Abstract
Aim:
Appoptosin (SLC25A38) is a pro-apoptotic protein, which is upregulated in Alzheimer's disease (AD) brains and plays an important role in promoting the pathological progress of AD. The aim of this study was to investigate the effects of curcumin from the rhizome of Curcuma longa on appoptosin-induced apoptosis in SH-SY5Y cells.
Methods:
SH-SY5Y cells were pretreated with curcumin, then transfected with appoptosin or vector. The apoptotic cells were detected with Annexin V staining analysis by flow cytometry. The expression of cleaved caspase-3, appoptosin, heme oxygenase-1 (HO-1) was examined using Western blotting. Intracellular level of ROS was measured with DCFH-DA staining by flow cytometry analysis. Mitochondrial membrane potential (ΔΨm) was detected with JC-1 staining under a fluorescence microscope and quantified by fluorescence ratio detection.Overexpression of appoptosin in SH-SY5Y cells markedly increased cell apoptosis accompanied by reduced HO-1 expression, increased intracellular heme level, ROS overproduction and ΔΨm impairment. Treatment of SH-SY5Y cells with curcumin (2.5–20 μmol/L) for 24 h did not significantly affect their viability. However, pretreatment with curcumin (2.5–20 μmol/L) dose-dependently attenuated all above-mentioned pathological changes in appoptosin-transfected SH-SY5Y cells.
Results:
Overexpression of appoptosin in SH-SY5Y cells markedly increased cell apoptosis accompanied by reduced HO-1 expression, increased intracellular heme level, ROS overproduction and ΔΨm impairment. Treatment of SH-SY5Y cells with curcumin (2.5–20 μmol/L) for 24 h did not significantly affect their viability. However, pretreatment with curcumin (2.5–20 μmol/L) dose-dependently attenuated all above-mentioned pathological changes in appoptosin-transfected SH-SY5Y cells.
Conclusion:
Curcumin inhibits appoptosin-induced apoptosis in SH-SY5Y cells by upregulating the expression of HO-1, reducing the production of intracellular heme and ROS, and preventing the ΔΨm loss.
Similar content being viewed by others
Introduction
As a most common dementia, Alzheimer's disease (AD) is characterized by neuropathological hallmarks of extracellular senile plaques, intracellular neurofibrillary tangles, progressive loss of neurons, synaptic dysfunction, and disequilibrium of multiple neurotransmitter systems1,2,3. Although the central hypothesis for the pathogenesis of AD is the amyloid hypothesis, the exact mechanism underlying AD pathogenesis remains unclear4. A growing number of studies have documented that apoptosis play an important role in the onset and progression of AD, which can be triggered by multiple factors including oxidative stress, mitochondrial dysfunction, dysregulation of ion-homeostasis, reduced clearance of toxin, protein aggregation and so on5,6,7,8.
As a new β-amyloid precursor protein (APP)-interacting pro-apoptotic protein, SLC25A38 has recently been identified and designated as appoptosin for its pro-apoptotic feature. Appoptosin is upregulated in AD brains as well as in neurons treated with β-amyloid9. It belongs to the mitochondrial solute carrier family (SLC25), which is encoded by nuclear genes located on chromosome 3p22.1, synthesized in the cytosol and located in the inner mitochondrial membrane. Appoptosin acts as a transporter that shuttles glycine into mitochondria and 5-aminolevulinic acid out of mitochondria10,11. Further research has found that appoptosin regulates intrinsic caspase-dependent apoptosis by governing heme biosynthesis, hence inducing ROS overproduction, impairing mitochondrial membrane potential, promoting cytochrome c release, and activating caspase 9 and caspase 39. Therefore, preventing appoptosin-induced intrinsic caspase-dependent apoptosis would be a promising therapeutic alternative in AD treatment.
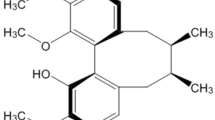
Curcumin, also known as diferuloylmethane (C21H20O6) is a golden component of turmeric isolated from the rhizome of Curcuma longa12. The chemical structure of curcumin consists of two aryl rings containing ortho-methoxy phenolic OH- groups linked to a β-diketone moiety symmetrically (Figure 1)13. It is a low molecular mass (368.37 g/mol) polyphenol compound and has long been used in medicinal preparation. It has been documented to possess multiple pharmaceutical properties such as antioxidation, anti-inflammation, anti-atherosclerosis, anticancer, and anti-arthritis14. Curcumin has desirable characteristics for being a neuroprotective drug, including antioxidant, anti-inflammatory, anti-protein-aggregated activities and so on15,16,17. Since oxidative stress plays an important role in the progress of appoptosin-induced cell apoptosis, it would be worth testing whether curcumin can protect against appoptosin-induced apoptosis.
Chemical structure of curcumin.
Materials and methods
Chemical reagents
pCMV-appoptosin plasmid, pCMV-vector plasmid, and anti-appoptosin were obtained from Institute of Neuroscience, College of Medicine, Xiamen University (Xiamen, China); TurboFect in vitro transfection reagent was from Fermentas Life Science (Burlington, ON, Canada); anti-cleaved caspase 3 and anti α-tubulin were from Cell Signaling Technology (Beverly, MA, USA); anti-heme oxygenase 1 (HO-1) was from Abcam (Cambridge, UK); HRP-conjugated secondary antibody was from Invitrogen (Carlsbad, CA, USA); MTT, curcumin, dimethyl sulfoxide (DMSO) were purchased from Sigma (St Louis, MO, USA); Heme Colorimetric Assay Kit was obtained from BioVision Inc (Milpitas, CA, USA); JC-1 mitochondrial membrane potential detection kit was from Cell Technology Inc (Mountain View, CA, USA); annexin V-FITC apoptosis detection kit was from Calbiochem of Merck Millipore (Billerica, MA, USA); and reactive oxygen species assay kit was from Beyotime Biotechnology (Haimen, China). Curcumin was dissolved in DMSO to prepare an 8 mmol/L stock solution which was later stored at −20 °C.
Cell culture and treatment
SH-SY5Y cells were maintained in a high-glucose DMEM containing 10% fetal bovine serum (FBS). To exclude the heme interference from the serum, the cells were cultured in serum-free neurobasal medium in a condition of 95% filtered air and 5% CO2 at 37 °C for heme assays.
In experiments, the cells were cultured at a density of 2×105 cells/well in 6-well plates (or 2×104 cells/well in 24-well ones, 5×103 cells/well in 96-well ones) the day before treatment. Cells were pretreated with curcumin or vehicle 1 h before transfection. The medium was replaced with fresh medium containing corresponding concentration of curcumin 6 h after transfection. All measurements were performed 24 h after transfection. The control cells received no treatment. Three parallel experiments were performed in every test. To excluse the effect of DMSO (as dissolvent for curcumin), the final concentration of DMSO in the solution of each group including the control one was adjusted as the concentration of DMSO in the solution of 20 μmol/L curcumin-treated group, which was 0.25%.
Cell viability assay
Cell viability was determined by the MTT assay according to manufacturer's instructions. Briefly, cells were seeded into 96-well plates in 100 μL of medium. Twelve hours later, the cells were incubated with various concentrations of curcumin for another 24 h or 48 h. Then 10 μL of 5 mg/mL MTT (dissolved in 0.01 mol/L PBS) was added to the medium in every well. Four hours after the addition, the medium containing MTT was discarded and 150 μL of DMSO was added to dissolve the formazan product. The absorbance was read with a Bio-Tek EPOCH Microplate Reader (Bio-Tek Instruments Inc, USA) at the wavelength of 490 nm. The control cells received no treatment. The result was expressed as a percentage relative to the control.
Annexin V staining analysis
SH-SY5Y cells were seeded into six-well culture plates the day before treatment. The appoptosin-transfected cells were pretreated with various concentrations of curcumin 1 h prior to transfection. Vector-transfected cells were pretreated with vehicle. Twenty-four hours after transfection, both types of cells were collected and stained with annexin V-FITC. Briefly, cells were collected (detached by 0.25% trypsinization without EDTA) in the 1.5 mL centrifuge tube by centrifugation. After being washed with 0.01 mol/L PBS, cells were resuspended in 500 μL of binding buffer and incubated with 1.25 μL of annexin V-FITC in the dark at room temperature for 15 min. Following centrifugation, cells were resuspended in 500 μL of binding buffer again and incubated with 10 μL of propidium iodide. Fifteen minutes after incubation, the staining results were detected by flow cytometry analysis. The control cells received no treatment.
Western blotting analysis
SH-SY5Y cells were pretreated with various concentrations of curcumin, and then transfected with appoptosin or vector 1 h later. They were collected 24 h after transfection and lysated. Briefly, the cell protein lysates were quantified by BCA assay. Then equal amount of cell protein lysates were separated by SDS-poly-acrylamide gel electrophoresis and transferred to PVDF membranes, which were blocked with 5% skim milk at room temperature for 1 h. The PVDF membranes were then correspondingly incubated with rabbit cleaved caspase 3 antibody (1:1000), rabbit anti-heme oxygenase-1 (HO-1) antibody (1:1000), rabbit anti-appoptosin (1:1000), and mouse anti-α-tubulin antibody (1:2000) at 4 °C over-night. After a 10-min wash in TBST washing buffer for three times, the PVDF membranes were further incubated with HRP-conjugated secondary antibody for 90 min and detected for enhanced chemiluminescence after a 10-min wash with TBST washing buffer for three times.
Intracellular reactive oxygen species (ROS) measurement
SH-SY5Y cells were pretreated with curcumin or vehicle 1 h prior to transfection. Appoptosin-transfected cells received 10 μmol/L curcumin or vehicle while vector-tranfected ones received vehicle. Twenty-four hours after transfecion, cells were collected in 1.5 mL centrifuge tube, resuspended in DMEM with 10 μmol/L dichlorofluorescein diacetate (DCFH-DA), and then incubated in the dark at 37 °C. Thirty minutes later, they were washed with 0.01 mol/L PBS three times and resuspended in 0.01 mol/L PBS. Then the relative levels of fluorescence were determined by flow cytometry analysis at excitation wavelength of 488 nm and emission wavelength of 525 nm. The intracellular ROS was evaluated by fluorescence of DCF. The control cells received no treatment. And cells treated with ROS-up acted as the positive control whose intracellular ROS level was indeed overaccumulation in our experiment. ROS-up is some substance that can induce intracellular ROS level significant elevation in 20–30 min after stimulation usually.
Mitochondrial membrane potential (ΔΨm) assay
SH-SY5Y cells were cultured in 24-well plates with a glass cover slip the day before treatment. Then cells were pretreated with curcumin or vehicle 1 h prior to transfection. Appoptosin-transfected cells received 10 μmol/L curcumin or vehicle while vector-tranfected ones received vehicle. The control cells received no treatment. Twenty-four hours after transfection, ΔΨm was detected with the JC-1 mitochondrial transmembrane potential detection kit according to the manufacturer's instructions. Briefly, the culture media were replaced with enough JC-1 reagent to cover the cells. Then the cells were incubated in a 5% CO2 incubator at 37 °C, washed with assay buffer 15 min later and observed immediately under a fluorescence microscope using a “dual-bandpass” filter designed to simultaneously detect fluorescein and rhodamine or fluorescein and Texas Red. ΔΨm was assessed on the basis of the color of fluorescence. In normal cells, the intact mitochondrial membrane potential allows the dye to enter the mitochondrial matrix. When the dye accumulating in mitochondrial matrix exceeds the threshold concentration, it aggregates appearing fluorescent red. So the mitochondria are stained bright red. In apoptotic cells, the mitochondrial membrane potential collapses, the JC-1 cannot accumulate in the mitochondria and remains in its monomeric form in the cytoplasm appearing green. To further quantify ΔΨm by fluorescence ratio detection, SH-SY5Y cells were seeded into 6-well culture plates and received the same treatment above. Then cells were collected to a sterile centrifuge tube before incubating with JC-1 reagent in a 5% CO2 incubator at 37 °C for 15 min. After incubation, cells were washed with assay buffer for 3 times, and then resuspended in 300 μL of assay buffer. An amount of 100 μL of the cell suspension was transferred into wells of a black 96-well plate. Fluorescence was measured (red fluorescence at excitation 550 nm, emission 600 nm, and green fluorescence at excitation 485 nm, emission 535 nm) with a fluorescence plate reader FlexStation 3 (Molecular Devices Inc, USA).
Intracellular heme measurement
SH-SY5Y cells were cultured in 96-well plates the day before treatment. One hour before transfection, cells were pretreated with various concentrations of curcumin. Then cells were transfected with appoptosin or vector. The control cells received no treatment. Twenty-four hours after transfection, intracellular heme measurement was performed with Heme Colorimetric Assay Kit. Briefly, the medium was removed and 50 μL reaction mix (containing 2 μL probe, 2 μL substrate, 3 μL enzyme mix and 43 μL assay buffer) was added to each well which contained the heme standard or test samples. The mixture was incubated in the dark for 30 min at room temperature before the OD measurement at 570 nm.
Statistical analysis
Every result was obtained from at least three independent experiments, and all values were presented as the mean±standard error of the mean (SEM). Statistical analysis was conducted with the GraphPad Prism 5 software. One-way or two-way analysis of variance (ANOVA) was used for all data analyses. P value less than 0.05 was accepted as statistical significance.
Results
Effects of curcumin on SH-SY5Y cell viability
To find out the safe concentration of curcumin for SH-5YSY cells, we treated SH-SY5Y cells with various concentrations of curcumin (2.5–80 μmol/L) for 24 h or 48 h. Then cell viability was measured by MTT assay. The result showed that no cytotoxic effect of curcumin on cell viability was observed at the concentrations of 2.5–20 μmol/L for 24 h (P=0.8392) or at the concentrations of 2.5–10 μmol/L for 48 h (P=0.0509) (Figure 2). Thus 1.25–20 μmol/L curcumin for 24 h was chosen to study the effect of curcumin on appoptosin-induced apoptosis in SH-SY5Y cells.
Effect of curcumin on the viability of SH-SY5Y cells. Cells were treated with various concentrations of curcumin (2.5, 5, 10, 20, 40, and 80 μmol/L) for 24 h or 48 h. Cell viability was then determined by MTT assay. The control cells received no treatment. Values were presented as mean±SEM of six determinations, compared with control. cP<0.01 (24 h) or fP<0.01 (48 h).
Curcumin attenuates appoptosin-induced apoptosis in SH-SY5Y cells
SH-SY5Y cells were transfected with appoptosin or vector plasmids and treated with various concentrations of curcumin for 24 h. Cells were then harvested for Western blot. Compared with that of vector-transfected cells, appoptosin was highly expressed in cells transfected with appoptosin (P<0.01). Overexpression of appoptosin resulted in increased levels of cleaved caspase 3, indicating apoptosis. Curcumin treatments did not affect the transfection efficiency and expression of appoptosin (P=0.8235). However, curcumin treatments significantly attenuated the increase of cleaved caspase 3 levels induced by appoptosin overexpression in a concentration-dependent manner from 1.25 μmol/L to 20 μmol/L (Figure 3).
Curcumin inhibits appoptosin-induced apoptosis. SH-SY5Y cells were transfected with appoptosin or vector, and then treated with various concentrations (0, 1.25, 2.5, 5, 10, and 20 μmol/L) of curcumin for 24 h. Cells were collected and lysed. Equal amounts of cell protein lysates were immunoblotted for α-tubulin, appoptosin, cleaved caspase-3, and quantified by densitometry and normalized to corresponding α-tubulin for statistical comparison. (A) Representative Western blot bands of α-tubulin, appoptosin, cleaved caspase-3. (B) Quantitative analysis of appoptosin expression. Compared with vector-transfected SH-SY5Y cells, the expression of appoptosin in appoptosin-transfected cells increased significantly. Curcumin had no effect on the expression of appoptosin. (C) Quantitative analysis of cleaved caspase-3 expression in appotosin-transfected SH-SY5Y cells that were treated with various concentrations of curcumin. n=3. bP<0.05, cP<0.01.
To further confirm the protective effect of curcumin on appoptosin-induced apoptosis in SH-SY5Y cells, we analyzed appoptosin-induced apoptosis by flow cytometry through annexin V staining. Little cell apoptosis was observed in control cells (about 3.09%–4.81%) as well as in vector-transfected ones (about 3.80%–4.86%) (P=0.7064). Compared to the control cells, the percentage of apoptotic cells in appoptosin-transfected cells (about 34.37%–42.68%) was significantly increased (P=0.0022). Curcumin treatments attenuated cell apoptosis induced by appoptosin in a concentration-dependent manner (from 1.25 μmol/L to 20 μmol/L) (P<0.01) (Figure 4).
Curcumin attenuates appoptosin-induced apoptosis. SH-SY5Y cells were transfected with vector or appoptosin. Un-transfected cells were used as control, as well as vector-transfected cells that were treated with vehicle (0 μmol/L curcumin). Appoptosin-transfected cells were treated with various concentrations (0–20 μmol/L) of curcumin for 24 h. Cells were collected and stained with annexin V-FITC, and then analyzed by flow cytometry. Representative drawings and the percentage of annexin V-positive cells were presented. The percentage of annexin V-positive (apoptotic) cells was quantitated. n=3. cP<0.01. fP<0.01.
The protective effect of curcumin on appoptosin-induced apoptosis was most remarkable at high concentrations. Therefore, we chose the concentration of 10 μmol/L to evaluate the possible protective mechanism of curcumin in appoptosin-induced apoptosis.
Curcumin prevents cells from appoptosin-induced overproduction of intracellular ROS
Intracellular ROS has been reported to play an important role in appoptosin-induced apoptosis9. In this study, appoptosin-transfected SH-SY5Y cells were treated with 10 μmol/L curcumin or vehicle (0 μmol/L curcumin) for 24 h. ROS was then detected by flow cytometry. Compared with control, intracellular ROS was significantly elevated in appoptosin-transfected cells (P<0.01), but not in vector-transfected ones (P=0.3811). Curcumin remarkably reduced the intracellular ROS level of appoptosin-transfected cells (P=0.0036) (Figure 5).
Curcumin reduces intracellular ROS in appoptosin-transfected SH-SY5Y cells. SH-SY5Y Cells were transfected with vector or appoptosin. Vector-transfected cells were treated with vehicle (0 μmol/L curcumin) for 24 h. Appoptosin-transfected cells were treated with vehicle (0 μmol/L curcumin) or 10 μmol/L of curcumin for 24 h. Normal control cells received no treatment. ROS-up-treated cells served as the positive control. ROS-up is a substance that can induce intracellular ROS level significant elevation. Intracellular ROS was assayed by flow cytometry analysis and quantitated. n=3. cP<0.01. fP<0.01.
Curcumin attenuates ΔΨm loss in appoptosin-overexpressed cells
To evaluate whether curcumin protects against appoptosin-induced impairment of mitochondrial membrane potential, we treated appoptosin-transfected SH-SY5Y cells with 10 μmol/L curcumin or vehicle (0 μmol/L curcumin) for 24 h. The ΔΨm was assayed by fluorescence microscopy and fluorescence ratio detection. ΔΨm was evaluated under a fluorescence microscope, in which red fluorescence presents normal ΔΨm while green fluorescence indicates ΔΨm loss. The result showed that ΔΨm of vector-transfected cells appeared normal while that of appoptosin-transfected ones was insulted obviously. Curcumin attenuated appoptosin-induced ΔΨm loss (Figure 6A). Furthermore, ΔΨm was quantitatively analyzed by fluorescence ratio detection. As shown in Figure 6B, compared with that of control, ΔΨm of vector-transfected cells remained unaffected (P=0.3420), while that of appoptosin-transfected cells decreased significantly (P=0.0003). Curcumin noticeably inhibited appoptosin-induced ΔΨm loss (P=0.0013).
Effect of curcumin on rescuing appoptosin-induced mitochondrial membrane potential impairment. SH-SY5Y cells were transfected with vector or appoptosin. Vector-transfected cells were treated with vehicle (0 μmol/L curcumin) for 24 h. Appoptosin-transfected cells were treated with vehicle (0 μmol/L curcumin) or 10 μmol/L of curcumin for 24 h. Normal control cells received no treatment. The mitochondrial membrane potential was assayed with JC-1 staining. (A) The cells were detected by fluorescence microscopy. ΔΨm was evaluated on the basis of the color of fluorescence. Red fluorescence represented normal ΔΨm, while green fluorescence indicated ΔΨm insult. (B) ΔΨm was quantified by fluorescence ratio detection. The ratio of red to green fluorescence is decreased in dead cells and in cells undergoing apoptosis compared to healthy cells. n=3. cP<0.01. fP<0.01.
Curcumin reduces intracellular heme level in appoptosin-overexpressed SH-SY5Y cells
SH-SY5Y cells were transfected with appoptosin or vector plasmids and appoptosin-transfected cells were treated with various concentrations of curcumin. At the end of treatment, intracellular heme level was determined with Heme Colorimetric Assay Kit. As shown in Figure 7, compared with that of control or vector-transfected cells, intracellular heme level of appoptosin-transfected cells was significantly elevated (P=0.0008 and P=0.0019, respectively), but no difference was found between control and vector-transfected ones (P=0.7731). Compared with that of appoptosin-transfected cells which received no curcumin treatment, intracellular heme level decreased significantly (P<0.01) in appoptosin-transfected ones which were treated with various concentrations of curcumin, especially at high concentrations (from 5 μmol/L to 20 μmol/L).
Effect of curcumin on intracellular heme level in appoptosin-transfected SH-SY5Y cells. SH-SY5Y cells were transfected with appoptosin or vector. Vector-transfected cells were treated with vehicle (0 μmol/L curcumin) for 24 h. Appoptosin-transfected cells were treated with various concentrations (0, 1.25, 2.5, 5, 10, and 20 μmol/L) of curcumin for 24 h. Normal control cells received no treatment. Intracellular heme level was determined with Heme Colorimetric Assay Kit and quantitatively analyzed for statistical comparison. n=3. cP<0.01. eP<0.05, fP<0.01.
Effect of curcumin on HO-1 expression in appoptosin-overexpressed SH-SY5Y cells
Heme oxygenase (HO), especially HO-1, serves as a rate-limiting enzyme in the degradation of heme18,19. In this study, SH-SY5Y cells were transfected with appoptosin or vector. Appoptosin-transfected cells were treated with 10 μmol/L curcumin or vehicle (0 μmol/L curcumin) for 24 h. The expression of HO-1 in SH-SY5Y cells was analyzed by Western blot. Compared with that of the control, the expression of HO-1 was not affected in vector-transfected cells (P=0.9558) or normal cells treated with 10 μmol/L curcumin (P=0.8594), but significantly downregulated in appoptosin-transfected cells (P=0.0060). Treatments with curcumin attenuated the reduction of HO-1 in appoptosin-transfected cells (P=0.0063) (Figure 8).
Effect of curcumin on HO-1 expression in appoptosin-transfected SH-SY5Y cells. SH-SY5Y cells were transfected with vector or appoptosin. Vector-transfected cells were treated with vehicle (0 μmol/L curcumin) for 24 h. Appoptosin-transfected cells were treated with vehicle (0 μmol/L curcumin) or 10 μmol/L of curcumin for 24 h. To evaluate the effect of curcumin on HO-1, we treated normal cells with 10 μmol/L of curcumin for 24 h. Normal control cells received no treatment. For statistical comparison, equal amounts of cell protein lysates were immunoblotted for HO-1 and quantitated. n=3. cP<0.01. fP<0.01.
Discussion
In the present study, we confirmed that overexpression of appoptosin can induce heme biosynthesis, resulting in ROS overproduction, mitochondrial ΔΨm loss, and intrinsic caspase-dependent apoptosis. Importantly, we found that curcumin dramatically inhibited appoptosin-induced cytotoxicity as described above. In addition, curcumin treatments can upregulate HO-1 level and reduce intracellular heme level.
Heme is a complex of iron with protoporphyrin IX. It appears in aerobic cells ubiquitously, and plays key regulatory roles in cell biological processes including oxidation-reduction reactions, signal transduction and drug metabolism. Yet excessive levels of intracellular heme are toxic to cell. Redox-active iron of heme plays the central role for heme toxicity20. When heme, especial free heme accumulates, the heme detoxification systems are overwhelmed. Then heme exerts its damaging effects such as promoting lipid peroxidation, damaging protein, and DNA through oxidative stress by generating ROS, resulting in membrane injury and cell apoptosis21,22,23,24,25.
Heme has been confirmed to be biosynthesized in brain cells and greatly increased in AD brains26. Heme can colocalize with AD senile plaques27. Excessive heme, especially free heme in the brain, is confirmed as a common factor for related metabolic perturbations. As a primary agent in AD, amyloid-β peptide initiates the main cytopathologies of AD, such as oxidative stress, mitochondrial dysfunction, accumulation of iron in cells and so on28,29,30,31. Recent evidence shows that Aβ can induce intracellular heme synthesis and iron uptake in vitro9,32. Heme can bind to Aβ to form heme-Aβ, promoting pathological cellular processes including overproduction of ROS, abnormal iron homeostasis, mitochondrial dysfunction, and consequently neuron apoptosis24,26,33,34.
Appoptosin plays a critical role in the process of heme synthesis and is overexpressed in AD brains. It has been identified as a transporter of glycine/5-aminolevulinic acid across the mitochondrial inner membrane, a decisive process in heme synthesis. Mutations of appoptosin were confirmed as a cause of inherited sideroblastic anemia10. The intracellular level of heme increases with the upregulation of appoptosin and decreases with the downregulation of appoptosin. High level of intracellular heme, especial heme-b, is associated with a risk of oxidative damage since it is a prooxidant35. Over production and accumulation of heme in cells may exacerbate production of intracellular ROS and increase oxidative stress on cells, thus promoting cell apoptosis21,23. Recent studies in vitro have shown that appoptosin can induce heme biosynthesis, resulting in ROS overproduction, mitochondrial ΔΨm loss, and intrinsic caspase-dependent apoptosis, especially when cells overexpress appoptosin during 12 h to 36 h9. Accumulation of heme may play a key role in the pathological progress of appoptosin-induced apoptosis.
Antioxidant is a main medicinal characteristic of curcumin. Several studies show that curcumin can exert powerful oxygen free radical-scavenging effect and exhibit neuroprotective effect against oxidative damage in nervous system36,37,38,39. Curcumin may protect cortical neurons from tert-butyl hydroperoxide (t-BHP)-induced apoptosis in rat cortical neurons40. In the process of appoptosin-induced apoptosis, oxidative stress plays an important role, which is induced by overproduction of intracellular heme. In this study, we found that curcumin prevented intracellular heme from increasing and inhibited appoptosin-induced apoptosis in a concentration-dependent manner, but did not affect the expression of appoptosin. Our results also showed that curcumin can reduce intracellular ROS and relieve appoptosin-induced mitochondrial ΔΨm loss.
Heme oxygenase (HO) acts as the rate-limiting enzyme in the degradation of heme18,19, including two functionally active isoforms, HO-1 and HO-2. The former can predominantly catabolize intracellular heme and convert it to bilirubin, carbon monoxide (CO), and free iron, thus controlling the level of cellular heme41. In return, increased heme level can induce elevated HO-1 expression. Here we found that intracellular heme was accumulated and HO-1 was downregulated in appoptosin-overexpressing SH-SY5Y cells. We speculate that in the early stage, HO-1 expression is upregulated by increased heme to prevent intracellular heme from accumulating. But the persistent high level heme would consume HO-1 and lead to the downregulation at last. An overriding principle shows that HO-1 expression can initially rapidly increase in response to an increased cellular heme but subsequently decline to a low level20. Our further result showed that curcumin inhibited the downregulation of HO-1 and prohibited the increase of intracellular heme in appoptosin-overexpressing SH-SY5Y cells. However, curcumin didn't affect the expression of HO-1 of cells under normal physiological condition.
In conclusion, our data indicate that curcumin can protect SH-SY5Y cells against appoptosin-induced intrinsic caspase-dependent apoptosis by upregulating HO-1, attenuating accumulation of intracellular heme and ROS, inhibiting ΔΨm loss, and thereby blocking apoptosis.
Author contribution
Xiao-chun CHEN, Yun-wu ZHANG, and Kun-mu ZHENG designed research; Kun-mu ZHENG performed research; Kun-mu ZHENG, Jing ZHANG, and Cui-lin ZHANG contributed new reagents or analytic tools; Xiao-chun CHEN, Yun-wu ZHANG, and Kun-mu ZHENG analyzed data; Kun-mu ZHENG, Jing ZHANG, and Xiao-chun CHEN wrote the paper. Kun-mu ZHENG is the only first author of the article.
References
Selkoe DJ . Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 2001; 81: 741–66.
Gouras GK, Willen K, Faideau M . The inside-out amyloid hypothesis and synapse pathology in Alzheimer's disease. Neurodegener Dis 2014; 13: 142–6.
Castello MA, Soriano S . On the origin of Alzheimer's disease. Trials and tribulations of the amyloid hypothesis. Ageing Res Rev 2014; 13: 10–2.
Armstrong RA . The pathogenesis of Alzheimer's disease: a reevaluation of the “amyloid cascade hypothesis”. Int J Alzheimers Dis 2011; 2011: 630865.
Lee JH, Cheon YH, Woo RS, Song DY, Moon C, Baik TK . Evidence of early involvement of apoptosis inducing factor-induced neuronal death in Alzheimer brain. Anat Cell Biol 2012; 45: 26–37.
Mattson MP, Magnus T . Ageing and neuronal vulnerability. Nat Rev Neurosci 2006; 7: 278–94.
Anderson AJ, Stoltzner S, Lai F, Su J, Nixon RA . Morphological and biochemical assessment of DNA damage and apoptosis in Down syndrome and Alzheimer disease, and effect of postmortem tissue archival on TUNEL. Neurobiol Aging 2000; 21: 511–24.
Canu N, Calissano P . In vitro cultured neurons for molecular studies correlating apoptosis with events related to Alzheimer disease. Cerebellum 2003; 2: 270–8.
Zhang H, Zhang YW, Chen Y, Huang X, Zhou F, Wang W, et al. Appoptosin is a novel pro-apoptotic protein and mediates cell death in neurodegeneration. J Neurosci 2012; 32: 15565–76.
Guernsey DL, Jiang H, Campagna DR, Evans SC, Ferguson M, Kellogg MD, et al. Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat Genet 2009; 41: 651–3.
Kannengiesser C, Sanchez M, Sweeney M, Hetet G, Kerr B, Moran E, et al. Missense SLC25A38 variations play an important role in autosomal recessive inherited sideroblastic anemia. Haematologica 2011; 96: 808–13.
Ammon HP, Wahl MA . Pharmacology of Curcuma longa. Planta Med 1991; 57: 1–7.
Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R . Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 2013; 11: 338–78.
Ramsewak RS, DeWitt DL, Nair MG . Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I–III from Curcuma longa. Phytomedicine 2000; 7: 303–8.
Sharma OP . Antioxidant activity of curcumin and related compounds. Biochem Pharmacol 1976; 25: 1811–2.
Gilgun-Sherki Y, Melamed E, Offen D . Antioxidant treatment in Alzheimer's disease: current state. J Mol Neurosci 2003; 21: 1–11.
Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL . A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res 2005; 2: 131–6.
Maines MD . The heme oxygenase system and its functions in the brain. Cell Mol Biol (Noisy-le-grand) 2000; 46: 573–85.
Guo JZ, Zhou QX . The research into functions of heme oxygenase system in brain. Sheng Li Ke Xue Jin Zhan 2002; 33: 26–9.
Khan AA, Quigley JG . Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim Biophys Acta 2011; 1813: 668–82.
Ryter SW, Tyrrell RM . The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 2000; 28: 289–309.
Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, et al. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant 2003; 18: v8–12.
Halliwell B, Gutteridge JM . Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 1990; 186: 1–85.
Kumar S, Bandyopadhyay U . Free heme toxicity and its detoxification systems in human. Toxicol Lett 2005; 157: 175–88.
Rahman Q, Mahmood N, Khan SG, Arif JM, Athar M . Mechanism of asbestos-mediated DNA damage: role of heme and heme proteins. Environ Health Perspect 1997; 105: 1109–12.
Atamna H, Frey WH 2nd . A role for heme in Alzheimer's disease: heme binds amyloid beta and has altered metabolism. Proc Natl Acad Sci U S A 2004; 101: 11153–8.
Cullen KM, Kocsi Z, Stone J . Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging 2006; 27: 1786–96.
Parker WD Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK . Electron transport chain defects in Alzheimer's disease brain. Neurology 1994; 44: 1090–6.
Hardy J, Selkoe DJ . The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002; 297: 353–6.
Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM . Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 2005; 45: 675–88.
Smith MA, Nunomura A, Zhu X, Takeda A, Perry G . Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal 2000; 2: 413–20.
Atamna H, Boyle K . Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease. Proc Natl Acad Sci U S A 2006; 103: 3381–6.
Chuang JY, Lee CW, Shih YH, Yang T, Yu L, Kuo YM . Interactions between amyloid-beta and hemoglobin: implications for amyloid plaque formation in Alzheimer's disease. PLoS One 2012; 7: e33120.
Pramanik D, Dey SG . Active site environment of heme-bound amyloid beta peptide associated with Alzheimer's disease. J Am Chem Soc 2011; 133: 81–7.
Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF . Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab 2003; 23: 629–52.
Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B . Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav 2010; 96: 378–85.
Ezz HS, Khadrawy YA, Noor NA . The neuroprotective effect of curcumin and Nigella sativa oil against oxidative stress in the pilocarpine model of epilepsy: a comparison with valproate. Neurochem Res 2011; 36: 2195–204.
Du P, Tang HY, Li X, Lin HJ, Peng WF, Ma Y, et al. Anticonvulsive and antioxidant effects of curcumin on pilocarpine-induced seizures in rats. Chin Med J (Engl) 2012; 125: 1975–9.
Carmona-Ramirez I, Santamaria A, Tobon-Velasco JC, Orozco-Ibarra M, Gonzalez-Herrera IG, Pedraza-Chaverri J, et al. Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J Nutr Biochem 2013; 24: 14–24.
Zhu YG, Chen XC, Chen ZZ, Zeng YQ, Shi GB, Su YH, et al. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol Sin 2004; 25: 1606–12.
Ponka P . Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood 1997; 89: 1–25.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No 81110555, 91232709, 81171216, and 81161120496).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, Km., Zhang, J., Zhang, Cl. et al. Curcumin inhibits appoptosin-induced apoptosis via upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta Pharmacol Sin 36, 544–552 (2015). https://doi.org/10.1038/aps.2014.166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.166