Abstract
Aim:
To investigate the anticancer mechanisms of triptolide, a diterpenoid isolated from the plant Tripterygium wilfordii Hook F, against human breast cancer cells and the involvement of the estrogen receptor-α (ERα)-mediated signaling pathway in particular.
Methods:
Human breast cancer ERα-positive MCF-7 cells and ERα-negative MDA-MB-231 cells were tested. PrestoBlue assay was used to evaluate the cell viability. The levels of ERα mRNA and protein were detected with real-time PCR and immunoblotting, respectively. Mouse models of MCF-7 or MDA-MB-231 xenograft tumors were treated with triptolide (0.4 mg·kg−1·d−1, po) or a selective estrogen receptor modulator tamoxifen (mg·kg−1·d−1, po) for 3 weeks, and the tumor weight and volume were measured.
Results:
Triptolide (5–200 nmol/L) dose-dependently inhibited the viability of both MCF-7 and MDA-MB-231 cells, with a more potent inhibition on MCF-7 cells. Knockdown of ERα in MCF-7 cells by siRNA significantly attenuated the cytotoxicity of triptolide, whereas overexpression of ERα in MDA-MB-231 cells markedly enhanced the cytotoxicity. Triptolide dose-dependently decreased the expression of ERα in MCF-7 cells and MCF-7 xenograft tumors. Furthermore, treatment of MCF-7 cells with triptolide inhibited the phosphorylation of ERK1/2 in dose- and time-dependent manners. In the mice xenografted with MCF-7 cells, treatment with triptolide or tamoxifen resulted in significant reduction in the tumor weight and volume. Similar effects were not obtained in the mice xenografted with MDA-MB-231 cells.
Conclusion:
The anticancer activity of triptolide against ERα-positive human breast cancer is partially mediated by downregulation of the ERα-mediated signaling pathway.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignant disease affecting women worldwide. It has been reported that estrogen receptor-α (ERα) plays a critical role in the initiation and progression of breast cancer because approximately 70% of primary breast cancers are ERα-positive1. ERα is a member of a large superfamily of nuclear receptors and acts as a ligand-activated transcription factor2 by binding to 17β-estradiol (E2) and interacting with specific estrogen response elements (EREs) in the promoters of target genes in the nucleus3. Additionally, non-nuclear ERα rapidly activates downstream growth factor signals, such as extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinase (JNK) and signal transducer and activator of transcription 3 (STAT3)4. For patients with ERα-positive breast cancer, anti-ER therapeutic agents include selective estrogen receptor modulators (SERMs), selective estrogen receptor downregulators (SERDs) and disruptors of estrogen synthesis5. Although SERMs (such as tamoxifen) and SERDs (such as fulvestrant) are effective treatments for ERα-positive breast cancer, these treatments are commonly limited by drug resistance6. The drug resistance that results from long-term anti-ER treatment may be induced by the loss of ERα, an increase in the levels of nonnuclear ERα or growth factor receptor signaling, or the abnormal activities of signal transducers (such as ERK1/ERK2, JNK, and STAT3), cell-cycle regulators or apoptosis regulators. The mitogen-activated protein kinase (MAPK) pathways are involved in cell proliferation and survival. The best characterized MAPK is ERK, the activation of which is known to inhibit apoptosis and induce cell proliferation in several human malignancies7.
Triptolide is a diterpenoid isolated from the plant Tripterygium wilfordii Hook F, which is a member of the Celastraceae family. Triptolide has been shown to display a unique bioactive spectrum of immunosuppressive, anti-fertility and anti-cystogenesis activities8. Recent studies of triptolide have revealed many properties relevant to its anti-inflammatory and anticancer activities9,10,11. Many in vitro and in vivo studies have aimed to elucidate the mechanism underlying the anticancer activity of triptolide; however, the findings of these studies have been inconsistent. Previously, we have found that triptolide is much more sensitive to ERα-positive MCF-7 cells than that to ERα-negative MDA-MB-231 cells12. In this study, we further characterized the anticancer effects of triptolide in breast cancer cell lines and in an in vivo mouse model and further investigated the signaling mechanisms underlying the triptolide-induced inhibition of breast cancer growth. We found that triptolide inhibited breast cancer growth by blocking both the ERα and ERK1/ERK2 pathways. These findings suggest that triptolide represents a promising drug candidate for breast cancer intervention.
Materials and methods
Materials
Reagents were obtained as follows. PrestoBlue® Cell Viability Reagent(Invitrogen, Carlsbad, CA, USA) was purchased from Life Technologies(Grand Island, NY, USA), and Cell Viability Reagent and TRIzol reagent were purchased from Invitrogen(Carlsbad, CA, USA). The rabbit polyclonal anti-ERα, anti-ERK1, and anti-ERK2 and mouse monoclonal anti-p-ERK and anti-actin antibodies were obtained from Santa Cruz Biotechnology Inc(Santa Cruz, CA, USA); ICI 182 780 was purchased from Apex Bio Corporation(Boston, MA, USA). Tamoxifen was purchased from Sigma(St Louis, MO, USA). Triptolide (purity >98%) was a gift from the Dermatologic Disease Research Institute of the Chinese Academy of Medical Science (Nanjing, China).
Cell culture and triptolide treatment
MCF-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM), and MDA-MB-231 cells were maintained in RPMI-1640 medium. All cells were supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 10 mg/mL streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. The cells were starved for 24 h in medium containing 0.1% FBS before treatment with triptolide. The concentration of DMSO in the cell cultures was less than 0.1%.
Cell viability assay
Exponentially growing cells were seeded in a 96-well plate. Twenty-four hours after seeding, the cells were incubated in the absence or presence of triptolide for the indicated duration. Cell viability was evaluated using a PrestoBlue® Cell Viability Reagent (Invitrogen, Carlsbad, CA, USA).
Plasmids and transient transfection
Transient transfection was performed in a manner similar to that in a previous study13. Briefly, MDA-MB-231 cells were seeded at a density of 1×104 cells per well in a 24-well plate and then transfected with an ERα-GFP construct (AddGene) for 24 h using the transfection reagent Lipofectamine 2000 (Invitrogen). Following incubation for 6 h, the transfection medium was replaced with fresh medium, followed by an additional 24 h of incubation to allow for gene expression. The transfection efficiency was monitored based on the intensity of GFP fluorescence. The ERα expression levels were determined via Western blot analysis.
Small interfering RNA (siRNA) transfection
siRNA duplexes against ER-α (Santa Cruz Biotechnology, Santa Cruz, CA, USA; accession No sc-29305) were used for the targeted knockdown of ER-α protein expression. Non-targeting (NT) scrambled siRNA (Santa Cruz Biotechnology; accession No sc-37007) was used as a control. MCF-7 cells were seeded in 6-cm dishes and grown in culture medium. At 50%–60% confluence, the cells were transfected with 10 nmol/L ERα siRNA or NT siRNA using HiPerFect Transfection Reagent (QIAGEN, Valencia, CA, USA) in 1 mL of transfection medium (Santa Cruz Biotechnology). After 24 h, the medium was replaced with fresh medium, and the cells were cultured for an additional 24 h before Western blot analysis of ERα expression. In another set of experiments, the cells were treated with triptolide and an ER antagonist, ICI182 780, followed by cell viability assays.
Western blot analysis
Whole-cell lysates were prepared using lysis buffer containing 150 mmol/L NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris, pH 8.0, and protease and phosphatase inhibitors. The proteins were resolved on 10% SDS-PAGE gels under reducing conditions and then electrophoretically transferred to nitrocellulose membranes. The membranes were incubated in primary antibodies against ERα, t-ERK1/2, ERK1, ERK2, or β-actin at 4 °C overnight. Immunoreactive bands were detected using HRP-conjugated secondary antibodies and the Western Lightning Chemiluminescence Plus reagent.
RNA extraction and real-time PCR
Total cellular RNA was isolated from tumor xenografts using TRIzol reagent. Total RNA (2.5 μg) was used for first-strand cDNA synthesis using a RevertAid First Strand cDNA Synthesis Kit (Takara Bio Inc, Shiga, Japan). ERα mRNA in the tumor xenografts was quantified using the following primers: forward primer 5′-ATCCTGATGATTGGTCTCGTCT-3′ and reverse primer 5′-GGATATGGTCCTTCTCTTCCAG-3′. SsoFast™ EvaGreen® Supermix (Bio-Rad) was used as a fluorescent dye to detect double-stranded DNA. The mRNA level of each gene was normalized to that of the internal control β-actin using the following primers: forward primer 5′-CCTGGCACCCAGCACAAT-3′ and reverse primer 5′-GCCGATCCACACGGAGTACT-3′. The ratio of the normalized mean value for each treatment group to that for the vehicle DMSO control group was calculated.
Antitumor activity in vivo
Female, homozygous, 5- to 6-week-old athymic, inbred BALB/c-nu+/nu+ mice were supplied by the Laboratory Animal Center of the General Hospital of Nanjing Military Region (Nanjing, China), where they were bred and maintained in vinyl chambers under germ-free conditions. The animals were housed in a pathogen-free environment under controlled light and humidity and received food and water ad libitum. After the mice had been in quarantine for 1 week, they were subcutaneously (sc) injected with 5×106 MCF-7 or MDA-MB-231 cells (0.15 mL). To evaluate antitumor activity, the mice were stratified into three groups according to their tumor volume once the mean tumor volume had reached approximately 100-140 mm3 (d 0). Thereafter, triptolide (0.4 mg·kg−1·d−1) and a selective estrogen receptor modulator, tamoxifen (5 mg·kg−1·d−1), were administered via gavage daily to both the MCF-7 and MDA-MB-231 groups. After three weeks, the mice were euthanized, and the xenografted tumors were removed and weighed. All procedures of animal experiments were conducted in compliance with standard ethical guidelines and with the approval of the faculty ethical committee. The relative tumor volume (RTV) was calculated using the following formula: RTV=tumor volume on measured day/tumor volume on d 0.
Histopathology
For histological observation, the tumor xenografts were removed and blotted dry before weighing. The tissues were placed in 4% formalin for 2 d, embedded in paraffin, serially sectioned at 5 μm, and stained in hematoxylin and eosin for microscopic observation. The histological sections were used for qualitative evaluation of the tumor xenografts.
Immunohistochemistry
After endogenous peroxidase activity had been blocked, deparaffinized sections (5-μm thick) of the tumor xenografts were pretreated in 10 mmol/L citrate buffer (pH 6.0) by microwaving (500 W at full power) for 15 min. After cooling for 60 min, the sections were incubated in a primary antibody overnight at 4 °C in a moist chamber. Then, the sections were incubated in a biotinylated secondary antibody for 10 min, followed by incubation in an HRP-conjugated streptavidin complex (Beyotime, Haimen, China) for 10 min. To visualize immunoreactivity, diaminobenzidine tetrachloride (1 mg/mL) containing 0.1% hydrogen peroxide (30% w/v) was used. The immunostained slides were evaluated and photographed under a microscope (FV-1000; Olympus, Japan). The intensity of immunostaining was semiquantitatively designated as strong,weak,or none, and the ratio of highly stained cells was calculated.
Statistical analysis
The data from the inhibition assays were analyzed by non-linear curve fitting of the delta F% value versus the inhibitor concentration using PRISM version 5.0 software (GraphPad Software Inc, CA, USA). The data are expressed as the mean±SEM. The significance of the differences between the groups was evaluated via one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison post-test. The differences were considered to be significant at P<0.05.
Results
Triptolide suppresses breast cancer cell growth
To assess the effects of triptolide on breast cancer cell growth, breast cancer (MDA-MB-231 and MCF-7) cells were treated with triptolide at varying concentrations or for different durations. Cell viability was measured using the PrestoBlue cell proliferation assay. The MCF-7 cells positively expressed ERα, whereas the MDA-MB-231 cells did not14,15. Triptolide decreased the viabilities of both types of cancer cells and exerted a greater suppressive effect on the growth of the MCF-7 cells than on that of the MDA-MB-231 cells (Figure 1A). Triptolide dose-dependently (5–200 nmol/L) decreased the survival of the MCF-7 cells at 24, 48, and 72 h (Figure 1B). To explore the effects of triptolide in combination with estradiol, MCF-7 cells were exposed to increasing concentrations of triptolide in the presence of estradiol (10 nmol/L) (Figure 1C). In contrast with the stimulatory effects of estradiol alone, the presence of triptolide at 10, 25, or 50 nmol/L suppressed estradiol-stimulated cell growth by 9%, 20%, and 55% (P<0.05), respectively.
Triptolide reduces the proliferation of human breast cancer cells. (A) The IC50 values of MCF-7 and MDA-MB-231 cells treated with triptolide. GraphPad Prism 5 (GraphPad Software) was used for the calculations. (B) MCF-7 cells were treated with triptolide (from 0 to 200 nmol/L). Cell viability was measured using PrestoBlue following treatment for 24, 48, or 72 h. (C) The proliferation of MCF-7 cells in the presence of triptolide or E2 in vitro. The cells were cultured under hormone-free conditions or in E2, and cell growth was evaluated. All data are presented as the mean±SD of 3 repeated experiments. bP<0.05, cP<0.01.
The ERα signaling pathway partially mediates the triptolide-induced suppression of breast cancer cell growth
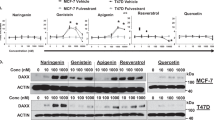
To investigate the effect of triptolide on ERα expression, real-time PCR and an immunoblotting assay were used to examine mRNA and protein expression, respectively, after MCF-7 cells were treated with triptolide for 24 h. Triptolide decreased ERα expression at both the mRNA and protein levels in a dose-dependent manner in the MCF-7 cells (Figure 2A, 2B). To determine the effects of triptolide on the ERα signaling pathway in vivo, we used an MCF-7 xenograft mouse tumor model and immunostained for ERα at the endpoint. The ERα expression levels were significantly reduced in the tumor-bearing mice treated with triptolide in a dose-dependent manner (Figure 2C, 2D).
Triptolide decreases ERα expression in MCF-7 cells and MCF-7 solid tumors. (A) Western blot analysis showing that triptolide reduced the ERα protein levels in MCF-7 cells. (B) Triptolide reduced the ERα mRNA levels as determined by RT-PCR. (C) Immunohistochemical staining for ERα in MCF-7 solid tumors treated with triptolide. (D) Sections immunostained for ERα were classified into three groups (highly stained, moderately stained, and not stained). The values indicate the mean±SD of the proportions of highly stained cells (n=6). bP<0.05, cP<0.01 compared with the control as determined by ANOVA.
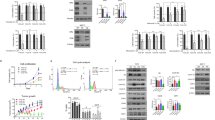
Knockdown of ERα expression using siRNA in MCF-7 cells significantly attenuated the inhibitory effects of triptolide on cell proliferation (Figure 3B). The IC50 value of triptolide in the MCF-7 cells transfected with siRNA targeting ERα was 90.14 nmol/L, whereas that in the cells transfected with control siRNA was 254.6 nmol/L. The pattern of proliferation of the MCF-7 cells treated with the estrogen receptor inhibitor ICI 182780 was similar to that of the cells treated with triptolide. In addition, the knockdown of ERα using siRNA in MCF-7 cells attenuated the inhibitory effects of ICI 182 780 on cell proliferation (Figure 3C). In contrast, triptolide exerted a strikingly greater inhibitory effect on the proliferation of MDA-MB-231 cells overexpressing ERα (Figure 3E) than on the proliferation of empty vector-treated control cells (IC50=40.57 and 216.96 nmol/L, respectively).
ERα signaling is essential for the triptolide-mediated suppression of breast cancer cell growth. (A) MCF-7 cells were transfected with siRNA duplexes against ERα or NT scrambled siRNA for 24 h. The cell lysates were subjected to Western blot analysis using anti-ERα antibodies. The cells were transfected with siRNA followed by treatment with triptolide (B) or ICI182780 (C) at the indicated concentration for 24 h. The MDA-MB-231 cells were transfected with an empty vector or the ERα-GFP construct for 24 h. The cell lysates were subjected to Western blot analysis using anti-ERα antibodies. The MDA-MB-231 cells were transfected with an ERα-GFP construct followed by treatment with triptolide (E) or ICI182780 (F) at the indicated concentration for 24 h. Cell viability was assessed via the PrestoBlue assay. The data are expressed as the mean±SD. bP<0.05, cP< 0.01 compared with the cells transfected with an empty vector according to ANOVA.
To further investigate the effect of triptolide on the ERα signaling pathway, we examined the estrogen-induced activation of ERK in MCF-7 cells. Treatment with 10 nmol/L E2 resulted in an increase in ERK1/2 phosphorylation in the MCF-7 cells, which was attenuated by triptolide treatment (Figure 4A). We also compared the effect of triptolide on ERK1/2 phosphorylation between MCF-7 and MDA-MB-231 cells. As shown in Figure 4B, treatment with triptolide significantly decreased ERK1/2 phosphorylation in the MCF-7 cells. Because triptolide-induced growth inhibition was observed in the MDA-MB-231 cells after triptolide treatment, we investigated the effect of this agent on ERK1/2 phosphorylation in these cells. As shown by immunoblotting, the levels of phosphorylated ERK1/2 proteins did not significantly change after triptolide treatment.
Effects of triptolide on the p-ERK and ERK protein levels in MCF-7 and MDA-MB-231 cells. (A) Time-dependent effects of triptolide on the phosphorylation of the ERK1/2 proteins in MCF-7 cells. ERK1/2 protein phosphorylation was measured by immunoblotting and was normalized to the expression of the control. (B) The MCF-7 and MDA-MB-231 cells were treated with different concentrations of triptolide for 12 h. The cells were analyzed via Western blot using anti-phospho-ERK or anti-ERK antibodies.
Triptolide inhibits the growth of MCF-7 cell xenografts in a mouse model
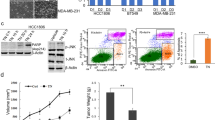
To determine the effects of triptolide on breast cancer in vivo, we compared its effects on tumor growth in the MCF-7 and MDA-MB-231 xenograft mouse tumor models. Triptolide treatment was initiated when the tumors were palpable and was continued for 21 d. As shown in Figure 5, the growth of MCF-7 tumors was significantly inhibited by triptolide administration for 21 consecutive days. At the end of this period, the MCF-7 tumor volume and weight in the triptolide-treated mice were reduced by 41% and 67%, respectively, compared with those in the control mice (P<0.01). The tumor weight and volume were also reduced in the mice treated with triptolide compared with the vehicle-treated control mice in the MDA-MB-231 group. However, the ability of triptolide to inhibit tumor growth in the MDA-MB-231 group was not as significant as that in the MCF-7 group. The MDA-MB-231 tumor volume and weight in the triptolide-treated mice were reduced by 11% (P>0.05) and 24% (P<0.05), respectively, compared with those in the control mice.
The breast tumor growth of MCF-7 cells is more sensitive to triptolide than that of MDA-MB-231 cells in mice. Triptolide and a selective estrogen receptor modulator, tamoxifen, were administered on d 0. The tumor volume was measured twice a week until d 21. (A) The tumor volume changes in the MDA-MB-231 cells in vivo. (B) The tumor volume changes in the MCF-7 cells in vivo. (C and D) The changes in the tumor weight in nude mice xenografted with MCF-7 and MDA-MB-231 cells. The tumor weights were measured on d 21. The values indicate the mean±SD of the tumor weights (n=6). bP<0.05, cP<0.01 compared with the control as determined by ANOVA.
Discussion
Breast cancer is a common cancer that displays a high mortality rate among women. Endocrine therapy has become the most effective treatment option for women with ER-positive breast cancer. Nevertheless, many breast cancer patients with tumors expressing high levels of ER are unresponsive to endocrine therapy, and all patients with advanced disease ultimately develop resistance to this therapy. The potential mechanisms underlying this intrinsic or acquired endocrine resistance involve ER-co-regulatory proteins and cross-talk between the ER pathway and other growth factor signaling networks16. The cross-talk between receptor tyrosine kinase (RTK) and ER signal transduction pathways may contribute to the acquisition of endocrine resistance by sensitizing breast tumor cells to estrogens or by circumventing the need for hormones. Based on this evidence, one strategy for improving the efficacy of current endocrine agents and for delaying the onset of resistance is to simultaneously target the ER and RTK signaling pathways. Several studies have proposed a role for the ERK signaling pathway in the initiation and pathogenesis of breast cancer17. The activation of the ERK cascade modulates the phosphorylation, and thus, the activity of several nuclear transcription factors, which in turn regulate genes involved in cell proliferation and survival. ERα is functionally regulated via phosphorylation by several protein kinases18. The phosphorylation of Ser118 of ERα is mediated by CDK7 in response to E2, and this residue is phosphorylated by pERK1/ERK2 in a ligand-independent manner19. In contrast, Ser167 is a target of AKT and p90RSK that is activated by pERK1/ERK2. Therefore, increased ERK1/ERK2 activity may result in endocrine resistance. Laboratory studies have demonstrated that the activation of the EGF receptor family and the ERK/MAPK pathway promotes estrogen-independent growth, which has led to several clinical trials using signal transduction inhibitors to enhance endocrine sensitivity20,21. There is currently increased interest in the application of endocrine agents in combination with signal transduction inhibitors that selectively block the activities of growth factor receptors and their downstream partners, such as ERK, AKT, mTOR and farnesyltransferase, and several clinical trials are underway22.
Triptolide has been shown to exert potent anticancer effects via multiple molecular targets and signaling pathways. In the present study, the inhibitory effects of triptolide were investigated in several breast cancer cell lines, specifically the ERα-positive MCF-7, ERα-negative MDA-MB-231 and HER2-positive BT-474 cell lines23. The molecular targets and signaling pathways include apoptosis-related genes (such as caspase-7 and -9), focal adhesion kinase (FAK), Wnt/β catenin and tumor suppressors (such as p53) were investigated23,24,25. In particular, MCF-7 is an estrogen receptor-positive cell line that lacks caspase-326, thus representing a cell model displaying compromised apoptotic machinery activity, and this deficiency might influence the cellular response to anti-cancer drug treatments. In this study, we demonstrated that triptolide inhibited the proliferation of ERα-positive breast cancer cells partially via ERα-related pathways in vitro and in vivo. Our conclusion was based on the following observations: first, triptolide more potently reduced the growth of the ERα-positive breast cancer cells (MCF-7) than that of the ERα-negative cells (MDA-MB-231). In addition, triptolide exerted suppressive effects on estrogen-stimulated MCF-7 cell proliferation and gene expression. The knockdown of ERα via siRNA transfection in MCF-7 cells attenuated these effects of triptolide. In contrast, the overexpression of ERα in MDA-MB-231 cells enhanced these effects. Similarly, significant reductions in tumor weight and volume were observed in the mice treated with triptolide compared with the vehicle-treated control mice in the MCF-7 group. However, the ability of triptolide to inhibit tumor growth in the MDA-MB-231 group was not as significant as that in the MCF-7 group. In summary, we characterized the anticancer properties of triptolide in both breast cancer cells and in vivo in tumor-bearing mice. The inhibitory effects of triptolide on estrogen receptor-positive breast cancer cells might occur via the ERα pathway.
Author contribution
Han Li, Guo-feng Pan, Li-xin SUN, and Lu-yong Zhang designed the study; Jing YANG, Zhen-zhou JIANG, and Han Li performed the research; Han Li and Li-xin SUN wrote the manuscript.
References
Yamashita H . Current research topics in endocrine therapy for breast cancer. Int J Clin Oncol 2008; 13: 380–3.
Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol Rev 2001; 81: 1535–65.
Bjornstrom L, Sjoberg M . Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005; 19: 833–42.
Musgrove EA, Sutherland RL . Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 2009; 9: 631–43.
Clemons M, Danson S, Howell A . Tamoxifen ("Nolvadex"): a review. Cancer Treat Rev 2002; 28: 165–80.
Osborne CK, Schiff R . Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233–47.
Seddighzadeh M, Zhou JN, Kronenwett U, Shoshan MC, Auer G, Sten-Linder M, et al. ERK signalling in metastatic human MDA-MB-231 breast carcinoma cells is adapted to obtain high urokinase expression and rapid cell proliferation. Clin Exp Metastasis 1999; 17: 649–54.
Sun L, Li H, Huang X, Wang T, Zhang S, Yang J, et al. Triptolide alters barrier function in renal proximal tubular cells in rats. Toxicol Lett 2013; 223: 96–102.
Sun L, Zhang S, Jiang Z, Huang X, Wang T, Li H, et al. Triptolide inhibits COX-2 expression by regulating mRNA stability in TNF-alpha-treated A549 cells. Biochem Biophys Res Commun 2011; 416: 99–105.
Xue M, Jiang ZZ, Liu JP, Zhang LY, Wang T, Wang H, et al. Comparative study on the anti-inflammatory and immune suppressive effect of Wilforlide A. Fitoterapia 2010; 81: 1109–12.
Li J, Zhu W, Leng T, Shu M, Huang Y, Xu D, et al. Triptolide-induced cell cycle arrest and apoptosis in human renal cell carcinoma cells. Oncol Rep 2011; 25: 979–87.
Liu J, Jiang ZZ, Xiao JW, Zhang Y, Lin SS, Duan WG, et al. Effects of triptolide on ERα and p53 expression in two human breast cancer cell lines. Phytomedicine 2009; 16: 1006–13.
Maurisse R, De Semir D, Emamekhoo H, Bedayat B, Abdolmohammadi A, Parsi H, et al. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnol 2010; 10: 9.
Nizamutdinova IT, Lee GW, Son KH, Jeon SJ, Kang SS, Kim YS, et al. Tanshinone I effectively induces apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells. Int J Oncol 2008; 33: 485–91.
Massarweh S, Schiff R . Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res 2007; 13: 1950–4.
Osborne CK, Schiff R . Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol 2005; 23: 1616–22.
Sivaraman VS, Wang H, Nuovo GJ, Malbon CC . Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest 1997; 99: 1478–83.
Ali S, Coombes RC . Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2002; 2: 101–12.
Chen D, Riedl T, Washbrook E, Pace PE, Coombes RC, Egly JM, et al. Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell 2000; 6: 127–37.
Jeng MH, Yue W, Eischeid A, Wang JP, Santen RJ . Role of MAP kinase in the enhanced cell proliferation of long term estrogen deprived human breast cancer cells. Breast Cancer Res Treat 2000; 62: 167–75.
Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M . Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 2003; 278: 30458–68.
Johnston SR, Leary A, Martin LA, Smith IE, Dowsett M . Enhancing endocrine response with novel targeted therapies: why have the clinical trials to date failed to deliver on the preclinical promise? Cancer 2008; 112: 710–7.
Owa C, Michael E, Messina J, Reginald H . Triptolide induces lysosomal-mediated programmed cell death in MCF-7 breast cancer cells. Int J Womens health 2013; 5: 557–69.
Tan BJ, Tan BH, Chiu GNC . Effect of triptolide on focal adhesion kinase and survival in MCF-7 breast cancer cells. Oncol Rep 2011; 26: 1315.
Hongmin S, Jinghua M, Tianhua G, Rongrong H . Triptolide induces apoptosis of breast cancer cells via a mechanism associated with the Wnt/β catenin signaling pathway. Exp Ther Med 2014; 8: 505–8.
Semenov DV, Aronov PA, Kuligina EV, Potapenko MO, Richter VA . Oligonucleosome DNA Fragmentation of caspase 3 deficient MCF-7 cells in palmitate-induced apoptosis. Nucleosides Nucleotides Nucleic Acids 2004; 23: 831–6.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No 81001564, 81102887, 81274146, and 81320108029), the Natural Science Foundation of Jiangsu Province (No BK 2010433) and Beijing Nova Program (No Z141107001814061).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, H., Pan, Gf., Jiang, Zz. et al. Triptolide inhibits human breast cancer MCF-7 cell growth via downregulation of the ERα-mediated signaling pathway. Acta Pharmacol Sin 36, 606–613 (2015). https://doi.org/10.1038/aps.2014.162
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.162
Keywords
This article is cited by
-
l-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells
Scientific Reports (2022)
-
Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine
Chinese Medicine (2019)
-
A Novel Immunocompetent Mouse Model of Pancreatic Cancer with Robust Stroma: a Valuable Tool for Preclinical Evaluation of New Therapies
Journal of Gastrointestinal Surgery (2016)