Abstract
Aim:
To investigate the effects and the molecular mechanisms of fucoxanthin, a major carotenoid found in edible seaweed, on HeLa cells.
Methods:
The cytotoxicity of fucoxanthin was evaluated using MTT assay. Cell cycle and apoptosis were evaluated using flow cytometric analysis. Autophagy was detected with acridine orange staining and transient transfection of the GFP-LC3 plasmid into the cells. Protein expression was detected with Western blotting.
Results:
Treatment of HeLa cells with fucoxanthin (10–80 μmol/L) for 48 h caused dose-dependent cytotoxicity with an IC50 value of 55.1±7.6 μmol/L. Fucoxanthin (10, 20, and 40 μmol/L) dose-dependently induced G0/G1 arrest, but did not change the apoptosis of HeLa cells. The same concentrations of fucoxanthin dose-dependently increased the protein expression of LC3 II (the autophagosome marker) and Beclin 1 (the initiation factor for autophagosome formation) in HeLa cells. Moreover, fucoxanthin dose-dependently decreased the levels of phosphorylated Akt and its downstream proteins p53, p70S6K, and mTOR, and increases the expression of PTEN in HeLa cells. Pretreatment of HeLa cells with 3-methyladenine (5 mmol/L) blocked the cytotoxic effect of fucoxanthin as well as fucoxanthin-induced autophagy.
Conclusion:
Fucoxanthin exerts autophagy-dependent cytotoxic effect in HeLa cells via inhibition of Akt/mTOR signaling pathway.
Similar content being viewed by others
Introduction
Epithelial ovarian cancer is the leading cause of death among gynecologic malignancies in developed nations. Treatment success of recurrent ovarian cancer is limited by the emergence of chemoresistance. Chemoresistance commonly results from dysregulation of the apoptotic pathway1. Therefore, agents that are identified to exert a novel cell death mode may have the potential to be effective against chemoresistant diseases.
Autophagy is primarity considered a process of protein recycling. Recently, interest in autophagy has been renewed in antitumor investigations because various anticancer drugs can induce autophagy in different types of cancer cells2. This type of non-apoptotic cell death is considered programmed cell death type II, which is mainly characterized by morphological changes, such as numerous autophagic vacuoles in the cytoplasm. In contrast, apoptosis is classified as programmed cell death type I. Many studies have demonstrated that the two types of cell death are predominantly distinctive but also that cross-talk exists between them3,4. In multiple studies, autophagy has been inhibited, resulting in contrasting outcomes, ie, survival or death, depending on the drugs and cell types analyzed. Interestingly, several tumor suppressors, such as p53, Beclin 1, and PTEN, also play an important role in autophagy5. Taken together, these accumulating data may lead to the development of a new cancer therapeutic strategy via drug-induced autophagy.
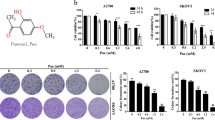
Fucoxanthin (Figure 1A) has a unique structure, including an allenic bond and a 5,6-monoepoxide, and is a major marine carotenoid found in edible seaweeds, such as Undaria pinnatifida, Hijikia fusiformis, and Sargassum fulvellum6. Recent studies have demonstrated that fucoxanthin has antitumor effects7, promotes apoptotic effects8, anti-inflammatory effects9, and radical scavenging activity10.
Antitumor effects resulting from a 48 h fucoxanthin treatment of HeLa cells. (A) Structure of fucoxanthin. (B) Cytotoxic activity resulting from a 48 h fucoxanthin treatment of HeLa cells. (C) Cell cycle distribution of HeLa cells after treatment with fucoxanthin for 48 h. (D) Apoptosis of HeLa cells after treatment with fucoxanthin for 48 and 72 h. Statistical analysis was performed using analysis of variance (ANOVA) followed by a q-test. bP <0.05 and cP <0.01 as compared to control (n=3).
Although it is often controversial whether autophagy in cancer cells causes cell death or cell protection, our results demonstrate that treatment with 3-methyladenine, which is a phosphatidylinositol 3-phosphate kinase inhibitor, not only reverses fucoxanthin-mediated autophagy but also partially reverses the cytotoxic effect of fucoxanthin, thereby suggesting that autophagy is the vital factor in fucoxanthin-mediated cytotoxic effects in HeLa cells. Mechanistically, fucoxanthin-mediated autophagy is dependent on the Akt/mTOR signaling pathway.
Materials and methods
Materials
RNase A, 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT), acridine orange (AO), fucoxanthin, bafilomycin A1 (Baf A1), ammonium chloride (NH4Cl), 3-methyl adenine (3-MA), propidium iodide (PI) and a monoclonal anti-p62 antibody were purchased from Sigma (St Louis, MO, USA). RPMI-1640 and fetal calf serum (FCS) were obtained from Gibco (Grand Island, NY, USA). An AnnexinV-FITC apoptotic detection kit and primary antibodies against phospho-Akt, Akt, p21, PTEN, p53, CDK2, cyclin D1, phospho-p70S6 kinase (p-p70S6K; Thr389) and phospho-mTOR (p-mTOR) were purchased from Cell Signaling Technology (Beverly, MA, USA). Primary antibodies against Beclin 1, LC3, and cathepsin D as well as horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). LysoTracker Red and Hoechst 33342 were purchased from Biyuntian (Haimen, China). All other chemicals used in the experiments were commercial products of reagent grade.
Cell culture and cytotoxicity assay
HeLa cells were purchased from Shanghai Institute for Biological Science (Shanghai, China) and were supplemented with 1 mmol/L glutamine and 10% FCS. The cells were cultured at 37 °C under 5% CO2 atmosphere.
The cytotoxic effect of fucoxanthin was evaluated in HeLa cells by the conversion of MTT to a purple formazan precipitate, as previously described11. After cells were seeded into 96-well plates at 5000 cells/well for 24 h, fucoxanthin was added, and the cells were incubated for an additional 48 h. The inhibition rate was calculated from plotted results using untreated cells as 100%11.
Cell cycle and cellular apoptotic evaluation
After treatment with fucoxanthin for 48 or 72 h, the cells were fixed with ice-cold 70% ethanol and then stained with PI (100 μg/mL) after removing the RNA from the cells by RNase A treatment (50 μg/mL). The analysis was performed using a FACScan (Becton Dickinson, USA) emitting at 488 nm. Data acquisition and analysis were controlled by Modifit software12.
Cell apoptosis was evaluated by an AnnexinV-FITC apoptotic detection kit using FACScan (Becton Dickinson, USA). Briefly, the cells were seeded in six-well plates, exposed to fucoxanthin for 48 h, harvested and stained according to the manufacturer's protocol12.
Autophagy detection with acridine orange staining
As a marker of autophagy, the volume of the cellular acidic compartment was visualized by acridine orange staining13. Cells were seeded into 6-well plates and treated as described above for the cytotoxicity assay. After 48 h of treatment, cells were incubated with medium containing acridine orange (1 mg/mL) for 15 min. For autophagy inhibitor analysis, cells were pretreated with 3-MA (5 mmol/L) for 2 h. Acridine orange was removed, and fluorescent micrographs were taken using a fluorescent microscope. A shift from green to red fluorescence indicated acidic vesicles consistent with autolysosomes. Western blotting analysis was performed for LC3-II and Beclin 1, which is an essential autophagy-related protein12.
Lysosome detection with LysoTracker Red staining
After the fucoxanthin incubation, cells were stained with LysoTracker Red (50 nmol/L), a specific red fluorescent dye for lysosomes, for 45 min at 37 °C, and the cells were then counterstained with Hoechst 33342 (1 μmol/L) for 15 min in the dark. Fluorescent micrographs were taken using a fluorescence microscope12.
Transient transfection and autophagy assays
HeLa cells were plated on coverslips at a density of 2×105 cells/coverslip and cultured to 60% confluence. Transient transfection of 2 μg/mL GFP-LC3 plasmid DNA in each dish was performed using Lipofectamine 2000 according to the manufacturer's protocol. After incubation in Opti-MEM medium for 6 h, the cells were incubated in RPMI-1640 containing 10% FBS. Autophagy was examined by analyzing the formation of fluorescent puncta of autophagosomes in GFP-LC3-transfected cells using fluorescence microscopy. When cells reached 90% confluence, fucoxanthin was added to the culture medium. After a 48 h treatment, the cells were fixed with 4% paraformaldehyde. The GFP-LC3 punctate dots per cell in GFP-LC3-positive cells were counted. Thirty cells per slide were counted, and three slides were used for each condition14.
Western blotting
After fucoxanthin treatment, HeLa cells were harvested and washed with PBS. Total cellular protein was isolated using a protein extraction buffer (containing 150 mmol/L NaCl; 10 mmol/L Tris, pH 7.2; 5 mmol/L EDTA; 0.1% Triton X-100; 5% glycerol; and 2% SDS). Protein concentration was determined using a protein assay kit (Biyuntian, Haimen, China). Equal amounts of protein (50 μg/lane) were separated using 8% SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with primary antibodies. After washing with PBS, the membranes were incubated with corresponding peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibodies followed by detection with enhanced chemiluminescence staining. β-Actin (45 kDa; cytosolic protein) was used to normalize protein loading12. The level of protein expression was quantified by densitometry analysis using a gel-imaging system.
Statistical analysis
All data are presented as the mean±SD and were analyzed using analysis of variance (ANOVA) followed by a q-test.
Results
Fucoxanthin exerted potent cytotoxic activity but did not induce cell apoptosis
After treatment for 48 h, the cytotoxic activity of fucoxanthin was significantly increased in a dose-dependent manner in HeLa cells. The IC50 value of fucoxanthin was 55.1±7.6 μmol/L (Figure 1B). Flow cytometric analysis demonstrated that fucoxanthin induced cell cycle arrest at the G0/G1 phase in a dose-dependent manner (Figure 1C). Further investigation showed that fucoxanthin increased the expression of p21 and decreased the expression of cyclin D1 and CDK2, which are G1-related proteins (Figure 4). Next, we investigated if fucoxanthin-induced cell death was caused by apoptosis. AnnexinV/PI double labeling was used for the detection of PS externalization, a hallmark of early phase apoptosis. Compared to control cells, the percentage of annexin V-positive cells was not significantly increased after a 48 or 72 h treatment with fucoxanthin (Figure 1D), thereby suggesting that fucoxanthin did not induce HeLa cell apoptosis.
Fucoxanthin induced HeLa cell autophagy
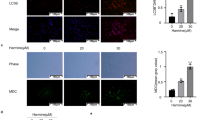
Autophagy can cause cell growth inhibition. The association of autophagic vacuole formation with autophagic cell death was evaluated. Based on acridine orange staining, there was a significant increase in autophagic vesicle (red fluorescence) formation in HeLa cells after being exposed to fucoxanthin, as shown in Figure 2A. During the process of autophagic vesicle formation, the number and activity of lysosomes increase; therefore, we further examined lysosomes using LysoTracker Red, a specific red fluorescent dye for lysosomes. As shown in Figure 2B, the fluorescence of LysoTracker Red increased after treatment with fucoxanthin in a dose-dependent manner. Furthermore, Western blotting results and quantitative analysis demonstrated that treatment with fucoxanthin for 48 h markedly upregulated Beclin 1 (initiation factor for autophagosome formation) and promoted the conversion of LC3 I to LC3 II (autophagosome marker) as compared to the control (Figure 2C). These results suggested that fucoxanthin induced autophagy in HeLa cells after treatment for 48 h.
Fucoxanthin induced HeLa cell autophagy after treatment for 48 h. (A) Representative micrographs of acridine orange staining of HeLa cells after treatment with fucoxanthin (Magnification ×20). (B) Representative micrographs of LysoTracker Red and Hoechst 33342 staining of HeLa cells after treatment with fucoxanthin (Magnification ×20). (C) Protein expression of Beclin-1 and LC3 in HeLa cells was detected by Western blotting after treatment with fucoxanthin for 48 h. (D) Representative Hoechst 33342 staining micrographs of transiently transfected HeLa cells with GFP-LC3 after treatment with fucoxanthin (Magnification ×20). Arrowheads indicate GFP-LC3 puncta.
Furthermore, the occurrence of autophagy after fucoxanthin exposure in HeLa cells was also validated by detecting the expression of GFP-LC3. Following fucoxanthin treatment, GFP-LC3-transfected cells showed punctate green fluorescence at 48 h. In contrast, control transfected cells without fucoxanthin treatment showed a diffuse distribution of green fluorescence (Figure 2D). These findings further demonstrated that fucoxanthin induced HeLa cell autophagy.
To assess autophagic flux, Baf A1, which is a potent and specific inhibitor of vacuolar H+-ATPases, was used to suppress the acidification of the lysosome and the autophagosome/lysosome15. As shown in Figure 3A, Baf A1 significantly enhanced fucoxanthin-mediated GFP-LC3 patches. Western blot analysis also demonstrated that the fucoxanthin-induced upregulation of LC3 II dramatically increased in the presence of Baf A1 (Figure 3B and 3C). To further detect autophagic flux, we treated cells with NH4Cl, an inhibitor of lysosomal acidification, which inhibits autophagic degradation of autophagic substrates. After treatment with fucoxanthin, the expression level of p62 was significantly decreased. However, the addition of NH4Cl caused the levels of p62 to recover, suggesting that p62 was degraded by the autophagy process (Figure 3D and 3E). Autophagy is a constitutive process that includes activation of lysosomal enzymes and subsequent degradation of their substrates. Therefore, the expression levels of cathepsin D, which is an aspartic protease localized inside the lysosomes, is increased during autophagy. Our results demonstrated that cathepsin D was upregulated after fucoxanthin treatment.
Autophagic flux was detected after treatment with fucoxanthin for 48 h. (A) Quantification of autophagy in fucoxanthin-treated HeLa cells transfected with GFP-LC3 in the presence or absence of 100 nmol/L Baf A1 for 48 h. Western blotting analysis (B) and quantitative analysis (C) of LC3 expression in HeLa cells after treatment with fucoxanthin in the presence or absence of 100 nmol/L Baf A1 for 48 h. Western blotting analysis (D) and quantitative analysis (E) of p62 expression in HeLa cells after treatment with fucoxanthin in the presence or absence of 50 mmol/L NH4Cl for 48 h. Western blotting analysis (F) and quantitative analysis (G) of cathepsin D expression in HeLa cells after treatment with fucoxanthin for 48 h. Mean±SD. bP <0.05, cP <0.01 compared to the control (n=3).
Fucoxanthin reduced phosphorylated Akt and its downstream targets
Recent studies have shown that the inhibition of Akt and its downstream target, mTOR, contribute to the initiation of autophagy16. Once activated, Akt transduces signals to downstream targets that control cell survival and autophagy17. To assess the involvement of the Akt pathway in fucoxanthin-induced autophagy, the related proteins were investigated by Western blotting. As shown in Figure 4, phosphorylated Akt significantly decreased after a 48 h treatment with fucoxanthin in a dose-dependent manner. In addition, we investigated the expression of downstream target proteins of Akt. Fucoxanthin significantly inhibited the phosphorylation of p53, p70S6K and mTOR, and it upregulated the expression of PTEN, thereby suggesting that the Akt signaling pathway was involved in fucoxanthin-mediated autophagy.
3-MA reversed cytotoxic effects and fucoxanthin-mediated autophagy
To investigate if the cytotoxic activity of fucoxanthin was dependent on autophagy, HeLa cells were pretreated with 3-MA before administration of fucoxanthin. As shown in Figure 5A, 3-MA partially reversed the fucoxanthin-mediated cytotoxic effects. In addition, fucoxanthin did not induce apoptosis in HeLa cells pretreated with 3-MA (Figure 5B). 3-MA also reversed fucoxanthin-mediated autophagy and inhibited the Akt signaling pathway (Figure 5C-5G), thereby suggesting that the antitumor activity of fucoxanthin was dependent on fucoxanthin-induced autophagy.
3-MA reversed fucoxanthin-induced autophagy in HeLa cells after treatment for 48 h. (A) 3-MA reversed fucoxanthin-induced cytotoxic effects on HeLa cells after treatment for 48 h. (B) Apoptosis of HeLa cells after treatment with fucoxanthin for 48 h. (C) Representative micrographs of acridine orange staining of HeLa cells after pretreatment with 3-MA (Magnification ×20). (D) Representative micrographs of LysoTracker Red and Hoechst 33342 staining of HeLa cells after pretreatment with 3-MA (Magnification ×20). (E) Representative micrographs of Hoechst 33342 staining of GFP-LC3-transfected HeLa cells after pretreatment with fucoxanthin (Magnification ×20). Arrowheads indicate GFP-LC3 puncta. (F) Protein expression in HeLa cells for Beclin-1 and LC3 was detected by Western blotting after pretreatment with fucoxanthin for 48 h (left). The level of protein expression was quantified by densitometry analysis using a gel-imaging system. (G) Expression of Akt signaling pathway-related proteins in HeLa cells was detected by Western blotting after pretreatment with fucoxanthin for 48 h (left). The level of protein expression was quantified by densitometry analysis using a gel-imaging system. Mean±SD. bP<0.05, cP<0.01 compared to the control (n=3).
Discussion
The role of the autophagic process in antitumor therapy has not been clearly elucidated18,19. After treatment with antitumor drugs, some cancer cells undergo autophagy as a temporary survival mechanism, and the suppression of autophagy leads to apoptosis, thus enhancing antitumor effects. In contrast, several antitumor drug treatments, including some chemotherapeutic agents, have been reported to induce autophagic cell death3,20. In the present study, fucoxanthin induced HeLa cell autophagy but not apoptosis, even with 3-MA pretreatment.
Autophagy is the regulated process by which cytoplasmic constituents are recruited to lysosomes for degradation18. The autophagic pathway begins with the formation of a double-membrane vesicle called the “autophagosome” that engulfs organelles or long-lived proteins and matures into an acidic single-membrane autophagosome that fuses with a lysosome to become the “autolysosome”21. The process is known to be accompanied by an increase in the acidity of the lumen, followed by the development of acidic vesicular organelles (AVOs)22. To detect the development of AVOs, HeLa cells treated with fucoxanthin were stained with acridine orange. Acridine orange concentrates in acidic vesicles, such as matured autophagosomes and autolysosomes, and it has been used as an indicator of autophagosomal maturation23. Our results demonstrated that the bright red fluorescence significantly increased after fucoxanthin treatment, indicating the development of AVOs. Furthermore, after treatment with fucoxanthin and then being stained with LysoTracker Red, the red fluorescence of HeLa cells significantly increased. In addition, Western blotting and GFP-LC3 transfection studies also demonstrated that fucoxanthin induced HeLa cell autophagy and that 3-MA reversed these effects. However, fucoxanthin did not induce HeLa cell apoptosis using the same dose and treatment time, even with 3-MA pre-incubation, thereby suggesting that fucoxanthin only induced HeLa cell autophagy and not apoptosis.
Efficient autophagy is dependent on the balance between the formation and elimination of autophagosomes, and a deficit in any part of this process will cause autophagic dysfunction. Thus, autophagy flux should be detected in fucoxanthin-mediated HeLa cell autophagy. Our results demonstrated that fucoxanthin enhanced autophagy flux, including an increase in autophagosome formation, lysosome activation and degradation of autophagic substrates. This effect was different from the effect of perifosine, which inhibits autophagy and enhances apoptosis24. However, Tafuku et al demonstrated that fucoxanthin induces B-cell malignancies, such as Hodgkin's lymphoma, Burkitt's lymphoma and Epstein-Barr virus-immortalized B-cells, through apoptosis by suppressing the NF-κB signaling pathway and cell cycle arrest at the G1 phase; however, they reported that fucoxanthin did not induce apoptosis in normal peripheral blood mononuclear cells25.
3-MA partially reversed the fucoxanthin-mediated cytotoxic effects, suggesting that the antitumor activity of fucoxanthin was autophagy-dependent. Several reports indicated that Akt/mTOR signaling negatively regulates autophagy through mTOR, which is a downstream target of Akt26,27. In the present study, we demonstrated that fucoxanthin inhibited Akt/mTOR signaling, as demonstrated by the inhibition of the phosphorylation of Akt, p70S6K and mTOR, resulting in the conversion of LC3 I to LC3 II, a hallmark of autophagy. In the context of induction of autophagy, Aoki et al showed that natural products induce autophagy through the inhibition of Akt/mTOR signaling, which agreed with the present results16.
Autophagy is a signal transduction pathway that can affect the G1 phase progression. The autophagic process during G1 arrest can repair cell damage to avoid cell death28. Some inhibitors of the AKT signaling pathway, such as NVP-BEZ235, induce tumor cell autophagy and cell cycle arrest29. Our results demonstrated that fucoxanthin induced cell cycle arrest at the G0/G1 phase via inhibiting the AKT signaling pathway and that fucoxanthin also regulated the expression of cell cycle-related proteins by upregulating p21 expression and downregulating CDK2 and cyclin D1 expression. These results were similar to previous reports that fucoxanthin induces cell cycle arrest at the G1 phase but not apoptosis in LNCap30, HepG2 and DU145 cells31. However, Satomi reported that fucoxanthin induces LNCap prostate cancer cell cycle arrest at the G1 phase via SAPK/JNK signal pathway activation30. In addition, Yoshiko and Hoyoku found that fucoxanthin induces HepG2 and DU145 cell cycle arrest at the G1 phase via induced GADD45A, a cell cycle-related gene. Accordingly, these data suggest that fucoxanthin-mediated tumor cell death and its molecular mechanism depend on the tumor cell type.
Recently, as a potential therapeutic approach for malignant tumors, targeting of the Akt/mTOR pathway has been suggested in the field of chemotherapy16. Hence, our results also support consideration of the potential use of fucoxanthin as an antitumor agent because fucoxanthin mediates autophagy via inhibition of the Akt/mTOR signaling pathway in HeLa cells.
Author contribution
Prof Song-qiang XIE designed the research and revised the manuscript; Li-li HOU and Chao GAO conducted the research; Liang CHEN helped with portions of the research; Guo-qiang HU performed the statistical analysis.
References
Shigetomi H, Higashiura Y, Kajihara H, Kobayashi H . Targeted molecular therapies for ovarian cancer: an update and future perspectives. Oncol Rep 2012; 28: 395–408.
Carew JS, Kelly KR, Nawrocki ST . Autophagy as a target for cancer therapy: new developments. Cancer Manag Res 2012; 4: 357–65.
Gordy C, He YW . The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell 2012; 3: 17–27.
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 2012; 45: 487–98.
Abrahamsen H, Stenmark H, Platta HW . Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett 2012; 586: 1584–91.
D'Orazio N, Gemello E, Gammone MA, de Girolamo M, Ficoneri C, Riccioni G . Fucoxantin: a treasure from the sea. Mar Drugs 2012; 10: 604–16.
Wang J, Chen S, Xu S, Yu X, Ma D, Xu X, et al. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs 2012; 10: 2055–68.
Yu RX, Hu XM, Xu SQ, Jiang ZJ, Yang W . Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur J Pharmacol 2011; 657: 10–9.
Heo SJ, Yoon WJ, Kim KN, Oh C, Choi YU, Yoon KT, et al. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem Toxicol 2012; 50: 3336–42.
Takashima M, Shichiri M, Hagihara Y, Yoshida Y, Niki E . Capacity of fucoxanthin for scavenging peroxyl radicals and inhibition of lipid peroxidation in model systems. Free Radic Res 2012; 46: 1406–12.
Xie SQ, Wang JH, Ma HX, Cheng PF, Zhao J, Wang CJ . Polyamine transporter recognization and antitumor effects of anthracenymethyl homospermidine. Toxicology 2009; 263: 127–33.
Xie SQ, Zhang YH, Li Q, Xu FH, Miao JW, Zhao J, et al. 3-Nitro-naphthalimide and nitrogen mustard conjugate NNM-25 induces hepatocellular carcinoma apoptosis via PARP-1/p53 pathway. Apoptosis 2012; 17: 725–34.
Huang S, Sinicrope FA . Celecoxib-induced apoptosis is enhanced by ABT-737 and by inhibition of autophagy in human colorectal cancer. Autophagy 2010; 6: 256–69.
Hu D, Wu J, Xu L, Zhang R, Chen L . A method for the establishment of a cell line with stable expression of the GFP-LC3 reporter protein. Mol Med Report 2012; 6: 783–6.
Zhang XJ, Chen S, Huang KX, Le WD . Why should autophagic flux be assessed? Acta Pharmacol Sin 2013; 34: 595–9.
Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y . Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol 2007; 72: 29–39.
Zou CY, Smith KD, Zhu QS, Liu J, McCutcheon IE, Slopis JM, et al. Dual targeting of AKT and mammalian target of rapamyc, in: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther 2009; 8: 1157–68.
Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A . Autophagy in lysosomal storage disorders. Autophagy 2012; 8: 719–30.
Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B . Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif 2012; 45: 466–76.
Marquez RT, Xu L . Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2012; 2: 214–21.
Kaushik S, Cuervo AM . Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 2012; 22: 407–17.
Hundeshagen P, Hamacher-Brady A, Eils R, Brady NR . Concurrent detection of autolysosome formation and lysosomal degradation by flow cytometry in a high-content screen for inducers of autophagy. BMC Biol 2011; 9: 38.
Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010; 465: 942–6.
Fu L, Kim YA, Wang X, Wu X, Xue P, Lonial S, et al. Perifosine inhibits mammalion target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res 2009; 69: 8967–76.
Tafuku S, Ishikawa C, Yasumoto T, Mori N . Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt's and Hodgkin's lymphoma cells. Oncol Rep 2012; 28: 1512–8.
Dickstein RJ, Nitti G, Dinney CP, Davies BR, Kamat AM, McConkey DJ . Autophagy limits the cytotoxic effects of the AKT inhibitor AZ7328 in human bladder cancer cells. Cancer Biol Ther 2012; 13: 1325–38.
Singh BN, Kumar D, Shankar S, Srivastava RK . Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol 2012; 84: 1154–63.
Platini F, Pérez-Tomás R, Ambrosio S, Tessitore L . Understanding autophagy in cell death control. Curr Pharm Des 2010; 16: 101–13.
Wang WJ, Long LM, Yang N, Zhang QQ, Ji WJ, Zhao JH, et al. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol Sin 2013; 34: 681–90.
Satomi Y . Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res 2012; 32: 807–13.
Yoshiko S, Hoyoku N . Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. In vivo 2007; 21: 305–9.
Acknowledgements
This work was supported by Projects of Basic and Frontier of Henan (No 102300410095) and China Postdoctoral Science Foundation Funded Project (No 20090450092; 201003395).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, Ll., Gao, C., Chen, L. et al. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol Sin 34, 1403–1410 (2013). https://doi.org/10.1038/aps.2013.90
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.90
Keywords
This article is cited by
-
Fucoxanthin, a Functional Food Ingredient: Challenges in Bioavailability
Current Nutrition Reports (2023)
-
The Link of Marine Products with Autophagy-Associated Cell Death in Cancer Cell
Current Pharmacology Reports (2019)
-
The anticancer effects and mechanisms of fucoxanthin combined with other drugs
Journal of Cancer Research and Clinical Oncology (2019)
-
Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas
Scientific Reports (2017)
-
G226, a novel epipolythiodioxopiperazine derivative, induces autophagy and caspase-dependent apoptosis in human breast cancer cells in vitro
Acta Pharmacologica Sinica (2014)