Abstract
Aim:
7,8-Dihydroxy-4-(3-hydroxy-4-methoxyphenyl)-2H-chromen-2-one (DW532) is one of simplified analogues of hematoxylin that has shown broad-spectrum inhibition on tyrosine kinases and in vitro anti-cancer activities. The aim of this study was to identify DW532 as a agent targeting both kinases and tubulin, and to investigate its anti-cancer and anti-angiogenesis activities.
Methods:
In vitro tyrosine kinases activity was examined with ELISA, and tyrosine kinases activity in cells was evaluated with Western blot analysis. Tubulin turbidity assay, surface plasmon resonance and immunofluorescence technique were used to characterize the tubulin inhibitory activity. Cell proliferation was examined with SRB assay, and cell apoptosis and cell cycle distribution were analyzed with Annexin-V/PI staining and flow cytometry. Tube formation, aortic ring and chick chorioallantoic membrane assays were used to evaluate the anti-angiogenesis efficacy.
Results:
DW532 inhibited EGFR and VEGFR2 in vitro kinase activity (the IC50 values were 4.9 and 5.5 μmol/L, respectively), and suppressed their downstream signaling. DW532 dose-dependently inhibited tubulin polymerization via direct binding to tubulin, thus disrupting the mitotic spindle assembly and leading to abnormal cell division. In a panel of human cancer cells, DW532 (1 and 10 μmol/L) induced G2/M phase arrest and cell apoptosis, which subsequently resulted in cytotoxicity. Knockdown of BubR1 or Mps1, the two core proteins of the spindle assembly checkpoint dramatically decreased DW532-induced cell cycle arrest in MDA-MB-468 cells. Moreover, treatment with DW532 potently and dose-dependently suppressed angiogenesis in vitro and in vivo.
Conclusion:
DW532 is a dual inhibitor against tubulin and tyrosine kinases, and deserves further development as a novel anti-cancer agent.
Similar content being viewed by others
Introduction
Protein tyrosine kinases (PTKs) play crucial roles in signal transduction that regulate various cellular functions, such as cell proliferation, the cell cycle, differentiation and cell survival, and angiogenesis. Dysregulation of kinase activity through overexpression or mutation is associated with the development of a number of different cancers1. Thus, tyrosine kinases have been proven to be important targets for cancer therapy. Several small molecule kinase inhibitors targeting different tyrosine kinases such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) have already been used in the clinic, and more are currently in different stages of preclinical or clinical development2,3.
Microtubules are components of the cytoskeleton with key roles in cellular functions, including intracellular transport, cell shape maintenance, polarity, and mitosis. Microtubule targeting agents (MTAs) have also been recognized as a class of the most effective medications in cancer treatment, and several MTAs have been approved for the treatment of solid and hematologic malignancies. MTAs are usually classified into two main groups4. One group is known as the microtubule-destabilizing agents, eg, vincristine (VCR), which inhibits microtubule polymerization5. The other group is known as the microtubule-stabilizing agents, eg, Taxol, which stimulates microtubule polymerization. MTAs can arrest cells in mitosis, particularly rapidly dividing cancer cells, and apoptosis and cell death likely follows6.
Cancer is a rather complex disease with robust and often redundant biological networks. Therefore, it is increasingly recognized that modulating more than one target may be a better clinical approach than using single-targeting agents7. Clinical trials for cancer treatment using a combination of tubulin and kinase inhibitors were investigated with promising therapeutic effects. For example, combination of the EGFR kinase inhibitor lapatinib and the microtubule-stabilizing agent Taxol was found to significantly improve the progression-free survival (FPS) and overall response (OR) of gastric cancer patients in a phase III clinical trial8. Meanwhile, agents that target kinases and tubulin also have gained increasing attention and achieved some progress. For example, Kadcyla, an antibody-drug conjugate that targets both HER2 and tubulin, was approved by the FDA for the treatment of HER2-positive metastatic breast cancer. KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, is being tested in a Phase II study of prostate cancer patients9. Tivantinib, the first MET inhibitor in clinical research, was discovered to also affect tubulin polymerization10. Moreover, researchers are reportedly attempting to modify the chemical structure of SU5416, an inhibitor of VEGFR, to obtain a dual inhibitor of VEGFR and tubulin11. All of these efforts highlight the importance of identifying and developing new inhibitors targeting kinases and tubulin.
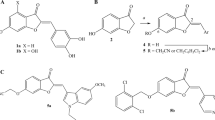
Hematoxylin, a natural product extracted from the heartwood of the the logwood tree (Haematoxylum campechianum) (Figure 1), was previously discovered by our group for its broad-spectrum inhibition of tyrosine kinases and in vitro anti-tumor activity12. Hematoxylin has a tetracyclic compound structure with four hydroxyl groups, and it is barely soluble in water because the tetracyclic configuration often accounts for the poor solubility of compounds. Although hematoxylin has interesting biological activity, its physical properties are sub-optimal for clinical use. Moreover, from the structure of hematoxylin, we found that it contains the key pharmacophore combretastatin (CA-4) (Figure 1), a well-known tubulin inhibitor, which includes two phenyl groups with substituted hydroxyls or methoxy groups. Therefore, we designed and synthesized a series of simplified analogues to achieve two purposes: 1) target kinases and tubulin and 2) simultaneously decrease the complexity of the tetracyclic system of hematoxylin. One of the compounds that possess excellent bioactivities is 7,8-dihydroxy-4-(3-hydroxy-4-methoxyphenyl)-2H-chromen-2-one (DW532 in Figure 1). Biological results further confirmed that DW532 is an agent targeting both tubulin and kinases. DW532 treatment resulted in growth inhibition, cell cycle arrest and apoptosis in cancer cells, and it also exhibited the suppression of angiogenesis in vitro and in vivo.
The structure of hematoxylin, combretastin, and DW532 Reagents and conditions for the synthesis of DW532: (a) Na, CO(OEt)2, reflux; (b) AcOH, reflux; (c) (CF3SO2)2O, 0 °C; (d) 3-(benzyloxy)-4-methoxyphenylboronic acid, Pd(PPh3)4, CuI, K2CO3, DMF, 120 °C, 20 min; (e) CF3COOH.
Materials and methods
Drugs and chemicals
Synthesis of DW532 (Figure 1).
General
All chemicals obtained from Sigma-Aldrich (St Louis, MO, USA) and the Sinopharm Chemical Reagent Co, Ltd (Shanghai, China) were used without further purification. Melting points (uncorrected) were measured with a Büchi B-510 melting point apparatus. 1H NMR spectra were recorded with a Varian Mercury 300 NMR spectrometer (Varian, Palo Alto, CA, USA); tetramethylsilane and residual solvent signals were used as internal standards. Chemical shifts are reported in ppm (δ). Low-resolution mass spectra (MS) and high-resolution mass spectra (HRMS) were obtained with an ionizing voltage of 70 eV using a Finnigan/MAT95 spectrometer. The purity of the new derivatives was determined with an Agilent Technologies 1260 series HPLC system (Agilent Technology, Santa Clara, CA, USA) using a ZORBAX Eclipse Plus column (C18, 4.6×100 mm, 3.5 Micron). DW532 had a purity of greater than 98%.
Ethyl 3-(3,4-bis(benzyloxy)phenyl)-3-oxopropanoate (2)
Sliced sodium (1.0 g; 43.06 mmol) was added to a mixture of distilled diethyl carbonate (20 mL, 165.08 mmol) and 1 (1.5 g, 474.16 mmol), and the resulting mixture was stirred at 120 °C for 20 min. After the reaction mixture was cooled to room temperature and quenched with ethanol (10 mL), its pH value was adjusted to 6 with 6 mol/L hydrochloric acid. The resulting mixture was extracted with ethyl acetate (3×30 mL). The combined extracts were dried over anhydrous sodium sulfate and concentrated in a vacuum. The residue was purified by flash chromatography (dichloromethane: methanol=40:1) to produce 2 as a white solid (1.40 g, 80.1%): mp: 156–158 °C; 1H NMR (300 MHz, DMSO-d6) δ: 11.83 (s, 1H), 77.63 (d, J=9.0 Hz, 1H), 7.43–7.27 (m, 10H), 6.81 (d, J=9.3 Hz, 1H), 5.24 (s, 2H), 4.95 (s, 2H), 4.14 (s, 2H), 4.11 (d, J=6.0 Hz, 1H), 1.17 (t, J=7.1 Hz, 3H); MS (EI): 420 m/z [M+]; HRMS (EI) calcd for C25H24O6 [M+]: 420.1573, found: 420.1572.
7,8-Bis(benzyloxy)-4-hydroxy-2H-chromen-2-one (3)
A solution of 2 (1 g, 2.38 mmol) in acetic acid (5 mL) was heated at reflux for 6 h. The reaction mixture was evaporated to dryness, and the resulting residue was purified by flash chromatography (dichloromethane: methanol=60:1) to produce 3 as a yellow solid (0.83 g, 92.7%): mp: 183–185 °C; 1H NMR (300 MHz, DMSO-d6) δ: 12.41–12.36 (m, 1H), 7.53 (d, J=9.2 Hz, 1H), 7.50 – 7.28 (m, 12H), 7.19 (d, J=9.1 Hz, 1H), 5.47 (s, 1H), 5.26 (s, 2H), 5.07 (s, 2H); MS (EI): 374 m/z [M+]; HRMS (EI) calcd for C23H18O5 [M+]: 374.1154, found: 374.1159.
7,8-Bis(benzyloxy)-2-oxo-2H-chromen-4-yl trifluoromethanesulfonate (4)
A solution of trifluoromethanesulfonic anhydride (0.11 mL, 1.61 mmol) was added dropwise to a mixture of 3 (150 mg, 0.41 mmol) and triethylamine (0.17 mL, 1.21 mmol) in dichloromethane (12 mL). After addition, the mixture was stirred at 0 °C for 12 h, and it was then quenched with brine and extracted with dichloromethane (3×10 mL). The combined extracts were dried over anhydrous sodium sulfate and concentrated in a vacuum. The resulting residue was purified by chromatography (petroleum ether: ethyl acetate=5:1) to produce 4 as a white solid (153 mg, 75.4%): mp: 112–113 °C; 1H NMR (300 MHz, CDCl3) δ: 7.38 (dd, J=28.7, 16.9 Hz, 11H), 7.00 (d, J=9.1 Hz, 1H), 6.33 (s, 1H), 5.23 (s, 2H), 5.19 (s, 2H); MS (EI): 506 m/z [M+]; HRMS (EI) calcd for C24H17SF3O7 [M+]: 506.0647, found: 506.0653.
7,8-Bis(benzyloxy)-4-(3-(benzyloxy)-4-methoxyphenyl)-2H-chromen-2-one (5)
A mixture of 4 (80 mg; 0.16 mmol), tetrakis(triphenylphosphine) palladium (10 mg; 0.01 mmol), cuprous iodide (34 mg; 0.18 mmol), sodium carbonate (118 mg; 1.20 mmol), and (3-(benzyloxy)-4-methoxyphenyl) boronic acid (82 mg; 0.32 mmol) in 1,4-dioxane (15 mL) was degassed three times with argon. The resulting mixture was heated in an argon atmosphere at 120 °C for 20 min. After cooling to room temperature, the reaction mixture was filtered to remove insoluble substances. The filtration was evaporated to dryness, and the resulting residue was purified by flash chromatography (dichloromethane: methanol=40:1) to produce 5 as a brown solid (124 mg, 78.0%): mp: 163–165 °C; 1H NMR (300 MHz, CDCl3) δ: 7.55 (dd, J=7.5, 2.4 Hz, 2H), 7.41–7.26 (m, 13H), 7.01 (s, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.67 (d, J=9 Hz, 1H), 6.16 (s, 1H), 5.22 (s, 2H), 5.20 (s, 2H), 5.19 (s, 2H), 3.97 (s, 3H); MS (EI): 570 m/z [M+]; HRMS (EI) calcd for C37H30O6 [M+]: 570.2042, found: 570.2036.
7,8-Dihydroxy-4-(3-hydroxy-4-methoxyphenyl)-2H-chromen-2-one (DW532)
A mixture of 4 (30 mg; 0.06 mmol) in trifluoromethanesulfonic acid (2 mL) was stirred at 55 °C for 2 h. The mixture was then evaporated to dryness, and the resulting residue was purified by flash chromatography (dichloromethane: methanol=90:1) to produce DW532 as a yellow solid (11 mg, 70.4%): mp: 118–120 °C; 1H NMR (300 MHz, DMSO-d6) δ:10.16 (s, 1H), 9.39 (s, 1H), 9.37 (s, 1H), 7.05 (s, 1H), 6.89 (d, J=7.8 Hz, 3H), 6.79 (d, J=8.9 Hz, 1H), 6.04 (s, 1H), 3.84 (s, 3H); MS (EI): 300 m/z [M+]; HRMS (EI) calcd for C16H12O6 [M+]: 300.0634, found 300.0637.
Combretastatin, Taxol, ispinesib, SB743921 and vincristine (VCR) were purchased from Sigma-Aldrich (St Louis, MO, USA). Aurora inhibitor II was purchased from Calbiochem (San Diego, CA, USA). All of the chemicals were prepared at 10 mmol/L in 100% dimethyl sulfoxide (DMSO) as stock solutions, and the aliquots were stored at −20 °C.
Cell culture
The human cancer cell lines HT-29, K562, BT-474, T47D, MCF-7, PC-3, HCT-116, A549, A431, A375, KB, BxPC3, MDA-MB-231, and MDA-MB-468 were obtained from the American Type Culture Collection (Manassas, VA), SMMC-7721 was obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). All of the cell lines were cultured according to the suppliers' instructions.
Sulforhodamine B (SRB) assays
Cell proliferation was evaluated using the SRB (Sulforhodamine B) assay as previously described13. Briefly, cells were seeded in 96-well plates and grown for 24 h. The cells were then treated with various concentrations of test compounds with each concentration tested in triplicate and grown for an additional 72 h. The cells were then fixed with 10% pre-cooled trichloroacetic acid (TCA) for 1 h at 4 °C and stained for 15 min at room temperature with 100 μL of 4 mg/mL SRB solution (Sigma) in 1% acetic acid. The SRB solution was dissolved in 150 μL of 10 mmol/L Tris base for 5 min and measured at 515 nm using a multiwell spectrophotometer (VERSAmax, Molecular Devices). The inhibition rate for cell proliferation was calculated as [1–(A515 treated/A515 control)]×100%. The IC50 value was obtained using the Logit method.
Enzyme-linked immunosorbent assay (ELISA)
A total of 20 μg/mL Poly(Glu, Tyr)4:1 (Sigma, St Louis, MO) was precoated in 96-well ELISA plates as a substrate. Active kinases were incubated with indicated drugs in 1×reaction buffer (50 mmol/L HEPES pH 7.4, 20 mmol/L MgCl2, 0.1 mmol/L MnCl2, 0.2 mmol/L Na3VO4, 1 mmol/L DTT) containing 5 μmol/L ATP at 37 °C for 1 h. After incubation, the wells were washed with PBS and then incubated with an anti-phosphotyrosine (PY99) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by a horseradish peroxidase (HRP)-conjugated secondary antibody. The wells were visualized using o-phenylenediamine (OPD) and read with a multiwell spectrophotometer (VERSAmax™, Molecular Devices, Sunnyvale, CA, USA) at 492 nm.
Western blot analysis
Cell samples were lysed in 1×SDS lysis buffer. Western blot analysis was subsequently performed using standard procedures. Antibodies directed against the following proteins were used: p-KDR (Tyr1123), VEGFR, p-EGFR (Tyr1068), EGFR, p-ERK, ERK, p-AKT (Ser473), AKT, p-H3 (Ser-10), H3, cyclin B1, and p-CDK1 (Tyr15), which were obtained from Cell Signaling Technologies (Cambridge, MA, USA), and GAPDH and α-tubulin, which were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence Assays
Cells were seeded onto glass coverslips overnight and treated with the indicated drugs for a certain period of time. After treatment, the cells were fixed for 15 min with 4% paraformaldehyde and then permeabilized for 15 min with 0.2% Triton X-100 followed by blocking with 3% bovine serum albumin. The cells were then incubated with the primary antibodies α–tubulin (1:200) and α–pericentrin (1:100) for 1 h. The cells were then stained with Alexa Fluor®488- or Alexa Fluor®633-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) for an additional hour. Finally, the cells were stained with DAPI, and the images were evaluated by microscopy.
RNA interference (RNAi)
BubR1 and Mps1 protein expression were knocked down with small interfering RNA (siRNA) duplexes. The transfection of siRNAs was performed according to the manufacturer's instructions using the Oligofectamine transfection reagent (Invitrogen, Carlsbad, CA). The BubR1 siRNA sequences were as follows: 1250: 5′-CUGCACAACAGCCAGUUAUTT-3′ (sense) and 5′-AUAACUGGCUGUUGUGCAGTT-3′ (antisense) and 3133: 5′-GGGUCCUUCUGGAAACUUATT-3′ (sense) and 5′-UAAGUUUCCAGAAGGACCCTT-3′ (antisense). The Mps1 siRNA sequences were as follows: 1813: 5′-CCGGAACGAAAUAGCUUAUTT-3′ (sense) and 5′-AUAAGCUAUUUCGUUCCGGTT-3′ (antisense) and 2561: 5′-GGUCUGAAUUCUCCUAACUTT-3′ (sense) and 5′-AGUUAGGAGAAUUCAGACCTT-3′ (antisense). The mock siRNA sequences were as follows: 5′-UUCUCCGAACGUGUCACGUDTDT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAADTDT-3′ (antisense). All of the RNA duplexes were synthesized by Genepharma Co (Shanghai, China).
Cell cycle and apoptosis analysis
Cells were treated with or without DW532 for the indicated times. After treatment, cells were harvested and fixed in 70% ethanol at −20 °C for 1 h followed by PBS washes. The cells were then incubated with propidium iodide (PI) and/or Annexin-V. After staining, the cell cycle distribution or apoptosis was determined using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed with CELLQUEST software (BD Biosciences, Franklin Lakes, NJ, USA).
Mitotic cell analysis
The percent of mitotic cells was quantified by flow cytometry as previously described14. Briefly, MDA-MB-468 cells were treated with or without DW532 or siRNA for the indicated time. After treatment, the cells were harvested and fixed in 90% methanol at −20 °C for 1 h followed by PBS washes. Then, the cells were incubated with the p-H3 antibody (1:200, CST, Cambridge, MA, USA) for 1 h at 37 °C. Afterward, the cells were labeled with an Alexa Fluor®488-conjugated goat anti-mouse IgG antibody (Invitrogen, Carlsbad, CA, USA), and their DNA was stained with propidium iodide (PI). Cytometric data were collected with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using CELLQUEST software (BD Biosciences, Franklin Lakes, NJ, USA).
Cellular microtubule stabilization assays
After treatment with the indicated drugs, MDA-MB-468 cells were harvested in lysis buffer (100 mmol/L PIPES pH 6.9, 1 mmol/L EGTA, 1 mmol/L MgCl2, 30% glycerol, 5% DMSO, 1% NP-40, 5 mmol/L GTP and protease inhibitors). The cell lysates were centrifuged at 180 000×g at 37 °C for 1 h, the supernatants containing soluble tubulin were separated from the pellets containing polymerized tubulin. These two fractions were adjusted to the same volume using SDS-PAGE loading buffer. Finally, the amount of α-tubulin from equal aliquots of the polymerized tubulin fraction and free tubulin fraction was determined by Western blotting.
Tubulin turbidity assays
Tubulin turbidity was measured with the HTS-Tubulin Polymerization Assay Kit (Cytoskeleton, Inc, Denver, CO, USA). Briefly, tubulin proteins (>99% purity) were suspended in G-PEM buffer containing 80 mmol/L PIPES, 2 mmol/L MgCl2, 0.5 mmol/L EDTA, 1.0 mmol/L GTP (pH 6.9), and 5% glycerol with or without the indicated drugs in a 96-well plate, and tubulin polymerization was initiated and monitored by turbidity changes at 340 nm using a spectrometer (SpectraMAX250, Molecular Devices, Sunnyvale, CA).
Surface plasmon resonance (SPR)-binding assay
Tubulin binding assays were performed with an SPR technology-based ProteOn TM XPR36 Protein Interaction Array System (Bio-Rad, Hercules, CA, USA) as previously described. Briefly, tubulin was dissolved in 10 mmol/L sodium acetate buffer (pH 3.5) and immobilized to a ProteOn TM CM5 sensor chip. The final immobilization level was 8000 RU (Response unit). DW532 was diluted two-fold from 50 μmol/L to 3.6 μmol/L in HBS-T buffer (10 mmol/L HEPES, 150 mmol/L NaCl, 3.4 mmol/L EDTA, 0.005% Tween 20, pH 7.4). Different concentrations of DW532 were then injected at a flow rate of 300 μL/min for 200 s followed by a 300 s dissociation phase. Data were analyzed with the ProteOn™ Manager software and fitted to the 1:1 Langmuir model.
Aortic ring assay
Aortic rings were obtained from 6-week-old Sprague-Dawley rats. The Sprague-Dawley rats, weighing 280 to 320 g, were obtained from the Shanghai Institute of Materia Medica (Shanghai, China). All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) and performed according to institutional ethical guidelines on animal care. Each aorta was cut into 1 mm slices, which were embedded with 55 μL Matrigel, incubated with M199 medium containing 10% FBS and various concentrations of DW532, and then photographed on day 7 with an inverted phase contrast microscope (IX-51, Olympus, Japan). The quantity of the microvessels was evaluated by the relative area covered by microvessels using Image-Pro Express.
Tube formation assay
Ninety six-well plates were coated with 55 μL Matrigel and then seeded with human umbilical vein endothelial cells (HUVECs) in M199 medium containing 20% FBS and the indicated drugs. After adherence for 6 h, tubes were examined with an inverted phase contrast microscope (DP70, Olympus, Japan).
Chick chorioallantoic membrane (CAM) assay
Fertilized chicken eggs were incubated (7 day) in a humidified Roll-X base egg incubator (Lyon Electric Company, CA, USA). Gentle suction was applied to the hole located at the broad end of the egg to create a false air sac directly over the chicken chorioallantoic membrane, and a 1 cm2 window was immediately removed from the eggshell. Glass coverslips (0.5 cm×0.5 cm) saturated with compounds or normal saline were placed on areas between pre-existing vessels, and the embryos were further incubated for 48 h. The neighboring neo-vascular zones were photographed with a stereomicroscope (MS5; Leica, Heerbrugg, Switzerland).
Data analysis
Data are presented as the mean±SD of data from at least three independent experiments. Differences were considered significant at P<0.05 as determined by Student's t-test.
Results
Chemistry
Based on the structure of hematoxylin, a multi-kinase inhibitor, we rationally designed and synthesized a series of compounds by decreasing the complexity of the hematoxylin structure for the purpose of obtaining a dual inhibitor that can target both kinases and tubulin. DW532 (Figure 1) was a distinct derivative that was selected for further study. The synthesis of DW532 began with the starting material 1-[3,4-bis(benzyloxy)-2-hydroxyphenyl]ethanone (1), which was prepared from benzene-1,2,3-triol using published procedures with some modification (Figure 1). A condensation of 1 with diethyl carbonate in the presence of sodium produced β-keto-phenylpropanoate 2, which was cyclized in refluxed acetic acid to create Chromenone 3. Triflation of 3 followed by Suzuki reaction with 3-(benzyloxy)-4-methoxyphenylboronic acid led to the precursor 5. Finally, debenzylation of 5 in trifluoroacetic acid led to DW532.
DW532 inhibits the EGFR and VEGFR in vitro kinase activity
Because DW532 was designed and expected to be a dual inhibitor targeting kinases and tubulin, we first examined the activity of DW532 against different types of tyrosine kinases using an in vitro kinase assay. The results showed that DW532 effectively inhibited EGFR and VEGFR2 activities with IC50 values of 4.9 and 5.5 μmol/L, respectively (Figure 2A and 2B). This drug also exhibited inhibitory activity for c-Src kinase with less potency (IC50 at 8.6 μmol/L). In contrast, it showed no obvious inhibition of other kinases tested, including PDGFR-α, PDGFR-β, Akt1, p70S6K, EPH-A2, and EPH-B2 even at 10 μmol/L (data not shown). The results showed that as a simplified derivative of hematoxylin, DW532 maintained kinase inhibitory activity.
DW532 inhibited EGFR and VEGFR2 kinase activity. (A) and (B) DW532 inhibited EGFR and VEGFR2 in vitro kinase activity. The kinase profiling assay was conducted as described in the Experimental section by using ELISA kinases assay. IC50 values were obtained by Logit method based on the data obtained from three independent experiments and expressed as mean±SD. (C) and (D) effects of DW532 on VEGF-induced VEGFR2 phosphorylation in HUVEC cells (C) and EGF-induced EGFR phosphorylation in A431 cell line (D). Cells were starved for 12 h and treated with indicated concentration of DW532 for 2 h and then stimulated by 50 ng/mL EGF and 100 ng/mL VEGF for 15 min. Cells were harvested and subjected to Western blot analysis.
DW532 inhibits EGFR and VEGFR activation and downstream signal transduction in cells
We further evaluated whether DW532 could inhibit the ligand-induced activation of kinases and downstream signaling transduction in A431 human epithelial cancer cells and human umbilical vein endothelial cells (HUVEC), which express high levels of EGFR or VEGFR2, respectively15,16. As demonstrated in Figure 2C, DW532 dose-dependently inhibited the VEGF-induced phosphorylation of VEGFR2 and its downstream signaling, which was indicated by phosphorylated Akt, in HUVEC cells. Similar inhibition potency on the EGF-induced phosphorylation of EGFR and its downstream signaling was also observed in A431 cells treated with DW532 (Figure 2D). These results demonstrated that DW532 possesses VEGFR2 and EGFR inhibitory activity at the cellular level.
DW532 inhibits tubulin polymerization in vitro by directly binding with tubulin
We then examined whether DW532 also affects tubulin polymerization as expected by using an in vitro turbidity assay with recombinant tubulin protein in the presence of various concentrations of DW532. DW532 was shown to inhibit tubulin polymerization in a concentration-dependent manner, which is similar to the activity of the classic microtubule destabilizers VCR and combretastatin (CA4) but quite different than the microtubule-stabilizing agent Taxol (Figure 3A). At a concentration of 20 μmol/L, DW532 suppressed tubulin polymerization by 30%, and at 40 μmol/L, tubulin polymerization was almost completely inhibited. The results demonstrated that DW532 could inhibit tubulin polymerization as a microtubule destabilizer but not as a microtubule stabilizer.
DW532 inhibited tubulin polymerization through directly binding with tubulin protein and disrupted mitotic spindle assembly. (A) DW532 inhibited tubulin polymerization in vitro. DW532 and reference compounds were incubated with purified tubulin protein at 37 °C, and then polymerization were kinetic monitored by SpectraMAX250 at 340 nm every 1 min for 30 min at 37 °C. (B) DW532 bound to tubulin directly. The tubulin binding affinity was assessed by SPR assay. Different concentrations of DW532 were prepared as indication and then injected to Biacore 3000 instrument with tubulin coated chip, and the data were analyzed with the ProteOn™ Manager software. (C) and (D) DW532 inhibited tubulin polymerization in cancer cells. MDA-MB-468 cells were treated with indicated concentrations for 12 h and then harvested by lysis buffer. After centrifuged 180 000×g 37 °C for 1 h, the free and polymerized tubulin [soluble (S) and polymerized (P)] were separated and detected by Western blotting (C). A549 cells were treated with indicated concentration of drugs for 6 h. The cells were then processed for immunofluorescence staining and confocal microscopy (×600) (D). (E) DW532 disrupted mitotic spindle assembly. A549 cells were treated with indicated concentration of drugs for 12 h. The cells were then processed for immunofluorescence staining and confocal microscopy (×600).
To identify how DW532 interacts with tubulin protein, we investigated its potential binding affinity using an in vitro SPR assay. The results revealed that DW532 treatment led to RU increase in a concentration-dependent manner (Figure 3B). When fitted to the 1:1 Langmuir model, DW532 displayed high binding affinity with an equilibrium dissociation constant (KD value) of 3.6 μmol/L (Figure 3B). Therefore, these data indicate that DW532 could directly bind tubulin and thus inhibit tubulin polymerization.
DW532 inhibits tubulin polymerization in cancer cells and causes disordered mitosis
Tubulin inhibitors could change the dynamics of cellular microtubules by promoting (ie, microtubule stabilizer) or inhibiting (ie, microtubule destabilizer) tubulin polymerization4. We then evaluated whether DW532 affected tubulin polymerization in cancer cells using an ultracentrifugation method (Figure 3C). The treatment of MDA-MB-468 cells with DW532 substantially decreased polymerized tubulin (P) in pellets in a dose-dependent manner, which was in accordance with upregulation of soluble tubulin (S) in the supernatant, indicating that DW532 promotes the transition of cellular tubulin from the polymerized state to the free state (Figure 3C). Treatment with the positive control microtubule destabilizer VCR resulted in similar results as DW532, whereas the microtubule stabilizer Taxol led to the upregulation of polymerized tubulin, which is consistent with previous reports (Figure 3C).
Immunofluorescence staining was then performed to detect microtubule networks in cancer cells treated with DW532. As demonstrated in Figure 3D, untreated cells exhibited an intact and stretched network, whereas DW532 treatment resulted in dispersed tubulin staining, which is similar to that found in VCR-treated cells. In contrast, Taxol treatment caused a significant increase in microtubule bundles. The above results further illustrated that DW532 disrupted the microtubule networks in cancer cells by destabilizing microtubules.
Because microtubules are required for assembly of the mitotic spindle, which is essential for accurate chromosome segregation17, we further investigated changes in the centrosomes and spindles of cancer cells treated with or without DW532 as indicated by staining with pericentrin and α-tubulin, respectively. As shown in Figure 3E, the cellular chromosomes were scattered in the cytoplasm, and multipolar spindles were formed with DW532 treatment, which is consistent with other traditional microtubule destabilizers. Interestingly, a small proportion of monopolar spindles was also observed in DW532-treated but not in control cells, which is rarely caused by tubulin inhibitors and deserves further investigation.
DW532 potently inhibits the proliferation of tumor cells via cell cycle arrest and apoptosis induction
The presence of both tyrosine kinase and tubulin inhibition could result in cell-cycle arrest, apoptosis and growth inhibition. Therefore, we further examined these activities in DW532-treated cancer cells. The in vitro antitumor activity of DW532 was evaluated in a panel of 16 cancer cell lines with different tissue origins. As illustrated in Figure 4A, DW532 inhibited the proliferation of the tested cancer cells with an average IC50 of 1.82 μmol/L (range: 0.35 to 7.32 μmol/L). DW532 displayed non-selective in vitro anti-proliferative activity against different types of tumor cells.
DW532 inhibited the proliferation of tumor cell lines and induced cell mitosis disorder. (A) DW532 inhibited the proliferation of a panel of human cancer cells. Proliferation of the tumor cells were assessed by SRB assays after 72 h treatments with DW532. All the Results were expressed as mean±SD of three independent experiments. (B) and (C) DW532 induced cell apoptosis. Cells were treated with indicated concentration of DW532 for 48 h and harvested for flow cytometry assays (B) and Western blot (C), respectively.
We then evaluated whether DW532 caused apoptosis in cancer cells by the Annexin V/PI staining method. The results revealed that treatment with DW532 led to a dose-dependent increase in apoptosis in the different types of cancer cells tested, including KB mouth epidermal carcinoma cells, MDA-MB-468 human breast carcinoma cells and A431 human epithelial carcinoma cells (Figure 4B). For example, approximately 59.7% of KB cells underwent apoptosis following treatment with DW532 at 1 μmol/L for 48 h. Approximately 70% of the cells underwent apoptosis when the concentration given was 10 μmol/L. We further examined the mechanisms involved in the apoptosis induced by DW532, and the results showed that with increasing DW532 concentrations, the cleavage of PARP, caspase-3 and caspase-9 concomitantly increased (Figure 4C), demonstrating that DW532 induced apoptosis through a caspase-related mechanism.
To clarify the effects of DW532 on the cell cycle, six types of sensitive cell lines were selected for treatment to test their cell cycle distribution. The results showed that 1 μmol/L DW532 treatment nearly arrested all six cell lines at the G2/M phase (Figure 4D). In agreement with these results, the level of two mitotic markers, cyclin B1 and phosphorylated histone H3, was significantly elevated, and the negative regulatory phosphorylation site in CDK1 was also distinctly decreased (Figure 4E). From previous reports, it is known that tubulin inhibitors generally cause cell cycle arrest in G2/M phase18,19, while protein kinase inhibitors induce G1 phase arrest20. It is evident that DW532 can arrest different tumor cell lines at the G2/M phase, supporting the notion that tubulin inhibition might play a major role in the antitumor activity of DW532.
(D) DW532 induced mitosis arrest at G2/M phase. Six cell lines were treated with DW532 at 1 and 10 μmol/L for 24 h followed by flow cytometry analysis. (E) DW532 affected mitotic associated protein. Six cell lines were treated with DW532 at 1 and 10 μmol/L for 24 h followed by Western blot to examine the expression of the proteins.
Therefore, our results demonstrated that DW532 potently inhibited the in vitro proliferation of tumor cells via cell cycle arrest and apoptosis induction.
DW532-induced abnormal division depends on the spindle assembly checkpoint
The above mentioned findings showed that tubulin inhibition might play a major role in the cell cycle arrest induced by DW532. Generally, disruption of the mitotic spindle through tubulin inhibition reduces the microtubule tension or attachment on kinetochores, which then activates the spindle assembly checkpoint (SAC) and delays the onset of anaphase17. To identify the role of DW532-mediated SAC activation in cell cycle arrest, we further evaluated the role of BubR1 and Mps121,22,23, two core SAC proteins, in the cell cycle arrest activity mediated by DW532 using gene knockdown assays. The efficiency of siRNA knockdown was first examined (Figure 5A, 5B), and it was confirmed that siRNA transfection had no obvious effects on the cell cycle (upper panel of Figure 5C, 5D). As expected, the percentage of mitotic cells induced by DW532 was dramatically decreased in cells transfected with siRNAs directed against BubR1 or Mps1 compared with control cells (Figure 5C, 5D, lower panel). These results indicate that tubulin inhibition and the subsequent SAC activation contribute to the mitotic arrest induced by DW532 treatment.
Knockdown of the spindle assembly checkpoint component decreased the DW532 induced mitotic arrest. (A) and (B) efficiency of BubR1 and MPS1 siRNA in MDA-MB-468 cells. (C) and (D) percentage of mitotic cells after knockdown of spindle assembly checkpoint component. After transfected with BubR1 siRNA or Mps1 siRNA (50 nmol/L) or control siRNA for 24 h, MDA-MB-468 cells were treated with DW532 (1 μmol/L) for another 24 h. Mitotic cells were analyzed by p-H3 and PI labeled flow cytometry assays.
DW532 exhibits anti-angiogenesis activity in vitro and in vivo
As VEGFR2 and tubulin play key roles in the process of angiogenesis24,25, we further evaluated the anti-angiogenesis activity of DW532 in vitro and in vivo. The formation of functional tubules in HUVECs plated on Matrigel was first determined. As shown in Figure 6A, HUVEC cells form a complete network structure within 6 h with serum stimulation. After DW532 treatment, the tubule formation was suppressed in a dose-dependent manner. At a concentration of 0.1 μmol/L DW532, the inhibition was approximately 41%, and at 10 μmol/L, there was nearly complete suppression (Figure 6B). We further tested the angiogenesis activity of DW532 using the aortic ring assay, which is a unique ex vivo method that recapitulates the key steps in the angiogenesis process26. As illustrated in Figure 6C, DW532 showed potent inhibition of the microvessel formation of the aortic ring with 18.7% inhibition observed at 0.1 μmol/L and 60.9% and 84.1% inhibition at 1 and 10 μmol/L, respectively (Figure 6D). Moreover, we also examined the effects of DW532 on the new blood vessel formation process using the CAM assay, a model used to study in vivo neovascularization since the early 1970s when it was adapted by Folkmann et al 27 The results showed that vascularization in chick embryos was significantly inhibited when they were treated with DW532 (0.1 or 1 nmol per egg) compared with a no treatment control (Figure 6E). Therefore, these results suggest that as an agent targeting both tubulin and VEGFR2, DW532 potently inhibits angiogenesis in vitro and in vivo.
DW532 had anti-angiogenesis activity. (A) DW532 inhibited the in vitro tube formation of HUVECs. HUVECs containing indicated drugs were seeded in 96-well plates which was coated with Matrigel. After 6 h align, the tube was examined with an inverted phase contrast microscope. (B) Relative tube formation was quantitated and plotted. (C) DW532 inhibited microvessel outgrowth arising from rat aortic rings. Aortic rings were embedded in Matrigel in 96-well plates, and then fed with medium containing various concentrations of DW532 for 7 d. (D) The area of microvessels was quantified and normalized to untreated controls. (E) DW532 showed anti-angiogenesis in a chorioallantoic membrane model. Glasscover-slip saturated with DW532 or normal saline was placed areas between pre-existing vessels in the fertilized chicken eggs and incubated for 48 h. The coverglass saturated with or without DW532 was placed on the right side of per field. Arrows indicated the edge line of the coverglass. cP<0.01 vs control.
Discussion
The aim of this study was to design and identify novel inhibitors targeting both kinases and tubulin. Based on the chemical structure of hematoxylin, a natural product that we previously reported to have kinase inhibitory activity, we rationally designed and synthesized a series of derivatives and evaluated their in vitro antitumor activities. One of the representative derivatives, DW532, showed distinct antitumor activity against cancer cells in preliminary screening (data not shown) and thus was selected for further investigation. This compound has moderate inhibitory activity toward the EGFR and VEGFR2 kinases in vitro and in cancer cells. In addition, DW532 exhibited more potent inhibitory activity on tubulin polymerization by directly binding with tubulin protein. Further experimental results revealed that this molecule disrupted the microtubule networks in cancer cells. This effect is quite similar to VCR, demonstrating that it belongs to the microtubule-destabilizing group of MTAs4. Therefore, our results demonstrated that DW532 could inhibit kinase activity and tubulin polymerization concurrently, which indicates the success of our original concept of the chemical designing of a dual-functional agent for tumor therapy. Until now, only a few agents have been reported to possess dual inhibitory effects on both kinases and tubulin8,9,10,28,29. These reported agents are structurally different from DW532. Therefore, DW532 is a dual inhibitor of kinases and tubulin with a new scaffold, and it deserves further investigation.
Inhibition of tubulin or kinases can result in many effects on tumor cells, such as cell-cycle arrest, apoptosis and growth inhibition6. Our results show that DW532 treatment causes cell cycle arrest, apoptosis and subsequently anti-proliferation activity in a panel of cancer cells. The results from flow cytometry revealed that DW532 treatment led to G2/M phase cell cycle arrest in different type of cancer cells with concomitant alterations in the mitotic cell cycle-associated proteins cyclin B1, p-CDK1, and p-H330. Generally, tyrosine kinase inhibitors trigger G1 phase arrest, while tubulin inhibitors induce G2/M phase arrest, and our results support the idea that tubulin inhibition plays a more important role in DW532 anti-tumor activity than kinase inhibition, reinforcing the results from the tubulin and kinase assays performed in vitro in which much higher concentrations of DW532 were required to inhibit kinases than tubulin. As it is recognized that microtubule inhibitors can directly alter microtubule polymerization at high concentration, they can influence the dynamics of microtubules and activate the SAC to block cells in mitosis, eventually inducing cell death at lower concentrations31. We found that knocking down BubR1 and Mps1, which are core proteins of the SAC, decreased the cancer cell sensitivity to DW532, further indicating that tubulin inhibition and the subsequent activation of SAC contributes to the mitosis induced by DW532.
Angiogenesis is an important and necessary phenomenon in tumors. Cancerous cells stimulate the formation of vasculature around themselves in a process called tumor-driven angiogenesis for survival and growth. Anti-angiogenesis has been proven to be an effective and efficient anti-tumor strategy32. The treatment of cancers with both a tumor angiogenesis inhibitor, such as a VEGFR inhibitor, and tumor vascular-targeting drugs, such as a tubulin-targeting agent, could produce anti-angiogenesis effects. Our study demonstrates that DW532 not only inhibits the in vitro tube formation of HUVEC cells and ex vivo microvessel outgrowth of the aortic ring, it also shows potent anti-angiogenesis activity in vivo as evidenced by the inhibition of the blood vessel formation in CAM assays. Therefore, as a dual inhibitor of these two targets, DW532 exhibited potent inhibition of angiogenesis, which contributes to the antitumor activity of the compound.
Taken together, we have identified DW532 as a novel agent that has dual effects, targeting both protein kinases and tubulin polymerization. This compound showed significant antitumor activity in vitro and exhibited anti-angiogenesis effects in vitro and in vivo. These characteristics, combined with better solubility and a convenient approach to synthesis, make DW532 a promising anti-cancer agent in cancer therapy.
Author contribution
Ting PENG, Wen-hu DUAN, Hua XIE, and Jian DING participated in the research design; Ting PENG, Jian-rui WU, Lin-jiang TONG, Meng-yuan LI, Kun HAN, Rong QU, Yi SU, and Yi CHEN conducted experiments; Ting PENG, Jian-rui WU, Fang CHEN, and Yi-xin LENG contributed new reagents or analytic tools; Ting PENG, Jian-rui WU, Wen-hu DUAN, and Hua XIE performed the data analysis; Ting PENG, Jian-rui WU, Wen-hu DUAN, and Hua XIE wrote or contributed to the writing of the manuscript.
References
Giamas G, Man YL, Hirner H, Bischof J, Kramer K, Khan K, et al. Kinases as targets in the treatment of solid tumors. Cell Signalling 2010; 22: 984–1002.
Yarden Y, Pines G . The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12: 553–63.
Shibuya M . Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013; 153: 13–9.
Jordan MA, Wilson L . Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253–65.
Liu ZL, Tian W, Wang Y, Kuang S, Luo XM, Yu Q . A novel sulfonamide agent, MPSP-001, exhibits potent activity against human cancer cells in vitro through disruption of microtubule. Acta Pharmacol Sin 2012; 33: 261–70.
Chan KS, Koh CG, Li HY . Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 2012; 3: e411.
Gossage L, Eisen T . Targeting multiple kinase pathways: a change in paradigm. Clin Cancer Res: offic J Am Assoc Cancer Res 2010; 16: 1973–8.
Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 2008; 26: 5544–52.
Antonarakis ES, Heath EI, Posadas EM, Yu EY, Harrison MR, Bruce JY, et al. A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol 2013; 71: 883–92.
Katayama R, Aoyama A, Yamori T, Qi J, Oh-hara T, Song Y, et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res 2013; 73: 3087–96.
Ren X, Dai M, Lin LP, Li PK, Ding J . Anti-angiogenic and vascular disrupting effects of C9, a new microtubule-depolymerizing agent. Br J Pharmacol 2009; 156: 1228–38.
Lin LG, Xie H, Li HL, Tong LJ, Tang CP, Ke CQ, et al. Naturally occurring homoisoflavonoids function as potent protein tyrosine kinase inhibitors by c-Src0based high-throughput screening. J Med Chem 2008; 51: 4419–29.
Zhang X, Peng T, Ji X, Li J, Tong L, Li Z, et al. Design, synthesis and biological evaluation of novel 4-anilinoquinazolines with C-6 urea-linked side chains as inhibitors of the epidermal growth factor receptor. Bioorg Med Chem 2013; 21: 7988–98.
Zhang Z, Meng T, He J, Li M, Tong LJ, Xiong B, et al. MT7, a novel compound from a combinatorial library, arrests mitosis via inhibiting the polymerization of microtubules. Investigat New Drugs 2010; 28: 715–28.
DiStefano JF, Cotto CA, Lane B, Hagag N . Role of epidermal growth factor in the expression of A431 cancer cell protease and red blood cell cytotoxicity. Cancer Res 1989; 49: 179–84.
Imaizumi T, Itaya H, Nasu S, Yoshida H, Matsubara Y, Fujimoto K, et al. Expression of vascular endothelial growth factor in human umbilical vein endothelial cells stimulated with interleukin-1alpha — an autocrine regulation of angiogenesis and inflammatory reactions. Thromb Haemost 2000; 83: 949–55.
Kline-Smith SL, Walczak CE . Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell 2004; 15: 317–27.
Gan PP, McCarroll JA, Po'uha ST, Kamath K, Jordan MA, Kavallaris M . Microtubule dynamics, mitotic arrest, and apoptosis: drug-induced differential effects of betaIII-tubulin. Mol Cancer Ther 2010; 9: 1339–48.
Ma YM, Zhou YB, Xie CM, Chen DM, Li J . Novel microtubule-targeted agent 6-chloro-4-(methoxyphenyl) coumarin induces G2-M arrest and apoptosis in HeLa cells. Acta Pharmacol Sin 2012; 33: 407–17.
Ling YH, Li T, Yuan Z, Haigentz M Jr, Weber TK, Perez-Soler R . Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27KIP1 up-regulation and nuclear translocation in association with cell growth inhibition and G1/S phase arrest in human non-small-cell lung cancer cell lines. Mol Pharmacol 2007; 72: 248–58.
De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol 2005; 15: 214–25.
Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 2003; 161: 281–94.
Weiss E, Winey M . The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol 1996; 132: 111–23.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA . Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56: 549–80.
Bijman MN, Amerongen GP, Laurens N, van Hinsbergh VW, Boven E . Microtubule-targeting agents inhibit angiogenesis at subtoxic concentrations, a process associated with inhibition of Rac1 and Cdc42 activity and changes in the endothelial cytoskeleton. Mol Cancer Ther 2006; 5: 2348–57.
Nicosia RF, Ottinetti A . Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Laboratory investigation; J Tech Methods Pathol 1990; 63: 115–22.
Auerbach R, Arensman R, Kubai L, Folkman J . Tumor-induced angiogenesis: lack of inhibition by irradiation. Int J Cancer J Int Cancer 1975; 15: 241–5.
Anbalagan M, Ali A, Jones RK, Marsden CG, Sheng M, Carrier L, et al. Peptidomimetic Src/pretubulin inhibitor KX-01 alone and in combination with paclitaxel suppresses growth, metastasis in human ER/PR/HER2-negative tumor xenografts. Mol Cancer Ther 2012; 11: 1936–47.
Rochais C, Cresteil T, Perri V, Jouanne M, Lesnard A, Rault S, et al. MR22388, a novel anti-cancer agent with a strong FLT–3 ITD kinase affinity. Cancer Lett 2013; 331: 92–8.
Malumbres M, Barbacid M . Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9: 153–66.
Dumontet C, Jordan MA . Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 2010; 9: 790–803.
Kubota Y . Tumor angiogenesis and anti-angiogenic therapy. The Keio J Med 2012; 61: 47–56.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81173080, 81273365, and 81321092) and the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” of the People's Republic of China (No 2012ZX09103101-024).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Peng, T., Wu, Jr., Tong, Lj. et al. Identification of DW532 as a novel anti-tumor agent targeting both kinases and tubulin. Acta Pharmacol Sin 35, 916–928 (2014). https://doi.org/10.1038/aps.2014.33
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.33
Keywords
This article is cited by
-
New antiproliferative 3-substituted oxindoles inhibiting EGFR/VEGFR-2 and tubulin polymerization
Molecular Diversity (2023)
-
DW10075, a novel selective and small-molecule inhibitor of VEGFR, exhibits antitumor activities both in vitro and in vivo
Acta Pharmacologica Sinica (2016)
-
Antitumor action of CDK inhibitor LS-007 as a single agent and in combination with ABT-199 against human acute leukemia cells
Acta Pharmacologica Sinica (2016)
-
Deacetylisovaltratum disrupts microtubule dynamics and causes G2/M-phase arrest in human gastric cancer cells in vitro
Acta Pharmacologica Sinica (2016)