Abstract
Aim:
To study the effects of tanshinone IIA (TIIA) on lipopolysaccharide (LPS)-induced acute lung injury in mice and the underlying mechanisms.
Methods:
Mice were injected with LPS (10 mg/kg, ip), then treated with TIIA (10 mg/kg, ip). Seven hours after LPS injection, the lungs were collected for histological study. Protein, LDH, TNF-α and IL-1β levels in bronchoalveolar lavage fluid (BALF) and myeloperoxidase (MPO) activity in lungs were measured. Cell apoptosis and Bcl-2, caspase-3, NF-κB and HIF-1α expression in lungs were assayed.
Results:
LPS caused marked histological changes in lungs, accompanied by significantly increased lung W/D ratio, protein content and LDH level in BALF, and Evans blue leakage. LPS markedly increased neutrophil infiltration in lungs and inflammatory cytokines in BALF. Furthermore, LPS induced cell apoptosis in lungs, as evidenced by increased TUNEL-positive cells, decreased Bcl-2 content and increased cleaved caspase-3 content. Moreover, LPS significantly increased the expression of NF-κB and HIF-1α in lungs. Treatment of LPS-injected mice with TIIA significantly alleviated these pathological changes in lungs.
Conclusion:
TIIA alleviates LPS-induced acute lung injury in mice by suppressing inflammatory responses and apoptosis, which is mediated via inhibition of the NF-κB and HIF-1α pathways.
Similar content being viewed by others
Introduction
Acute lung injury (ALI), a severe complication with high rates of morbidity and mortality caused by stress situations such as trauma, burns and sepsis, is characterized by alveolar-capillary membrane damage. Serious ALI can lead to acute respiratory distress syndrome (ARDS)1, respiratory failure2, and ultimately death. Despite recent improvements in therapies and tools, the prognosis of patients with ALI/ARDS remains poor. Therefore, there is an urgent need to develop novel therapies to improve the treatment of ALI/ARDS.
The pathophysiological mechanism of ALI is complex. The inflammatory response is a crucial process during this period. Inflammatory cells, primarily neutrophils, first accumulate and are activated in the lung3,4,5. Simultaneously, pro-inflammatory cytokines, such as TNF-α and IL-1β, are produced mainly by inflammatory cells and are found at high levels in the lung. Both neutrophils and inflammatory cytokines can directly or indirectly damage lung cells. Moreover, earlier studies have shown that apoptosis of lung epithelial cells and pulmonary capillary endothelial cells, regulated mainly by the caspase family and Bcl-2 family, represent a potentially important mechanism in the development of ALI6. Results show that the activation of NF-κB and HIF-17 signaling pathways is an important step in modulating ALI.
Tanshinone IIA (TIIA), a phenanthrenequinone derivative extracted from Salvia miltiorrhiza Bunge, is widely used in China for the treatment of many diseases. TIIA may exert a series of biochemical effects, such as anti-oxidant and anti-inflammatory effects. Our previous work has demonstrated that TIIA was able to alleviate ALI induced by lipopolysaccharide (LPS)8,9 and seawater exposure10,11,12,13, indicating that TIIA may be a potential agent to treat ALI.
Although we have previously found that TIIA was able to prevent the occurrence of ALI to a certain extent with a pretreatment method, little is known about its therapeutic effect. The development of ALI is a cascade reaction, which is involved in many mechanisms; as a result, although pretreatment with TIIA may attenuate lung injury, it is unclear whether it has therapeutic effects when lung injury has already occurred. However, as most patients in hospitals have already been diagnosed with ALI rather than are at risk of developing ALI, there is also a tremendous need to explore the therapeutic effect of TIIA on lung injury. In the present study, to accelerate its clinical use, we examined whether TIIA was able to therapeutically reduce LPS-induced ALI and explored the underlying molecular mechanisms in mice. Our results demonstrate that TIIA alleviated LPS-induced lung injury, attenuated lung inflammatory responses, and reduced lung cell apoptosis, which was via the inhibition of NF-κB and HIF-1α signaling pathways.
Materials and methods
Chemicals
Tanshinone IIA (sulfonate, purity is 99%) was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The structure of TIIA is shown in Figure 1. The kit for determining myeloperoxidase (MPO) activity was obtained from Jiancheng Bioengineering Institute (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and IL-1β were obtained from R&D Systems (Minneapolis, MN, USA). In situ cell death detection kits and proteinase were obtained from Roche Molecular Biochemicals (Indianapolis, IN, USA). Bcl-2 and caspase-3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies specific for total and phosphorylated NF-κB, and HIF-1α were purchased form Millipore (Bedford, MA, USA). Monoclonal β-actin antibody, Escherichia coli endotoxin LPS (O55:B5), Evans blue dye and all the other reagents were obtained from Sigma-Aldrich Inc (St Louis, MO, USA). The purity of all chemical reagents was at least in analytical grade.
The chemical structure of TIIA.
Animal preparation
Male BALB/c mice, which weighed 18–22 g, were obtained from the Animal Center (the 88th Hospital of PLA, Taian, China). Mice were kept in a temperature-controlled house with 12-h light-dark cycles and were fed standard laboratory diet and water ad libitum. All experiments were approved by Animal Care and Use Committee at the 88th Hospital of PLA and were in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No 85–23, revised 1985).
Model and grouping
Mice were randomly divided into four groups: 1) saline control group (n=18): mice received saline 1 h after the first saline administration; 2) TIIA control group (n=18): mice received TIIA (10 mg/kg) 1 h after saline administration; 3) LPS group (n=18): mice received saline 1 h after LPS (10 mg/kg) administration; and 4) LPS/TIIA group (n=18): mice received TIIA (10 mg/kg) 1 h after LPS (10 mg/kg) administration. In all groups, administration was via intraperitoneal injection and all measurements were made 7 h after LPS administration. The current dose of TIIA and timing of TIIA administration were applied on the basis of our preliminary experiments8. At the end of the experiments, blood samples were obtained to analyze blood gases. We selected mice that met the standard of ALI for the subsequent assays.
Histological study
At the end of experiment, the right lungs of all groups (n=6, respectively) were removed and fixed with 4% paraformaldehyde. The tissue blocks obtained from midsagittal slices of the lungs were stained with hematoxylin-eosin (H&E) to characterize lung injury under a microscope. Sections were scored for edema, neutrophil infiltration, hemorrhage, bronchiole epithelial desquamation and hyaline membrane formation according to previous reports14,15.
Wet-to-dry weight ratio (W/D)
At the end of the experiment, after lungs were separated from the thoracic cavity, the left lungs (n=6, respectively) were weighed, then dried to constant weight at 50 °C for 72 h and weighed again. The ratio of W/D was finally calculated by dividing the wet weight by the dry weight.
MPO assay
At the end of the experiment, the left lungs (n=6, respectively) were removed from mice of all groups and homogenized. The homogenates were then used to measure MPO activity. Briefly, the weighed lungs were frozen and homogenized in homogenate medium. The homogenates were then performed according to the manufacturer's instructions. The final reagents were then placed in a spectrophotometer for reading at 460 nm.
Preparation of bronchoalveolar lavage fluid (BALF) and measurements
At the end of the experiment, bronchoalveolar lavage was performed [1 mL of phosphate-buffered saline (PBS, 140 mmol/L NaCl, 3 mmol/L KCl, 6 mmol/L Na2HPO4, and 1 mmol/L KH2PO4, pH 7.4) three times] in all groups (n=6, respectively). In each mouse, 90% (2.7 mL) of the total injected volume was consistently recovered. After BALF was centrifuged at 450×g for 10 min, total and differential cell counts and protein concentration in the BALF were determined from the cell fraction16,17. The supernatant was used for measurements of LDH and inflammatory cytokines (TNF-α and IL-1β) by ELISA according to the corresponding manufacturer's instructions.
TUNEL staining
Paraffin-embedded tissue slices were dewaxed, washed with PBS, and digested with proteinase K in the wet box for 30 min at 37 °C. After being washed with PBS, the slides were dipped in TUNEL reaction mixture, and then incubated for 1 h at 37 °C in the wet box. After washing, the sections were incubated with converter-AP for 30 min at 37 °C in the wet box, and then washed with PBS. Subsequently, the sections were stained with NBT/BCIP substrate solution for 1 h, and signals were observed with a microscope. All cells with purple nuclei were considered to be dead.
Protein extraction and Western blotting
Tissues from the right lungs (n=6) were removed from mice from all groups, washed with ice-cold PBS, homogenated and pelleted by centrifugation. Whole-cell extracts were prepared using lysis buffer [100 mmol/L Tris, 5 mmol/L EDTA, 1% Triton X-100, 1% deoxycholate acid, 0.1% SDS, 2 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate, 2 mmol/L DTT, 20 μg/mL leupeptin, and 20 μg/mL pepstatin (pH 7.4)]. After protein concentrations were assayed using BCA assay, 50 μg protein was boiled in loading buffer, resolved on 10% SDS-polyacrylamide gels, electrotransferred to nitrocellulose membranes, and subjected to immunoblotting analysis. Monoclonal HIF-1α antibody was used at a dilution of 1:200 and anti-β-actin antibody was used at a dilution of 1:10 000. Bcl-2, caspase-3, p-NF-κB, and total-NF-κB antibodies were diluted at 1:1000. The blots were blocked in 1× Tris-buffered saline plus Tween 20 containing 5% nonfat dry milk for 2 h at room temperature, followed by an incubation with the appropriately diluted primary antibodies overnight at 4 °C. Immunoreactivity was visualized with corresponding peroxidase-conjugated secondary antibodies and the relative content of target proteins was detected by chemiluminescence.
Statistical analysis
Data are expressed as the mean±SEM from three replicate experiments, and statistical analysis (SPSS 15.0) was performed with analysis of variance (ANOVA), followed by Dunnett's test for multiple comparisons. Differences were considered statistically significant if P<0.05.
Results
TIIA improved LPS-mediated lung histopathologic changes
To investigate the therapeutic effect of TIIA on lung injury, we first observed the histological changes in the lung by microscope. Results showed that normal lung tissue structure and clear pulmonary alveoli were observed in saline and in TIIA control groups (Figure 2A and 2B). LPS administration caused acute inflammation, congestion and edema (Figure 2C). By contrast, compared with the LPS group, TIIA treatment significantly reduced the destruction of lung structure in the LPS/TIIA group (Figure 2D). Lung injury scores are presented in Table 1.
Effects of TIIA on LPS-mediated lung histopathologic changes (H&E stain, ×400). Mice were sacrificed 7 h after LPS injection, and a histopathologic examination of the lungs was performed. (A) Saline control group; (B) TIIA control group; (C) LPS group; (D) LPS/TIIA group.
TIIA alleviated LPS-induced lung injury
To evaluate the ALI model, various parameters related to the acute phase response, such as cell injury, lung edema, vascular and protein leakage were determined. First, we used BALF LDH content to assess the extent of cell injury in ALI model. Results showed that there were no differences in BALF LDH content between saline and TIIA control groups, but BALF LDH content was significantly increased in the LPS group, indicating that LPS induced ALI in the treated mice (Figure 3A, P<0.01). However, TIIA markedly reduced LPS-induced LDH content in BALF (Figure 3A, P<0.05).
Effects of TIIA on LPS-induced lung injury. Mice were randomly divided into four groups and all measurements were performed 7 h after LPS injection as described in the Model and Grouping section. (A) BALF LDH; (B) Lung W/D ratio; (C) Evans blue leakage; (D) BALF protein. Values are expressed as mean±SEM. bP<0.05, cP<0.01 vs saline control group. eP<0.05 vs LPS group.
Next, we measured the lung W/D ratio to assess the lung water content and edema. Results showed that no significant differences were observed between saline and TIIA control groups, whereas the lung W/D ratio was markedly increased in the LPS group compared with the control group (Figure 3B, P<0.05), but was significantly reduced in the LPS/TIIA group (Figure 3B, P<0.05).
The leak index of Evans blue was then used to measure the epithelial-endothelial barrier function. Figure 3C summarizes the analyzed data of Evans blue leakage. Vascular leakage was clearly elevated in the LPS group (Figure 3C, P<0.01). Compared with the LPS group, Evans blue leakage was significantly decreased as a result of the treatment with TIIA in the LPS/TIIA group (Figure 3C, P<0.05).
As protein content in BALF denotes the severity of lung permeability, protein leakage from blood vessels and lung injury, we therefore measured BALF protein content (Figure 3D). LPS administration increased protein amount in BALF (Figure 3D, P<0.01), but TIIA attenuated BALF protein in the LPS/TIIA group (Figure 3D, P<0.05).
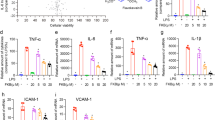
TIIA attenuated LPS-induced lung inflammatory response
In the present study, we measured neutrophil infiltration and inflammatory cytokines to assess lung inflammatory response. To assess neutrophil infiltration into the lungs, we investigated MPO activity in lung homogenates (Figure 4A) and neutrophils in BALF (Figure 4B). We found that no significant differences in MPO activity and BALF neutrophils were observed between saline- and TIIA-treated groups. Furthermore, lung MPO activity and BALF neutrophils were markedly increased by LPS administration compared with saline administration (P<0.01, respectively), whereas their levels were significantly reduced in the LPS/TIIA group (P<0.05 and P<0.01, respectively).
Effects of TIIA on LPS-induced lung inflammatory response. Mice were randomly divided into four groups and all measurements were performed at 7 h after LPS injection as described in the Model and Grouping section. (A) MPO activity in lung homogenates; (B) BALF neutrophils; (C) BALF TNF-α; (D) BALF IL-1β. Values are expressed as mean±SEM. cP< 0.01 vs saline control group. eP<0.05, fP<0.01 vs LPS group.
Next, we measured the levels of inflammatory cytokines such as TNF-α and IL-1β by ELISA. As shown in Figure 4C and 4D, we observed that TNF-α and IL-1β in BALF were only minimally expressed in the two control groups. After LPS administration, the expressions of TNF-α and IL-1β in BALF were markedly increased (P<0.01), and these levels were significantly attenuated by TIIA treatment (P<0.01 and P<0.05, respectively).
TIIA reduced LPS-induced lung apoptosis
To determine whether TIIA could affect LPS-induced apoptosis in lungs, we performed TUNEL staining on lung sections. Results showed that few cells staining positive for TUNEL were detected in saline and TIIA control groups (Figure 5A and 5B). LPS induced more TUNEL-positive cells in lungs in the LPS group (Figure 5C), but TIIA treatment resulted in a marked reduction in the number of these cells compared with the LPS group (Figure 5D). These data suggest that TIIA could therapeutically reduce LPS-induced cell apoptosis in lungs.
Effects of TIIA on LPS-induced lung apoptosis. Mice were randomly divided into four groups and lungs were harvested 7 h after LPS injection to detect apoptosis by TUNEL staining as described in the Materials and methods section. (A) Saline control group; (B) TIIA control group; (C) LPS group; (D) LPS/TIIA group.
To further confirm the effect of TIIA on apoptosis, we used Western blotting to measure two apoptosis-related factors, namely, an anti-apoptotic factor Bcl-2 and a pro-apoptotic factor caspase-3. The results showed that LPS resulted in a significant reduction in the expression of Bcl-2 in the LPS group (Figure 6A, P<0.01). However, in the LPS/TIIA group, the level of Bcl-2 was significantly increased compared with the LPS group (Figure 6A, P<0.05). Moreover, compared with the two control groups, LPS induced a marked increase in the cleaved caspase-3 (Figure 6B, P<0.01), which was attenuated in the LPS/TIIA group compared with LPS group (Figure 6B, P<0.05).
Effects of TIIA on apoptosis-related factors in lungs from LPS-treated mice. Mice were randomly divided into four groups, and lungs were harvested 7 h after LPS injection to detect apoptosis-related factors by Western blot as described in the Materials and methods section. (A) Bcl-2; (B) Caspase-3. Values are expressed as mean±SEM. cP<0.01 vs saline control group. eP<0.05 vs LPS group.
TIIA inhibited LPS-induced NF-κB and HIF-1α activation
To explore the mechanisms responsible for the therapeutic effect of TIIA, we investigated two important pathways, NF-κB and HIF-1α, which play key roles in LPS-induced lung injury. Western blotting revealed that a basal level of p-NF-κB and HIF-1α was detected in saline and TIIA control groups, but p-NF-κB and HIF-1α were significantly increased in the LPS group (Figure 7A and 7B; P<0.01, respectively). Moreover, we found that the amount of p-NF-κB and HIF-1α were reduced in the LPS/TIIA group (Figure 7A and 7B; P<0.05, respectively).
Effects of TIIA on LPS-induced NF-κB and HIF-1α activation. Mice were randomly divided into four groups, and lungs were harvested 7 h after LPS injection to detect the activation of NF-κB and HIF-1α by Western blot as described in the Materials and methods section. (A) NF-κB; (B) HIF-1α. Values are expressed as mean±SEM. cP<0.01 vs saline control group. eP<0.05 vs LPS group.
Discussion
Danshen, a herbal drug derived from the dried root or rhizome of Salvia miltiorrhizae Bunge, has been used clinically to manage many diseases, such as angina pectoris, myocardial infarction and stroke. TIIA, one of the key components of Danshen, has been reported to possess the majority of Danshen's properties with few side effects. Previous study have demonstrated that TIIA inhibits the production of pro-inflammatory mediators such as NO, TNF-α, IL-1β, and IL-618 by the inhibition of NF-κB activation in RAW 264.7 cells stimulated with LPS, which are mediated by estrogen receptor activation19. In brain microvascular endothelial cells, TIIA concentration-dependently inhibits the synthesis of endothelin-converting enzyme-1 and thus the production of endothelin-1 induced by TNF-α20. Additionally, Salvia miltiorrhizae is able to reduce the contents of plasma endotoxin and serum IL-6, promote the expression of Bax protein in pancreas, improve the pathological changes in the pancreas, and decrease the mortality rate of rats, thereby showing a therapeutic effect on rats with severe acute pancreatitis21.
ALI caused by sepsis is a major and increasing cause of mortality and morbidity worldwide22,23. ALI is a manifestation of systemic inflammatorome induced by various factors in the lung and leads to a noncardiogenic respiratory failure24, which is characterized by lung inflammation and increased permeability-induced functional disturbance of gas exchange. Because of the unclear pathogenesis and lack of effective treatment, ALI has high mortality and remains a common but incurable critical disease.
Once LPS, an exogenous toxin, enters the bloodstream, it can elicit systemic inflammation that mimics many of the initial clinical features of ALI25. Lung is the most frequently involved organ, and ALI/ARDS induced by LPS is the major cause of death26. Therefore, the ALI model induced by LPS is commonly used experimentally. Notably, one widely used murine model of ARDS is produced by LPS administered intraperitoneally27. In this model, LPS induced many features characteristic of ALI, including early (1–2 h) expression of inflammatory mediators, leukocyte accumulation in lung tissue, pulmonary edema and mortality. As a result, we used this model to reproduce clinical ALI.
Previously, we found that pharmacologic pretreatment with TIIA could significantly reduce lethality in LPS-treated mice and attenuate LPS-induced lung injury both in vivo and in vitro by the inhibition of lung PLA2 activity, which is possibly associated with the inhibition of NF-κB activation8. We also showed that pretreatment with TIIA greatly decreased the production of pro-inflammatory cytokines in both LPS-treated mice and cultured macrophages, which most likely correlated with the inhibition of HIF-1α expression by TIIA9. Moreover, we further indicated that pretreatment with TIIA could significantly attenuate the severity of ALI in seawater-challenged rats through the depression of macrophage migration inhibitory factor12, aquaporins (AQP) 1 and AQP5 expression11 and apoptosis13 and up-regulation of Na+,K+-ATPase activity10.
In the present study, we performed experiments to investigate the effect of TIIA treatment in mice after ALI was induced by LPS. According to previous reports, lung tissue damage reaches peaks at 6–8 h and 12–24 h caused by two waves of neutrophil infiltration and sequestration into the lung28,29,30. As our present study mainly focused on early lung injury, we chose the time point for all measurements 7 h after LPS insult. Results showed that TIIA treatment therapeutically inhibited LPS-induced lung injury in mice, as demonstrated by the improvement in lung morphology and the inhibition of cell injury (assessed by BALF LDH), lung edema (assessed by lung W/D ratio), vascular leakage (assessed by the leak index of Evans blue) and protein leakage (assessed by BALF protein content). These results indicated that TIIA may be useful as a treatment for when ALI has already occured.
It is known that inflammatory responses contribute to lung injury following LPS challenge. After the onset of lung injury by various insults, inflammatory cells are recruited into the lung. Activated neutrophils release inflammatory cytokines, reactive oxygen species, cationic peptides, and hydrolytic proteinase31,32,33, which display cytotoxic effect on lung tissue cells. Pro-inflammatory cytokines, such as TNF-α and IL-1β, are key mediators and participate in the development of ALI. Therefore, we next examined whether TIIA could therapeutically attenuate LPS-induced inflammatory responses in the lungs. Our results demonstrated that TIIA significantly reduced neutrophil infiltration into the lungs, as evidenced by the decrease in MPO activity in lung homogenates (Figure 4A) and neutrophil counts in BALF (Figure 4B). Moreover, we also observed that the expression of TNF-α and IL-1β was inhibited by TIIA (Figure 4C and 4D). These results indicated that TIIA may reduce immune response in mice with LPS-induced ALI.
Previous studies have shown that apoptosis contributes to ALI/ARDS pathogenesis33,34,35,36. Apoptotic cells were found in the damaged alveolar epithelium of patients with ARDS37, and in murine models of pulmonary fibrosis and LPS34,35,38 and seawater exposure-induced ALI13. Although TIIA could prevent and treat lung injury induced by LPS, little is known about the effect of TIIA on apoptosis in mice with ALI. Therefore, we studied the effect of TIIA on lung apoptosis induced by LPS, which had not been investigated even in the pretreatment experiment.
Apoptosis can be initiated by two alternative convergent pathways: the extrinsic pathway, which is mediated by cell surface death receptors; and the intrinsic pathway, which is mediated by mitochondria. In both pathways, cysteine aspartyl-specific proteases (caspases) are activated, and they then cleave cellular substrates, resulting in the characteristic morphological and biochemical alterations of apoptosis39,40. The point at which most of these pathways culminate is the activation of caspase-3, leading caspase-3 to be considered a pro-apoptotic factor. A number of Bcl-2 family members are important players in apoptosis and in the interactions between the anti-apoptotic members and pro-apoptotic members, which provide an inhibitive factor in modulating apoptotic cell death41,42. Therefore, to further verify the effect of TIIA on apoptosis, in addition to TUNEL staining, we measured two apoptosis-related factors, namely, an inhibitive factor of apoptosis Bcl-2 and a pro-apoptotic factor caspase-3, to observe the occurrence of apoptosis. We showed that TIIA reduced lung tissue cell apoptosis (Figure 5), which was confirmed by the enhancement of Bcl-2 and the inhibition of caspase-3 (Figure 6). These observations are similar with those seen in the seawater-induced ALI13,43, which further confirms that the inhibition of apoptosis contributes to the therapeutic effect of TIIA on ALI.
Notable, previous reports show that LPS exposure can upregulate Bcl-2 regardless of airway location and promote the differentiation of proliferating and preexisting epithelial cells into mucous cells44,45. Chronic LPS exposure may cause persistently elevated mucous cell numbers and contribute to mucous hypersecretion and airway obstruction as in chronic lung disease, such as cystic fibrosis or chronic bronchitis44,45. Although these previous reports and our present results appear to be contradictory, they are, in fact, two sides of the same coin. Although LPS can damage some types of cells, such as pulmonary epithelial cells, resulting in lung injury, it also increases the number inflammation-related cells, such as mucous cells, resulting in the chronic lung disease described above. Thus, we may infer that although LPS modulates Bcl-2 expression in mucous cells and pulmonary epithelial cells differently, the final outcomes are similar in that they both lead to lung dysfunction.
To explore the mechanisms responsible for the effect of TIIA, we investigated two main pathways, namely NF-κB and HIF-1α, which are key for the inflammation and apoptosis during LPS-induced ALI. NF-κB, a key pathway in modulating inflammation-related disease, is crucial for the regulation of the inflammatory response and apoptosis in LPS-induced ALI36,37. HIF-1α contributes to the production of inflammatory cytokines, such as TNF-α and IL-67,46 and modulates cell apoptosis47. Remarkably, our results showed that TIIA greatly decreased the activation of NF-κB and HIF-1α pathways (Figure 6). These results indicated that the therapeutic effect of TIIA on LPS-induced lung injury was most likely through the inhibition of NF-κB and HIF-1α activation.
Although our present study indicated that the inhibition of HIF-1α and NF-κB pathways contributed to the attenuation of TIIA on LPS-induced ALI, we should state that the effect of TIIA is not exclusively selective for the HIF-1α and NF-κB pathways. MAPKs, which are other crucial pathways in mediating LPS-induced inflammation, could also be inhibited by TIIA. A previous study showed that TIIA inhibited the phosphorylation of P38, ERK, and JNK48, which was in an estrogen receptor (ER) subtype-dependent manner19 in RAW 264.7 cells. As a result, we may infer that TIIA has multiple targets, which at least include the HIF-1α, NF-κB, and MAPKs pathways.
In summary, our study provides evidence that TIIA therapeutically inhibits LPS-induced ALI by the depression of inflammation and apoptosis in mice. The mechanisms accounting for the therapeutic effect of TIIA were most likely via the inhibition of the NF-κB and HIF-1α pathways. At present, although further studies need to be performed in the clinic, these results provide a basis for employing TIIA in the treatment of ALI.
Authors contribution
Min XU conceived the study, participated in the design and helped draft the manuscript; Fa-le CAO participated in the design, established animal models and helped draft the manuscript; Yu-fei ZHANG participated in the design, performed the statistical analysis and helped draft the manuscript; Liang SHAN performed the statistical analysis; Xiao-ling JIANG performed H&E and TUNEL staining and microscopic examination; Xiao-jing AN and Wei XU performed the Western blotting; Xiu-zhi LIU performed the ELISA assays; and Xiao-yan WANG performed the other chemical assays. All authors read and approved the final manuscript.
References
Touqui L, Arbibe L . A role for phospholipase A2 in ARDS pathogenesis. Mol Med Today 1999; 5: 244–9.
Pittet JF, Mackersie RC, Martin TR, Matthay MA . Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med 1997; 155: 1187–205.
Klebanoff SJ . Myeloperoxidase: friend and foe. J Leukoc Biol 2005; 77: 598–625.
Bellingan GJ . The pulmonary physician in critical care * 6: The pathogenesis of ALI/ARDS. Thorax 2002; 57: 540–6.
Downey GP, Dong Q, Kruger J, Dedhar S, Cherapanov V . Regulation of neutrophil activation in acute lung injury. Chest 1999; 116: 46S–54S.
Ware LB, Matthay MA . The acute respiratory distress syndrome. New Engl J Med 2000; 342: 1334–49.
Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V . Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol 2007; 178: 7516–9.
Xu M, Dong MQ, Cao FL, Liu ML, Wang YX, Dong HY, et al. Tanshinone IIA reduces lethality and acute lung injury in LPS-treated mice by inhibition of PLA2 activity. Eur J Pharmacol 2009; 607: 194–200.
Xu M, Cao F, Liu L, Zhang B, Wang Y, Dong H, et al. Tanshinone IIA-induced attenuation of lung injury in endotoxemic mice is associated with reduction of hypoxia-inducible factor 1alpha expression. Am J Respir Cell Mol Biol 2011; 45: 1028–35.
Xie XY, Zhang B, Li JH, Fan QX, Zhang Y, Mu DG, et al. Sodium tanshinone iia sulfonate attenuates seawater aspiration-induced acute pulmonary edema by up-regulating Na+,K+-ATPase activity. Exp Lung Res 2011; 37: 482–91.
Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D, et al. Tanshinone IIA ameliorates seawater exposure-induced lung injury by inhibiting aquaporins (AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol 2011; 176: 39–49.
Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li JH, et al. Tanshinone IIA attenuates seawater aspiration-induced lung injury by inhibiting macrophage migration inhibitory factor. Biol Pharm Bull 2011; 34: 1052–7.
Li JH, Xu M, Xie XY, Fan QX, Mu DG, Zhang Y, et al. Tanshinone IIA suppresses lung injury and apoptosis, and modulates protein kinase B and extracellular signal-regulated protein kinase pathways in rats challenged with seawater exposure. Clin Exp Pharmacol Physiol 2011; 38: 269–77.
Zhou GJ, Zhang H, Zhi SD, Jiang GP, Wang J, Zhang M, et al. Protective effect of raloxifene on lipopolysaccharide and acid- induced acute lung injury in rats. Acta Pharmacol Sin 2007; 28: 1585–90.
Zhou ZH, Sun B, Lin K, Zhu LW . Prevention of rabbit acute lung injury by surfactant, inhaled nitric oxide, and pressure support ventilation. Am J Respir Crit Care Med 2000; 161: 581–8.
Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, et al. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat Immunol 2000; 1: 42–6.
Yoshii C, Nagata N, Tao Y, Suematsu R, Nikaido Y, Kido M . Relationship between inflammatory cells in bronchoalveolar lavage fluid and pathologic changes in the lung interstitium. Respiration 1998; 65: 386–92.
Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH, Park R, et al. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-alpha, IL-1beta, and IL-6 in activated RAW 264.7 cells. Planta Med 2003; 69: 1057–9.
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol 2009; 113: 275–80.
Tang C, Wu AH, Xue HL, Wang YJ . Tanshinone IIA inhibits endothelin-1 production in TNF-alpha-induced brain microvascular endothelial cells through suppression of endothelin-converting enzyme-1 synthesis. Acta Pharmacol Sin 2007; 28: 1116–22.
Zhang R, Zhang X, Zhang J, Wu J, Ye Q, Xu R, et al. Efficacy and mechanism of Salvia miltiorrhizae injection in the treatment of rats with severe acute pancreatitis. Inflammation 2009; 32: 109–19.
Martin GS, Mannino DM, Eaton S, Moss M . The epidemiology of sepsis in the United States from 1979 through 2000. New Engl J Med 2003; 348: 1546–54.
Angus DC, Wax RS . Epidemiology of sepsis: an update. Crit Care Med 2001; 29: S109–16.
Kollef MH, Schuster DP . The acute respiratory distress syndrome. New Engl J Med 1995; 332: 27–37.
Glauser MP, Zanetti G, Baumgartner JD, Cohen J . Septic shock: pathogenesis. Lancet 1991; 338: 732–6.
Fenton MJ, Golenbock DT . LPS-binding proteins and receptors. J Leukoc Biol 1998; 64: 25–32.
Kabir K, Gelinas JP, Chen M, Chen D, Zhang D, Luo X, et al. Characterization of a murine model of endotoxin-induced acute lung injury. Shock 2002; 17: 300–3.
Baughman RP, Gunther KL, Rashkin MC, Keeton DA, Pattishall EN . Changes in the inflammatory response of the lung during acute respiratory distress syndrome: prognostic indicators. Am J Respir Crit Care Med 1996; 154: 76–81.
Kubo H, Graham L, Doyle NA, Quinlan WM, Hogg JC, Doerschuk CM . Complement fragment-induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood 1998; 92: 283–90.
van Eeden SF, Kitagawa Y, Klut ME, Lawrence E, Hogg JC . Polymorphonuclear leukocytes released from the bone marrow preferentially sequester in lung microvessels. Microcirculation 1997; 4: 369–80.
Rubio F, Cooley J, Accurso FJ, Remold-O'Donnell E . Linkage of neutrophil serine proteases and decreased surfactant protein-A (SP-A) levels in inflammatory lung disease. Thorax 2004; 59: 318–23.
Carney DE, Lutz CJ, Picone AL, Gatto LA, Ramamurthy NS, Golub LM, et al. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation 1999; 100: 400–6.
Hirche TO, Crouch EC, Espinola M, Brokelman TJ, Mecham RP, DeSilva N, et al. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J Biol Chem 2004; 279: 27688–98.
Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med 2001; 163: 762–9.
Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, Tanaka T, et al. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am J Pathol 2000; 157: 597–603.
Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, et al. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol 2001; 281: L328–35.
Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, et al. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol 1999; 163: 2217–25.
Fujita M, Kuwano K, Kunitake R, Hagimoto N, Miyazaki H, Kaneko Y, et al. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunol 1998; 117: 202–8.
Ashkenazi A, Dixit VM . Death receptors: signaling and modulation. Science 1998; 281: 1305–8.
Green DR, Reed JC . Mitochondria and apoptosis. Science 1998; 281: 1309–12.
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–56.
Danial NN, Korsmeyer SJ . Cell death: critical control points. Cell 2004; 116: 205–19.
Hong HJ, Liu JC, Cheng TH, Chan P . Tanshinone IIA attenuates angiotensin II-induced apoptosis via Akt pathway in neonatal rat cardiomyocytes. Acta Pharmacol Sin 2010; 31: 1569–75.
Foster JE, Gott K, Schuyler MR, Kozak W, Tesfaigzi Y . LPS-induced neutrophilic inflammation and Bcl-2 expression in metaplastic mucous cells. Am J Physiol Lung Cell Mol Physiol 2003; 285: L405–14.
Tesfaigzi Y, Fischer MJ, Martin AJ, Seagrave J . Bcl-2 in LPS- and allergen-induced hyperplastic mucous cells in airway epithelia of Brown Norway rats. Am J Physiol Lung Cell Mol Physiol 2000; 279: L1210–7.
Kim HY, Kim YH, Nam BH, Kong HJ, Kim HH, Kim YJ, et al. HIF-1alpha expression in response to lipopolysaccaride mediates induction of hepatic inflammatory cytokine TNFalpha. Exp Cell Res 2007; 313: 1866–76.
Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med 2005; 201: 105–15.
Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO . Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol 2006; 542: 1–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, M., Cao, Fl., Zhang, Yf. et al. Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice. Acta Pharmacol Sin 36, 179–187 (2015). https://doi.org/10.1038/aps.2014.112
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.112