be made based on the presence of more than three of the following disease-related components17: central obesity, defined as W over 90 cm; hypertriglyceridemia, defined as serum TG level≥1.70 mmol/L; low serum HDL-c, defined as HDL-c<1.04 mmol/L; hypertension, defined as BP≥130/85 mmHg or current therapy to address previously diagnosed hypertension; hyperglycemia, defined as FPG≥6.1 mmol/L and/or 2hPG≥7.8 mmol/L or previously diagnosed type 2 diabetes.
Statistical analysis
The SPSS (Statistical Package for the Social Sciences) statistical software suite, version 16.0, was used for all statistical analyses (SPSS Inc, Chicago, IL, USA). Data with normal distribution were expressed as the mean values±SD and were assessed by unpaired Student's t-test to evaluate inter-group (NAFLD vs non-NAFLD) differences. Data with skewed distribution were expressed as medians with corresponding interquartile ranges and were assessed by the Mann-Whitney U-test for the inter-group comparisons. Comparative analyses of categorical variables were carried out by the chi-square test. The relationship between FLI and demographic and clinical variables was evaluated by partial correlation testing. In addition, regression analysis was performed to identify independent factors of NAFLD and FLI. A two-tailed P value <0.05 indicated statistical significance.
Results
Demographic and clinical characteristics of study participants
A total of 514 adult Chinese males (age range: 22 to 79 years old; median (interquartile range): 57.62 (51.21–62.09) years) with normal BMI and liver enzymes were selected for study participation. The median (interquartile range) level of FLI was 19.96 (11.19–28.68), and the serum 25(OH)D3 level was 15.32±5.77 ng/mL for the entire study population.
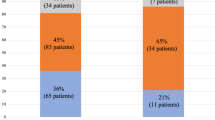
The study population was stratified by B-mode ultrasound diagnosis of NAFLD, and the demographic and clinical characteristics between the two groups were comparatively analyzed. As shown in Table 1, compared to the non-NAFLD group (n=438; 85.21%), the NAFLD group (n=76; 14.79%) was younger, had lower levels of serum 25(OH)D3 and HDL-c, had higher levels of BMI, W, FPG, 2hPG, HbA1c, TG, FINS, HOMA-IR (ie, the clinical profile for adverse cardiometabolic conditions) and FLI, and enhanced ALT and GGT (ie, markers of liver dysfunction) levels. In addition, the NAFLD group showed a higher frequency of MetS and of its components, with the exception of hypertension.
Comparison of serum 25(OH)D3 levels in different FLI level groups
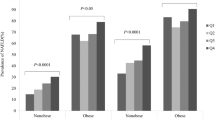
The stratification of the overall study population by FLI values showed 395 (76.85%) of the participants with a FLI level<30 and 119 (23.15%) with a FLI level≥30. When the latter were subjected to comparative analysis against those with a FLI level<30, participants with a FLI level≥30 were found to have lower serum 25(OH)D3 levels (15.65±5.86 ng/mL vs 14.22±5.34 ng/mL, P=0.018).
Correlation of FLI levels with clinical and metabolic parameters
The age- and BMI-adjusted associations of FLI with serum 25(OH)D3 levels and other cardiometabolic parameters are shown in Table 2. Significant positive correlations were found to exist between FLI and W, BP, 2hPG, TC, TG, FINS, HOMA-IR, ALT, and GGT. In contrast, significant negative correlations were found to exist between FLI and serum 25(OH)D3 levels and HDL-c.
Variables independently associated with NAFLD and FLI
To investigate the potential clinical value of serum 25(OH)D3 levels for diagnosing NAFLD, logistic regression analysis was conducted to evaluate the association of B-mode ultrasound-diagnosed NAFLD with serum 25(OH)D3 levels as well as with the various cardiometabolic variables (specifically age, BMI, W, BP, glucose levels, HbA1c, lipid profiles, HOMA-IR and CRP), liver enzymes and any current therapy for managing these disease-related components (including anti-diabetics, anti-hypertensives, and lipid-lowering medications). Serum 25(OH)D3 levels were shown to be independently correlated with B-mode ultrasound-diagnosed NAFLD (odds ratio=0.937, 95%CI: 0.884-0.993, P=0.028) (Table 3), together with 2hPG, HOMA-IR and ALT. When multiple stepwise regression analysis was carried out with the FLI set as the dependent variable and adjustments made for the aforementioned independent covariates, the serum 25(OH)D3 levels were identified as an independent protective factor of FLI (β=−0.055, P=0.040) (Table 4).
Discussion
To the best of our knowledge, the findings from the study described herein provide the first evidence of an association between serum 25(OH)D3 levels and B-mode ultrasound-diagnosed NAFLD in Chinese men who present with normal BMI and liver enzymes as detected by routine clinical testing. Specifically, the men with NAFLD in this study cohort had remarkably decreased serum 25(OH)D3 levels compared to their non-NAFLD counterparts. In addition, the serum 25(OH)D3 levels in these men were inversely associated with FLI, a novel observation recorded by this study.
While previous studies carried out in European and American populations have investigated the potential association of vitamin D with NAFLD, the results have been largely inconsistent; non-uniformity in the methods used to assess NAFLD among these various studies may explain this quandary. Indeed, when the studies are grouped for general comparison according to NAFLD diagnosis method or study population, some consistency is found. For example, the two studies using biopsy-based NAFLD diagnosis (from Italy and the USA) found a significantly lower serum vitamin D level in NAFLD patients and demonstrated a close association of vitamin D levels with both fibrosis and hepatocyte ballooning8,18. Furthermore, in two studies using B-mode ultrasound NAFLD diagnosis (again from Italy and the USA), serum vitamin D was also found to be an independent predictor of NAFLD19,20. However, the inverse relationship that was shown to exist between serum levels of vitamin D and an unexplained elevation in ALT9 was found to disappear in an adolescent population study after adjustment for obesity21. Moreover, when an Italian study of essential hypertension performed an analysis of NAFLD, the association with vitamin D deficiency was lost22.
It is also important to consider the various well-known influencing factors (such as ethnicity and geography) of serum vitamin D levels, which may have confounded the results from the various study populations. A study from Turkey23 (which represents a Eurasian ethnicity), demonstrated lower serum 25(OH)D3 levels in liver biopsy proven-NAFLD subjects. Yet, not all studies in Asian populations have yielded similar results. For example, two studies from Korea showed that the increased prevalence of NAFLD was accompanied by decreased serum 25(OH)D3 levels in non-type 2 diabetes24 and that this relationship was independent of visceral obesity25. However, one study from South China (using a population of factory employees in the Yunnan Province) found no association between vitamin D and NAFLD10. The differences in these results may be related to heterogeneity in environmental factors among and within the study populations.
In the present study of Chinese males recruited from Shanghai, we attempted to eliminate (or at least minimize) the impact of gender, BMI and liver enzymes on our analysis of serum 25(OH)D3 levels and NAFLD. The subjects were recruited as residents of Shanghai (latitude 31° north), and all clinical sampling was performed in seasons that had adequate sunlight. Therefore, we feel relatively confident in our results showing an inverse correlation between NAFLD and serum levels of 25(OH)D3 (13.99% lower than in the non-NAFLD subjects) and a significant relationship between serum 25(OH)D3 levels and FLI.
When considering the potential mechanisms that underlie the association between vitamin D and NAFLD, we theorized that processes related to insulin resistance (IR) and inflammation may be involved. Vitamin D has been reported to play a protective role in IR, which is a feature of MetS26,27, and NAFLD is characterized as the hepatic component of MetS28. In the present study, we observed a higher prevalence of MetS in the NAFLD group and confirmed a strong independent association between IR and NAFLD using statistical analyses. Vitamin D deficiency may play a role in the pathogenesis of autoimmune diseases and may accelerate liver fibrosis in the context of those disease conditions29. Vitamin D has also been shown to protect against the occurrence and development of NAFLD, and this mechanism has been shown to involve vitamin D-VDR signaling, leading to reductions in the expression of inflammatory factors, such as CRP, interleukin-6, and tumor necrosis factor-α30. Consistent with this observation, another result of our current study is the observation of a trend towards enhanced serum CRP levels in our NALFD subjects.
The findings of the present study should be interpreted with care considering the inherent weaknesses related to the study design. The primary strengths of the current study are represented by our efforts to minimize confounding factors. However, we did not measure parathyroid hormone (PTH). We attempted to counter this limitation in our study design by denying study participation to individuals with hypercalcemia. In addition, the cross-sectional design of the study limits our ability to make any causal inferences. Therefore, further large prospective studies are warranted.
In summary, this study demonstrated a strong association between serum 25(OH)D3 levels and B-mode ultrasound-diagnosed NAFLD in Chinese men with normal total body fat and liver enzymes. Future prospective studies are necessary to validate these findings and to confirm the clinical utility of this association as a strategy to improve NAFLD management.
Author contribution
Wei-ping JIA and Yu-qian BAO designed the study; Yu-qi LUO, Jie NI, Jian-xin DOU, and Ya-qin HU collected data; Ya-ping HAO and Xiao-jing MA analyzed data and wrote the manuscript; and Jia-an ZHU performed the liver ultrasound analysis.
References
Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, Vandorpe T, et al. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis 2012; 33: 1319–26.
Ford ES, Ajani UA, McGuire LC, Liu S . Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care 2005; 28: 1228–30.
Kamen DL, Tangpricha V . Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010; 88: 441–50.
Hao Y, Ma X, Shen Y, Ni J, Luo Y, Xiao Y, et al. Associations of serum 25-hydroxyvitamin D3 levels with visceral adipose tissue in Chinese men with normal glucose tolerance. PLoS One 2014; 9: e 86773.
Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET . Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89: 2569–75.
Uchil D, Pipalia D, Chawla M, Patel R, Maniar S . Narayani, et al. Non-alcoholic fatty liver disease (NAFLD) — the hepatic component of metabolic syndrome. J Assoc Physicians India 2009; 57: 201–4.
Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 2004; 164: 2169–75.
Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2007; 17: 517–24.
Liangpunsakul S, Chalasani N . Serum vitamin D concentrations and unexplained elevation in ALT among US adults. Dig Dis Sci 2011; 56: 2124–9.
Li L, Zhang L, Pan S, Wu X, Yin X . No significant association between vitamin D and nonalcoholic fatty liver disease in a Chinese population. Dig Dis Sci 2013; 58: 2376–82.
Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010; 59: 242–8.
Coney P, Demers LM, Dodson WC, Kunselman AR, Ladson G, Legro RS . Determination of vitamin D in relation to body mass index and race in a defined population of black and white women. Int J Gynaecol Obstet 2012; 119: 21–5.
Jian-gao F . Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Gan Zang Bing Za Zhi 2010; 18: 163–6.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6: 33.
Bao Y, Ma X, Yang R, Wang F, Hao Y, Dou J, et al. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J Clin Endocrinol Metab 2013; 98: 345–51.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9.
Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007; 35: 390–419.
Dasarathy J, Periyalwar P, Allampati S, Bhinder V, Hawkins C, Brandt P, et al. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int 2013. Epub 2014; 34: e118–27.
Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 2011; 9: 85.
Jablonski KL, Jovanovich A, Holmen J, Targher G, McFann K, Kendrick J, et al. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2013; 23: 792–8.
Katz K, Brar PC, Parekh N, Liu YH, Weitzman M . Suspected nonalcoholic Fatty liver disease is not associated with vitamin D status in adolescents after adjustment for obesity. J Obes 2010; 2010: 496829.
Catena C, Cosma C, Camozzi V, Plebani M, Ermani M, Sechi LA, et al. Non-alcoholic fatty liver disease is not associated with vitamin D deficiency in essential hypertension. High Blood Press Cardiovasc Prev 2013; 20: 33–7.
Eraslan S, Kizilgul M, Uzunlulu M, Colak Y, Ozturk O, Tuncer I . Frequency of metabolic syndrome and 25-hydroxyvitamin D3 levels in patients with non-alcoholic fatty liver disease. Minerva Med 2013; 104: 447–53.
Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, et al. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J 2013; 60: 743–52.
Seo JA, Eun CR, Cho H, Lee SK, Yoo HJ, Kim SG, et al. Low vitamin D status is associated with nonalcoholic Fatty liver disease independent of visceral obesity in Korean adults. PLoS One 2013; 8: e 75197.
Zhao G, Ford ES, Li C . Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among US adults without physician-diagnosed diabetes: NHANES, 2003–2006. Diabetes Care 2010; 33: 344–7.
Tao MF, Zhang Z, Ke YH, He JW, Fu WZ, Zhang CQ, et al. Association of serum 25-hydroxyvitamin D with insulin resistance and β-cell function in a healthy Chinese female population. Acta Pharmacol Sin 2013; 34: 1070–4.
Hurjui DM, Niţă O, Graur LI, Mihalache L, Popescu DS, Graur M . The central role of the non alcoholic fatty liver disease in metabolic syndrome. Rev Med Chir Soc Med Nat Iasi 2012; 116: 425–31.
Artaza JN, Norris KC . Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol 2009; 200: 207–21.
Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Kalayi A, et al. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 2012; 28: 424–30.
Acknowledgements
This work was funded by the 973 Program of China (2013CB530606), National Natural Science Foundation of China (81100563), National Key Technology R&D Program of China (2012BAI02B03), Key Project of Science and Technology of Shanghai (13XD1403000) and a grant from Shanghai Health and Family Planning Commission (2013ZYJB1001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, Yp., Ma, Xj., Luo, Yq. et al. Serum vitamin D is associated with non-alcoholic fatty liver disease in Chinese males with normal weight and liver enzymes. Acta Pharmacol Sin 35, 1150–1156 (2014). https://doi.org/10.1038/aps.2014.48
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.48
Keywords
This article is cited by
-
Fatty liver index (FLI): more than a marker of hepatic steatosis
Journal of Physiology and Biochemistry (2024)
-
Low vitamin D levels are linked with increased cardiovascular disease risk in young adults: a sub-study and secondary analyses from the ACTIBATE randomized controlled trial
Journal of Endocrinological Investigation (2024)
-
Low serum vitamin D concentrations are associated with obese but not lean NAFLD: a cross-sectional study
Nutrition Journal (2021)
-
Association and interaction between vitamin D level and metabolic syndrome for non-alcoholic fatty liver disease
Journal of Diabetes & Metabolic Disorders (2021)
-
Neck circumference as an independent indicator to non-alcoholic fatty liver disease in non-obese men
Nutrition & Metabolism (2015)