Abstract
Inflammation plays a critical role in mediating brain injury induced by neonatal hypoxic ischemic encephalopathy (HIE). The mechanisms underlying inflammatory responses to ischemia may be shared by neonatal and adult brains; however, HIE exhibits a unique inflammation phenotype that results from the immaturity of the neonatal immune system. This review will discuss the current knowledge concerning systemic and local inflammatory responses in the acute and subacute stages of HIE. The key components of inflammation, including immune cells, adhesion molecules, cytokines, chemokines and oxidative stress, will be reviewed, and the differences between neonatal and adult inflammatory responses to cerebral ischemic injury will also be discussed.
Similar content being viewed by others
Introduction
Perinatal hypoxic-ischemic encephalopathy (HIE) is a major cause of neonatal death and long-term disability. Approximately 15% to 25% of affected newborns die in the postnatal period and 25% develop severe and permanent neuropsychological sequelae1, including cerebral palsy, seizures, visual impairment, mental retardation, learning impairment and epilepsy2. Two phases of HIE-induced neuronal death have been identified in both clinical and experimental studies3,4,5. The immediate phase, primary neuronal death, is related to cellular hypoxia with exhaustion of the cell's high-energy stores (primary energy failure). The second phase, delayed neuronal death6, occurs after a latent period of at least six hours, and is associated with encephalopathy and increased seizure activity. Delayed neuronal death accounts for a significant proportion of final cell loss even after very severe insults. The mechanisms involved in delayed neuronal death include excitotoxicity, apoptosis and microglial activation7. Microglia are the resident immune cells in the brain, and microglial activation is the initial step in inflammatory responses of the central nervous system (CNS) to various stimuli, including stroke8. This initial step is followed by the infiltration of circulating monocytes, neutrophils and T-cells9, which amplifies the inflammatory response in a stimulated brain.
Cerebral ischemia induces an inflammatory response in both the parenchyma and the systemic circulation. Within hours after an insult to the brain of an adult, cytokines are produced in large amounts, and leukocytes are activated and migrate into the injured brain10,11,12,13,14. In neonates, however, cerebral ischemia initiates an immediate innate immune response even minutes after the insult15. Age differences in the mechanisms of stroke, some of them very striking, stem from immaturity of the CNS, including differences in the cross-talk between excitotoxic, oxidative and inflammatory injury mechanisms, creating “windows of susceptibility” to hypoxic-ischemic injury during embryonic and early postnatal brain development16. Here, we review the data on specific aspects of neuroinflammation in the acute and subacute stages of HIE, and will also introduce known similarities and differences in adult and neonatal cerebral ischemic injury. Because the chronic inflammatory response to HIE may last for years and varies according to the developmental stage of the brain, this topic is beyond the scope of this review and will not be discussed.
Immune cells
Microglia/macrophages
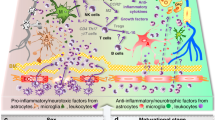
Microglia are a major glial component of the CNS and provide immuno-surveillance in the brain17. Resting microglia in a healthy brain, known as surveying microglia, are constantly extending and retracting their thin ramified processes to inspect the CNS microenvironment18,19. When an ischemic event occurs, microglia are activated and develop macrophage-like abilities including phagocytosis, the production of inflammatory and anti-inflammatory cytokines, antigen presentation and the release of matrix metalloproteinases (MMPs), which lead to blood-brain barrier (BBB) breakdown20. As a result, peripheral leukocytes infiltrate the brain, and the normally immune-privileged brain environment is exposed to systemic responses that further exacerbate inflammation and brain damage (Figure 1). The innate immune response is characterized by the classical activation (M1) of microglia and the subsequent production of specific cytokines, chemokines and reactive intermediates, followed by resolution and alternative activation (M2) that leads to anti-inflammatory signaling (M2a), the clearance of reactive oxygen (ROS) and nitrogen (RNS) species (M2b), and wound healing (M2c)21. During disease progression, microglial activation phenotypes switch from M1 to M2 or vice versa depending on inflammatory signaling22. The M1 phenotype of microglia can lead to increased neuronal death compared to the alternatively activated M2 microglial phenotype23; therefore, there is a growing interest in controlling the classical activation phenotype of microglia.
Schematic diagram of inflammatory responses in ischemic stroke. When stroke occurs, microglia are activated and develop macrophage-like capabilities including phagocytosis, cytokine and chemokine production, antigen presentation and the release of MMPs that weaken the BBB. As a result, peripheral leukocytes infiltrate into the brain, leading to exacerbation of inflammation and neuronal injury. MG, microglia; MP, macrophage; NE, neuron; NP, neutrophil; LC, lymphocyte; MN, monocyte; EC, erythrocyte.
In addition to microglia, macrophages also inhabit various regions (choroid plexus, peri-vasculature and meninges) of the CNS24. The heterogeneous population of tissue macrophages can be continuously replenished by circulating monocytes, unlike microglia, which are thought to reside in the adult CNS from early development25,26,27. The theory that a second wave of microglia is established in the brain during the postembryonic period and is derived from peripheral monocytic precursors that last into adulthood is a subject of ongoing debate25,28. However, one recent study suggested that a population of dying microglia in the ischemic brain could be replenished by peripheral monocytes or macrophages infiltrating the injured region and then acquiring microglial phenotypes29.
Microglial activation and aggregation are pathological markers for HIE in human infants30. Retrospective clinical studies on the postmortem examinations of 178 brains from neonates found that patients who died from HIE had a dense infiltrate of microglia in the hippocampal dentate gyrus, whereas those neonates who died of other acute causes (trauma or sepsis) had significantly fewer microglia30. Emerging experimental data from disease models also outline the importance of microglial activation in hypoxia-induced neuroinflammation. HIE in preterm sheep resulted in profound activation and proliferation of microglia in the hippocampus and the periventricular and subcortical white matter, followed by a significant influx of neutrophils into the brain31. Ameboid microglia in the developing brain respond vigorously to hypoxia and accumulate in injured tissue32,33,34,35, producing excess amounts of inflammatory cytokines (TNF-α, IL-1β, etc) along with glutamate, nitric oxide (NO) and ROS, which collectively cause oligodendrocyte death, axonal degeneration and disruption of the immature BBB32,33,36. Compared to adults, microglial activation in neonates is much more rapid following transient ischemia37,38 and excitotoxic injury39 and continues for weeks39,40,41.
Astrocytes
Both astrocytes and microglia are activated within minutes after injury by pro-inflammatory mediators, cytokines, and ROS that are secreted by injured neurons and glial cells42. The activation of astrocytes has both detrimental and beneficial roles in brain ischemia. Astrocyte support of neurons after a stroke can be achieved by several mechanisms, including the release of glutathione and superoxide dismutase (SOD)43,44,45, enhanced extra-synaptic glutamate uptake46,47,48, and the maintenance of ion gradients, such as that for potassium49,50. However, activated astrocytes can also produce pro-inflammatory cytokines, including IL-6, TNF-α, IL-1α, and β and interferon γ42,51,52. Rapid increases in the levels of these cytokines exacerbate an ischemic injury by directly inducing the apoptosis of neuronal cells53, increasing toxic NO levels and inhibiting neurogenesis54. Apart from cytokines, reactive astrocytes also secrete chemokines after ischemia, which results in the attraction of immune cells to the ischemic site and worsening of the brain injury55,56.
In the brains of human neonates, astrocytes do not readily become reactive and responsive to injury signals until 20 to 23 weeks of gestation57. Experimental studies regarding astrocytic responses to HIE or systemic LPS stimulation performed in fetuses from various species, eg, lamb58,59,60, baboon61, and kitten62, found astrocyte hypertrophy and hyperplasia. These studies concluded that astrocytes generally are resistant to damage during the neonatal period and that the astrocytes adjacent to regions of necrosis are ready to proliferate. Similar to the findings in adult ischemic models, astrocytes in P7 rat neonates are rarely observed within the ischemic core but are abundant in the penumbra area 24 h after HIE37. One unique role of neonatal astrocytes in HIE-induced inflammatory responses is that, in addition to the self-release of cytokines and chemokines, reactive astrocytes in neonatal brains have the ability to up-regulate the expression of inflammatory mediators in neuroblasts and angioblasts, which are chemotactic for bone marrow-derived immune cells63.
Neutrophils
During ischemia, neutrophils can exacerbate brain injury through multiple mechanisms, including ROS production35, decreased microvascular flow resulting from capillary plugging by neutrophils64, the enhanced release of cytoxic agents into the vasculature and brain parenchyma65,66, and MMP-9 secretion67. The accumulation of neutrophils in ischemic brain tissue occurs as early as 4 h to 6 h after the onset of ischemia in adult animals65,68,69,70 and lasts to 48 h post insult, during the period while the brain injury is evolving71,72,73. In contrast to the exacerbated neutrophil infiltration observed in adults, neonates have a diminished ability to mount a neutrophil response to ischemia. Neonatal neutrophils show reduced extravasation from blood vessels74,75. A previous study has shown that neutrophils did not transmigrate into the brains of P7 rats following HI injury within 42 h and were almost exclusively intravascular at all time periods examined76. Similarly, it has been reported that neutrophils were most often found within vessels and only transiently invaded brain tissue in the infarct region after induction by HI in P7 rats35. These studies indicated that neutrophils do not accumulate in ischemic brain parenchyma in neonatal rodents to the extent that they do in adults. Interestingly this concept translated well into the neuroprotection achieved with anti-neutrophil strategies; treatment with neutrophil inhibitory factor initiated after HI insult was neuroprotective in adult animals77,78,79 but was less efficacious in neonatal rats. Beneficial effects were only observed when neutropenia was induced before the HI insult80, making this a less clinically relevant target for treating neonatal injury.
Lymphocytes
Generally, lymphocytes are thought to play a negative role in acute ischemic brain pathogenesis. Yilmaz et al81 reported that Rag1−/− mice, deficient in both T cells and B cells, had significantly smaller infarcts and neurologic damage compared to WT mice when subjected to middle cerebral artery occlusion (MCAO). In the same study, Rag1−/− mice reconstituted with splenocytes from WT mice were no longer protected from stroke, suggesting that the peripheral lymphocytic response plays an important role in mediating post-stroke injury. Infiltration of T cells and B cells into the ischemic brain can be observed as early as a few hours82,83, and lasts days after injury in adult rodents84,85. However, in neonates the infiltration of these cells following HIE and focal stroke may be less profound35,86 or only briefly present in the parenchyma87. The minimal involvement of lymphocytes in ischemia-induced inflammatory responses in the neonatal brain may reflect the immaturity of lymphoid progenitor cells. Recent clinical studies showed that peripheral blood mononuclear cells of newborns are relatively undifferentiated and have a very low expression level of surface markers88. There are few studies investigating the role of lymphocytes in HIE. It is likely that a lymphocytic response is involved in the more chronic immunoinflammatory activation following HIE; the Hagberg group35 found that CD4 lymphocytes invaded the infarct region quite late after injury (7 d after HIE) and persisted in damaged areas for 14 d to 35 d. Whether this lymphocytic response enhances damage or, conversely, enhances post-stroke repair is not yet clear. It is also unknown whether the presence of lymphocytes can lead to the development of later CNS autoimmunity, as has been observed in adult injury models89.
Adhesion molecules
The recruitment of leukocytes in the cerebral vasculature and the subsequent migration to the ischemic brain tissue are initially mediated by three main groups of cell adhesion molecules: selectins, the immunoglobulin superfamily and integrins90. The recruitment process involves two stepwise stages, ie, an initial low affinity binding that is manifested as rolling and a later high affinity interaction that results in firm adhesion. Adhesion molecules may represent important therapeutic targets because inhibiting leukocyte adhesion with antibodies or inhibitors has improved histological and neurological outcomes in experimental stroke studies, whereas over-expression of adhesion molecules resulted in the exacerbation of infarcts91. Very few neonatal studies have reported the role of and changes in adhesion molecules in HIE; we have summarized the available data from studies in both HIE and other inflammatory diseases in Table 1.
Selectins
Selectins play a key role in the early (rolling) stages of leukocyte/endothelial interactions in the ischemic cerebral microvasculature. Although all three selectins, L-, P-, and E-selectin, have been implicated in neutrophil rolling, P-selectin is the most important during the initial induction of neutrophil rolling after endothelial cell stimulation92. Compared to adults, decreased P-selectin expression in neonates has been found in activated platelets93 and endothelial cells94. Similarly, L-selectin expression in term infant neutrophils is significantly lower than that in adult neutrophils either stimulated or unstimulated95. This may explain why the decreased adhesion of neutrophils to endothelial cells and delayed transendothelial cell migration of neutrophils have been consistently reported in neonatal animals and humans and may also contribute to susceptibility of neonates to infection96,97. In immature animal brains during acute inflammation, E-selectin seems less important than other selectins because the blockade of E-selectin has no effect on neutrophil recruitment to the brain parenchyma, whereas the administration of P-selectin blocking monoclonal antibody inhibited neutrophil recruitment by 85% compared with controls98.
Integrins and the immunoglobulin superfamily
The firm adhesion of leukocytes to the endothelium after rolling requires the activation and binding of leukocyte-expressed integrins to endothelial adhesion molecules99. Integrins are heterodimers consisting of a common β subunit and a variable α subunit100. The major integrins expressed on neutrophils are the β2 integrins LFA-1 (αLβ2, CD11a/CD18) and Mac-1 (αMβ2, CD11b/CD18). Monocytes adhere through the β1 integrins VLA-4 (α4β1, CD49d/CD29). To form a firm adhesion, integrins must bind to counter-receptors of the immunoglobulin superfamily expressed on inflamed endothelial cells, including ICAM-1, ICAM-2, VCAM-1, the mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), platelet-endothelial cell adhesion molecule-1 (PECAM-1), and the receptor for advanced glycation end products (RAGE)101,102,103. Although no age-related differences in basal and stimulated LFA-1 surface expression were found in human neonatal and adult neutrophils104,105,106,107,108, Mac-1 expression remains low during the prenatal and postnatal periods and reaches adult levels by 11 months108,109. The lower surface expression of Mac-1 on neonatal neutrophils has been directly linked to impaired transendothelial migration under chemotactic stimulation75,110 (Table 1).
Thus far, no data are available in neonates regarding the roles of integrins and the immunoglobulin superfamily in HIE. Experimental studies with adult stroke models have shown that blockade of LFA-1/Mac-1111,112,113,114,115 and ICAM-1116,117 had beneficial effects on stroke outcomes. However, clinical trials of stroke patients given humanized antibodies against these adhesion molecules showed no effect118,119 or a worse outcome120. There are several reasons (see review121) for the failure of antibodies against these adhesion molecules to translate into a clinically relevant treatment strategy. For example, the study designs in the clinical trials did not mirror the laboratory models (such as late treatment or the absence of documented recanalization to the occluded vessel). Another possibility is that changes in neutrophil integrins are different between humans and rodents. Indeed, recent work has highlighted the differences in the immune system between species122. These differences emphasize the importance of clinical biomarkers and early phase studies to confirm the targets in both adult stroke and neonatal HIE, particularly using accessible sources such as peripheral blood. Although intervention strategies targeting adhesion molecules appeared to be effective in preclinical studies, moving this work into humans remains a tremendous challenge. It is encouraging that natalizumab, a humanized monoclonal antibody against α4-integrin, has been used to treat multiple sclerosis for more than 5 years123 and has been reported to decrease the risk of disability progression by 42% to 54% and to reduce the annualized rate of relapse by 68%124. Natalizumab treatment is associated with a risk of progressive multifocal leukoencephalopathy (PML), an opportunistic brain infection caused by the JC virus125. However, because its clinical benefits outweigh the risks involved, natalizumab remains on the market in the US under a special prescription program using risk stratification algorithms and PML management strategies123.
Cytokines
Cytokines are important inflammatory mediators, and cerebral ischemic injury can trigger a cascade of cytokine induction that acts to orchestrate an in situ inflammatory reaction133 and maintains brain tissue homeostasis134. In general, the roles of cytokines are pleiotropic, and whether the overall effects are pro- or anti-inflammatory in the context of ischemic insults remains controversial even in adult models, for which there are more data than for HIE. The most studied cytokines related to the inflammatory responses to stroke are IL-1, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β)135.
IL-1β and TNF-α are among the best-characterized early response cytokines and are often expressed concurrently136. Several types of CNS cells secrete IL-1β and TNF-α, including microglia, astrocytes, and neurons, and these cytokines share potent pro-inflammatory actions. Human newborns with HIE have higher levels of IL-1β and TNF-α in peripheral blood samples at P1, P3, and P7 compared to controls, and the IL-1β levels correlate positively with HIE severity137. The neurotoxic consequences of IL-1 activation have been shown in experimental studies with HIE138,139,140 and other inflammatory disease models141,142,143. The most convincing evidence that IL-1 is functionally detrimental in the pathogenesis of HIE is provided by the neuroprotective potential of IL-1 receptor antagonist administration in HIE models in rodents144,145 (Table 2).
Chemokines
Chemokines, or chemoattractant cytokines, also play a pivotal role in cerebral damage in ischemic stroke, HIE and excitotoxic brain injury models146. Chemokines are classified based on the positions of key cysteine residues (C): C, CC, CXC, and CX3C, and act through specific and shared receptors belonging to the superfamily of G-protein-coupled receptors147. As their name indicates, chemokines play a central role in leukocyte physiology by controlling inflammatory cell trafficking. HIE modeled in P7 rats induces the up-regulation of alpha-chemokines [growth related gene and macrophage inflammatory protein-2 (MIP-2)] and beta-chemokines (MIP-1α, MIP-1β, CCL-5) preceding the expression of markers for lymphocytes in the infarcted area35. In the neonatal brain, acute excitotoxic injury stimulates the expression of both monocyte chemotactic protein-1 [MCP-1, also called chemokine ligand 2 (CCL2)] and its receptor CCR2, suggesting that MCP-1 regulates the microglial/monocyte response to acute brain injury and contributes to the pathogenesis of acute neonatal brain injury148,149. This has been confirmed by another study using the same model in which anti-MCP-1 antibody attenuated tissue injury in neonatal rats150 (Table 2). Few data are available on the potential role of CXC chemokines in perinatal stroke. In experimental adult stroke models, stromal cell-derived factor 1 (SDF-1 or CXCL12) is expressed perivascularly in the injured region up to 30 d after the injury, suggesting that it could be a therapeutic target for tissue repair strategies151. However, in P7 mice, stroke induced up-regulation of CXCL12 was only observed up to 7 d after the injury but not at a later time point63, indicating a significantly smaller temporal window for CXCL12-mediated repair after a perinatal stroke.
Oxidative stress
Oxidative stress has recently been recognized as a common pathway in which different inflammatory cells mediate post-ischemic injury159,160. After ischemic insults, the inflammatory cells in the brain are activated and then generate ROS via several enzyme systems to induce the expression of pro-inflammatory mediators including cytokines and adhesion molecules160. Superoxide is generated via cyclo-oxygenase (COX), xanthine dehydrogenase, xanthine oxidase, and NADPH oxidase, whereas myeloperoxidase (MPO) and monoamine oxidase (MAO) generate hypochlorous acid and H2O2121. Compared to adult mice, P7 pups show the increased accumulation of H2O2 in the brain after a HI injury, suggesting that the neonatal brain may be more damaged even after a milder degree of acute hypoxic-ischemic injury161 (Table 3). Glutathione peroxidase (GPX) is a key enzyme responsible for the degradation of H2O2162. The neonatal brain has limited GPX activity and is more susceptible to oxidative damage, as described in a study showing that H2O2 rapidly accumulates in human-superoxide dismutase-1 (hSOD1) transgenic P7 mice, thus resulting in exacerbated HI brain injury, which is reversed in hGPX1-Tg mice163. However, the role of ROS in neonatal inflammatory responses following HIE is controversial. Inhibition of NADPH oxidase, the most important source of ROS164, increases HI injury and the level of IL-1β in P9 mice165. In contrast, it has been well established that NADPH oxidase can exacerbate inflammatory responses and stroke outcomes in adult animal models (see review166). Therefore, the results obtained in adult animals are not completely relevant to newborns and the role of oxidative stress in HIE remains to be fully investigated.
Fetal inflammatory response syndrome (FIRS)
Originally defined in fetuses who experienced preterm labor and preterm premature rupture of the membranes (PROM), FIRS is a unique condition characterized by the systemic activation of the fetal innate immune system and by an elevation in fetal plasma IL-6 concentrations171. Currently, FIRS is characterized by a rapid increase in pro-inflammatory signaling (cytokines, chemokines, etc) and the mobilization of immune effector cells into the fetal circulation172. These pro-inflammatory mediators readily cross the BBB and induce the activation of microglia, which initiates a detrimental cerebral inflammatory response. The unique circumstances of the “patient” (fetus) and the environment (uterus) in FIRS make it distinguishable from other diseases; however, by definition, FIRS and inflammatory responses after HIE partly overlap in pathophysiology, and they share similar inflammatory mechanisms in the brain. There are multiple putative mechanisms by which the neonatal brain can sense FIRS signals in the systemic circulation, which will then lead to neuroinflammation. These mechanisms include the interface of macrophages in the circumventricular brain area, without a BBB, with circulating inflammatory molecules173, and the direct access of FIRS signals into the CNS through leakage of the BBB in the setting of peripheral inflammatory pain signaling through the vagal nerve174. The manner in which FIRS influences the response to HIE and whether HIE can induce FIRS and subsequent peripheral immune activation is an area of active study.
Summary
HIE triggers a robust inflammatory response and accumulating data have linked post-ischemic inflammation to the exacerbation of brain damage. Many inflammatory mechanisms and pathways after cerebral ischemia have been assessed in various studies performed in adult subjects; however, caution should be exercised when attempting to extrapolate these findings to neonates. The mechanisms underlying cerebral ischemic injury and the following immune response are likely very different between the neonates and the adults.
References
Lai MC, Yang SN . Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol 2011; 2011: 609813.
Vannucci RC, Perlman JM . Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997; 100: 1004–14.
Gluckman PD, Williams CE . When and why do brain cells die? Dev Med Child Neurol 1992; 34: 1010–4.
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 1994; 36: 699–706.
Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN, Aldridge RF, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia-ischemia. Pediatr Res 1996; 40: 6–14.
Williams CE, Gunn A, Gluckman PD . Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 1991; 22: 516–21.
Inder TE, Volpe JJ . Mechanisms of perinatal brain injury. Semin Neonatol 2000; 5: 3–16.
Yenari MA, Kauppinen TM, Swanson RA . Microglial activation in stroke: therapeutic targets. Neurotherapeutics 2010; 7: 378–91.
Zheng Z, Yenari MA . Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res 2004; 26: 884–92.
Wang LW, Chang YC, Lin CY, Hong JS, Huang CC . Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatr Res 2010; 68: 41–7.
Perego C, Fumagalli S, De Simoni MG . Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation 2011; 8: 174.
Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD . Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab 2006; 26: 654–65.
Garcia JH, Liu KF, Yoshida Y, Chen S, Lian J . Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (Wistar rat). Am J Pathol 1994; 145: 728–40.
Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, et al. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem 2007; 100: 893–904.
Algra SO, Groeneveld KM, Schadenberg AW, Haas F, Evens FC, Meerding J, et al. Cerebral ischemia initiates an immediate innate immune response in neonates during cardiac surgery. J Neuroinflammation 2013; 10: 24.
Ferriero DM . Neonatal brain injury. N Engl J Med 2004; 351: 1985–95.
Stoll G, Jander S . The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol 1999; 58: 233–47.
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752–8.
Nimmerjahn A, Kirchhoff F, Helmchen F . Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308: 1314–8.
Iadecola C, Anrather J . The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808.
Varnum MM, Ikezu T . The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer's disease brain. Arch Immunol Ther Exp (Warsz) 2012; 60: 251–66.
Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, et al. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci 2008; 28: 11650–61.
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–70.
McMenamin PG . Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 1999; 405: 553–62.
Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 2007; 10: 1544–53.
Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 2001; 14: 1651–8.
Lassmann H, Schmied M, Vass K, Hickey WF . Bone marrow derived elements and resident microglia in brain inflammation. Glia 1993; 7: 19–24.
Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol 2008; 86: 398–408.
Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A 2012; 109: 18150–5.
Del Bigio MR, Becker LE . Microglial aggregation in the dentate gyrus: a marker of mild hypoxic-ischaemic brain insult in human infants. Neuropathol Appl Neurobiol 1994; 20: 144–51.
Jellema RK, Passos VL, Zwanenburg A, Ophelders DR, De Munter S, Vanderlocht J, et al. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxic-ischemia in preterm sheep. J Neuroinflammation 2013; 10: 13.
Cowell RM, Xu H, Galasso JM, Silverstein FS . Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke 2002; 33: 795–801.
McRae A, Gilland E, Bona E, Hagberg H . Microglia activation after neonatal hypoxic-ischemia. Brain Res Dev Brain Res 1995; 84: 245–52.
Ivacko JA, Sun R, Silverstein FS . Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatr Res 1996; 39: 39–47.
Bona E, Andersson AL, Blomgren K, Gilland E, Puka-Sundvall M, Gustafson K, et al. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatr Res 1999; 45: 500–9.
Kaur C, Rathnasamy G, Ling EA . Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J Neuroimmune Pharmacol 2013; 8: 66–78.
Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke 2000; 31: 1752–61.
Derugin N, Dingman A, Wendland MF, Fox C, Bollen A, Vexler ZS . Magnetic resonance imaging as a surrogate measure for histological sub-chronic endpoint in a neonatal rat stroke model. Brain Res 2005; 1066: 49–56.
Dommergues MA, Plaisant F, Verney C, Gressens P . Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience 2003; 121: 619–28.
Renolleau S, Benjelloun N, Ben-Ari Y, Charriaut-Marlangue C . Regulation of apoptosis-associated proteins in cell death following transient focal ischemia in rat pups. Apoptosis 1997; 2: 368–76.
Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 2005; 25: 1138–49.
Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G . Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des 2008; 14: 3574–89.
Swanson RA, Ying W, Kauppinen TM . Astrocyte influences on ischemic neuronal death. Curr Mol Med 2004; 4: 193–205.
Bambrick L, Kristian T, Fiskum G . Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res 2004; 29: 601–8.
Sims NR, Nilsson M, Muyderman H . Mitochondrial glutathione: a modulator of brain cell death. J Bioenerg Biomembr 2004; 36: 329–33.
Anderson CM, Swanson RA . Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 2000; 32: 1–14.
Romera C, Hurtado O, Botella SH, Lizasoain I, Cardenas A, Fernandez-Tome P, et al. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci 2004; 24: 1350–7.
Schousboe A, Waagepetersen HS . Glial modulation of GABAergic and glutamatergic neurotransmission. Curr Top Med Chem 2006; 6: 929–34.
Walz W . Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int 2000; 36: 291–300.
Stanimirovic DB, Ball R, Durkin JP . Glutamate uptake and Na,K-ATPase activity in rat astrocyte cultures exposed to ischemia. Acta Neurochir Suppl 1997; 70: 1–3.
Orzylowska O, Oderfeld-Nowak B, Zaremba M, Januszewski S, Mossakowski M . Prolonged and concomitant induction of astroglial immunoreactivity of interleukin-1beta and interleukin-6 in the rat hippocampus after transient global ischemia. Neurosci Lett 1999; 263: 72–6.
Lau LT, Yu AC . Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma 2001; 18: 351–9.
Stoll G, Jander S, Schroeter M . Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 1998; 56: 149–71.
Monje ML, Toda H, Palmer TD . Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003; 302: 1760–5.
Sofroniew MV . Astrocyte failure as a cause of CNS dysfunction. Mol Psychiatry 2000; 5: 230–2.
Kim JS . Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci 1996; 137: 69–78.
Roessmann U, Gambetti P . Pathological reaction of astrocytes in perinatal brain injury. Immunohistochemical study. Acta Neuropathol 1986; 70: 302–7.
Ikeda T, Murata Y, Quilligan EJ, Choi BH, Parer JT, Doi S, et al. Physiologic and histologic changes in near-term fetal lambs exposed to asphyxia by partial umbilical cord occlusion. Am J Obstet Gynecol 1998; 178: 24–32.
Petersson KH, Pinar H, Stopa EG, Faris RA, Sadowska GB, Hanumara RC, et al. White matter injury after cerebral ischemia in ovine fetuses. Pediatr Res 2002; 51: 768–76.
Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I . White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res 2003; 28: 215–23.
Inder T, Neil J, Kroenke C, Dieni S, Yoder B, Rees S . Investigation of cerebral development and injury in the prematurely born primate by magnetic resonance imaging and histopathology. Dev Neurosci 2005; 27: 100–11.
Gilles FH, Murphy SF . Perinatal telencephalic leucoencephalopathy. J Neurol Neurosurg Psychiatry 1969; 32: 404–13.
Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD, et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci 2005; 6: 63.
Schmid-Schonbein GW . Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc 1987; 46: 2397–401.
Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K . Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab 1995; 15: 941–7.
Kochanek PM, Hallenbeck JM . Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke 1992; 23: 1367–79.
Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 2005; 289: H558–68.
Hallenbeck JM . Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl 1996; 66: 27–31.
Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res 1991, 29: 336–45.
Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ . Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol 1994; 144: 188–99.
Matsuo Y, Onodera H, Shiga Y, Nakamura M, Ninomiya M, Kihara T, et al. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke 1994; 25: 1469–75.
Zhang RL, Chopp M, Chen H, Garcia JH . Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci 1994; 125: 3–10.
Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke 1986; 17: 246–53.
Anderson DC, Hughes BJ, Smith CW . Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest 1981; 68: 863–74.
Anderson DC, Rothlein R, Marlin SD, Krater SS, Smith CW . Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood 1990; 76: 2613–21.
Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J . The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res 1997; 41: 607–16.
Jiang N, Chopp M, Chahwala S . Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res 1998; 788: 25–34.
Jiang N, Moyle M, Soule HR, Rote WE, Chopp M . Neutrophil inhibitory factor is neuroprotective after focal ischemia in rats. Ann Neurol 1995; 38: 935–42.
Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke 1995; 26: 1438–42.
Palmer C, Roberts RL, Young PI . Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic-ischemic brain injury. Pediatr Res 2004; 55: 549–56.
Yilmaz G, Arumugam TV, Stokes KY, Granger DN . Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006; 113: 2105–12.
Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, et al. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab 2010; 30: 1306–17.
Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G . Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab 1995; 15: 42–51.
Stoll G, Jander S, Schroeter M . Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol 2002; 513: 87–113.
Catania A, Lipton JM . Peptide modulation of fever and inflammation within the brain. Ann N Y Acad Sci 1998; 856: 62–8.
Northington FJ, Ferriero DM, Flock DL, Martin LJ . Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci 2001; 21: 1931–8.
Benjelloun N, Renolleau S, Represa A, Ben-Ari Y, Charriaut-Marlangue C . Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal Rat. Stroke 1999; 30: 1916–23.
Wang J, Lu Q . Expression of T subsets and mIL-2R in peripheral blood of newborns with hypoxic ischemic encephalopathy. World J Pediatr 2008; 4: 140–4.
Becker KJ . Activation of immune responses to brain antigens after stroke. J Neurochem 2012; 123: 148–55.
del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ . Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 2000; 10: 95–112.
Connolly ES Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res 1997; 81: 304–10.
Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med 1995; 181: 669–75.
Grosshaupt B, Muntean W, Sedlmayr P . Hyporeactivity of neonatal platelets is not caused by preactivation during birth. Eur J Pediatr 1997; 156: 944–8.
Lorant DE, Li W, Tabatabaei N, Garver MK, Albertine KH . P-selectin expression by endothelial cells is decreased in neonatal rats and human premature infants. Blood 1999; 94: 600–9.
Rebuck N, Gibson A, Finn A . Neutrophil adhesion molecules in term and premature infants: normal or enhanced leucocyte integrins but defective L-selectin expression and shedding. Clin Exp Immunol 1995; 101: 183–9.
Nussbaum C, Sperandio M . Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol 2011; 90: 74–81.
Mariscalco MM, Vergara W, Mei J, Smith EO, Smith CW . Mechanisms of decreased leukocyte localization in the developing host. Am J Physiol Heart Circ Physiol 2002; 282: H636–44.
Bernardes-Silva M, Anthony DC, Issekutz AC, Perry VH . Recruitment of neutrophils across the blood-brain barrier: the role of E- and P-selectins. J Cereb Blood Flow Metab 2001; 21: 1115–24.
Zarbock A, Ley K . Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol 2008; 172: 1–7.
Albelda SM . Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol 1991; 4: 195–203.
Danton GH, Dietrich WD . Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 2003; 62: 127–36.
Frommhold D, Kamphues A, Hepper I, Pruenster M, Lukic IK, Socher I, et al. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood 2010; 116: 841–9.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S . Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–89.
Moriguchi N, Yamamoto S, Isokawa S, Andou A, Miyata H . Granulocyte functions and changes in ability with age in newborns; Report No 2: activation of granulocyte functions by cytokines. Pediatr Int 2006; 48: 22–8.
Strunk T, Temming P, Gembruch U, Reiss I, Bucsky P, Schultz C . Differential maturation of the innate immune response in human fetuses. Pediatr Res 2004, 56: 219–26.
Kim SK, Keeney SE, Alpard SK, Schmalstieg FC . Comparison of L-selectin and CD11b on neutrophils of adults and neonates during the first month of life. Pediatr Res 2003; 53: 132–6.
Bikoue A, D'Ercole C, George F, Dameche L, Mutin M, Sampol J . Quantitative analysis of leukocyte membrane antigen expression on human fetal and cord blood: normal values and changes during development. Clin Immunol Immunopathol 1997; 84: 56–64.
McEvoy LT, Zakem-Cloud H, Tosi MF . Total cell content of CR3 (CD11b/CD18) and LFA-1 (CD11a/CD18) in neonatal neutrophils: relationship to gestational age. Blood 1996; 87: 3929–33.
Storm SW, Mariscalco MM, Tosi MF . Postnatal maturation of total cell content and up-regulated surface expression of Mac-1 (CD11b/CD18) in polymorphonuclear leukocytes of human infants. J Leukoc Biol 2008; 84: 477–9.
Anderson DC, Abbassi O, Kishimoto TK, Koenig JM, McIntire LV, Smith CW . Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. J Immunol 1991; 146: 3372–9.
Bowes MP, Rothlein R, Fagan SC, Zivin JA . Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology 1995; 45: 815–9.
Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, et al. Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol 1994; 35: 458–63.
Clark WM, Madden KP, Rothlein R, Zivin JA . Reduction of central nervous system ischemic injury in rabbits using leukocyte adhesion antibody treatment. Stroke 1991; 22: 877–83.
Garcia JH, Liu KF, Bree MP . Effects of CD11b/18 monoclonal antibody on rats with permanent middle cerebral artery occlusion. Am J Pathol 1996; 148: 241–8.
Bednar MM, Wright SD, Raymond-Russell SJ, Kohut JJ, Gross CE . IB4, a monoclonal antibody against the CD18 leukocyte adhesion protein, reduces intracranial pressure following thromboembolic stroke in the rabbit. Neurol Res 1996; 18: 171–5.
Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab 1998; 18: 1336–45.
Connolly ES Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, et al. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 1996; 97: 209–16.
Becker KJ . Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin 2002; 18: s18–22.
Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA . Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke 2003; 34: 2543–8.
Enlimomab A . Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57: 1428–34.
Vexler ZS, Tang XN, Yenari MA . Inflammation in adult and neonatal stroke. Clin Neurosci Res 2006; 6: 293–313.
Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110: 3507–12.
Rudick R, Polman C, Clifford D, Miller D, Steinman L . Natalizumab: bench to bedside and beyond. JAMA Neurol 2013; 70: 172–82.
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910.
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870–80.
Buhrer C, Graulich J, Stibenz D, Dudenhausen JW, Obladen M . L-selectin is down-regulated in umbilical cord blood granulocytes and monocytes of newborn infants with acute bacterial infection. Pediatr Res 1994; 36: 799–804.
Torok C, Lundahl J, Hed J, Lagercrantz H . Diversity in regulation of adhesion molecules (Mac-1 and L-selectin) in monocytes and neutrophils from neonates and adults. Arch Dis Child 1993; 68: 561–5.
Hailer NP, Jarhult JD, Nitsch R . Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia 1996; 18: 319–31.
Miao H, Jiang L, Huang L . Effects of simvastatin on the expression of intercellular adhesion molecule-1 mRNA in neonatal brain with hypoxic-ischemic damage. J Nanosci Nanotechnol 2005; 5: 1261–5.
Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA . TNF-alpha-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol 2003; 171: 4700–7.
Reiss Y, Hoch G, Deutsch U, Engelhardt B . T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur J Immunol 1998; 28: 3086–99.
Nadeau S, Baribeau J, Janvier A, Perreault T . Changes in expression of vascular endothelial growth factor and its receptors in neonatal hypoxia-induced pulmonary hypertension. Pediatr Res 2005; 58: 199–205.
Saliba E, Henrot A . Inflammatory mediators and neonatal brain damage. Biol Neonate 2001; 79: 224–7.
Hopkins SJ . The pathophysiological role of cytokines. Leg Med (Tokyo) 2003; 5: S45–57.
Han HS, Yenari MA . Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs 2003; 4: 522–9.
Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J . Cytokines and perinatal brain injury. Neurochem Int 1997; 30: 375–83.
Liu J, Feng ZC . Increased umbilical cord plasma interleukin-1 beta levels was correlated with adverse outcomes of neonatal hypoxic-ischemic encephalopathy. J Trop Pediatr 2010; 56: 178–82.
Girard S, Sebire H, Brochu ME, Briota S, Sarret P, Sebire G . Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries. Brain Behav Immun 2012; 26: 1331–9.
Girard S, Kadhim H, Larouche A, Roy M, Gobeil F, Sebire G . Pro-inflammatory disequilibrium of the IL-1 beta/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine 2008; 43: 54–62.
Cai Z, Lin S, Pang Y, Rhodes PG . Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res 2004; 56: 377–84.
Green HF, Treacy E, Keohane AK, Sullivan AM, O'Keeffe GW, Nolan YM . A role for interleukin-1beta in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci 2012; 49: 311–21.
Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol 2011; 70: 550–65.
Crampton SJ, Collins LM, Toulouse A, Nolan YM, O'Keeffe GW . Exposure of foetal neural progenitor cells to IL-1beta impairs their proliferation and alters their differentiation — a role for maternal inflammation? J Neurochem 2011; 120: 964–73.
Hagberg H, Gilland E, Bona E, Hanson LA, Hahin-Zoric M, Blennow M, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr Res 1996; 40: 603–9.
Martin D, Chinookoswong N, Miller G . The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol 1994; 130: 362–7.
Mirabelli-Badenier M, Braunersreuther V, Viviani GL, Dallegri F, Quercioli A, Veneselli E, et al. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost 2011; 105: 409–20.
Baggiolini M . Chemokines in pathology and medicine. J Intern Med 2001; 250: 91–104.
Galasso JM, Miller MJ, Cowell RM, Harrison JK, Warren JS, Silverstein FS . Acute excitotoxic injury induces expression of monocyte chemoattractant protein-1 and its receptor, CCR2, in neonatal rat brain. Exp Neurol 2000; 165: 295–305.
Szaflarski J, Ivacko J, Liu XH, Warren JS, Silverstein FS . Excitotoxic injury induces monocyte chemoattractant protein-1 expression in neonatal rat brain. Brain Res Mol Brain Res 1998; 55: 306–14.
Galasso JM, Liu Y, Szaflarski J, Warren JS, Silverstein FS . Monocyte chemoattractant protein-1 is a mediator of acute excitotoxic injury in neonatal rat brain. Neuroscience 2000; 101: 737–44.
Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 2004; 63: 84–96.
Szaflarski J, Burtrum D, Silverstein FS . Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke 1995; 26: 1093–100.
Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H . Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci 2002; 22: 5910–9.
Hedtjarn M, Mallard C, Iwakura Y, Hagberg H . Combined deficiency of IL-1beta18, but not IL-1alphabeta, reduces susceptibility to hypoxia-ischemia in the immature brain. Dev Neurosci 2005; 27: 143–8.
Yururer D, Teber S, Deda G, Egin Y, Akar N . The relation between cytokines, soluble endothelial protein C receptor, and factor VIII levels in Turkish pediatric stroke patients. Clin Appl Thromb Hemost 2009; 15: 545–51.
Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P . Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol 2000; 47: 54–63.
Mesples B, Plaisant F, Gressens P . Effects of interleukin-10 on neonatal excitotoxic brain lesions in mice. Brain Res Dev Brain Res 2003; 141: 25–32.
Mann SA, Versmold B, Marx R, Stahlhofen S, Dietzel ID, Heumann R, Berger R . Corticosteroids reverse cytokine-induced block of survival and differentiation of oligodendrocyte progenitor cells from rats. J Neuroinflammation 2008; 5:39.
Pun PB, Lu J, Moochhala S . Involvement of ROS in BBB dysfunction. Free Radic Res 2009; 43: 348–64.
Jin R, Yang G, Li G . Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–89.
Lafemina MJ, Sheldon RA, Ferriero DM . Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res 2006; 59: 680–3.
Armogida M, Nistico R, Mercuri NB . Therapeutic potential of targeting hydrogen peroxide metabolism in the treatment of brain ischaemia. Br J Pharmacol 2012; 166: 1211–24.
Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Tauber MG, et al. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res 2004; 56: 656–62.
Brandes RP, Kreuzer J . Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 2005; 65: 16–27.
Doverhag C, Keller M, Karlsson A, Hedtjarn M, Nilsson U, Kapeller E, et al. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiol Dis 2008; 31: 133–44.
Tang XN, Cairns B, Kim JY, Yenari MA . NADPH oxidase in stroke and cerebrovascular disease. Neurol Res 2012; 34: 338–45.
Yang L, Sameshima H, Yamaguchi M, Ikenoue T . Expression of inducible nitric oxide synthase and cyclooxygenase-2 mRNA in brain damage induced by lipopolysaccharide and intermittent hypoxia-ischemia in neonatal rats. J Obstet Gynaecol Res 2005; 31: 185–91.
Higuchi Y, Hattori H, Kume T, Tsuji M, Akaike A, Furusho K . Increase in nitric oxide in the hypoxic-ischemic neonatal rat brain and suppression by 7-nitroindazole and aminoguanidine. Eur J Pharmacol 1998; 342: 47–9.
Tsuji M, Higuchi Y, Shiraishi K, Kume T, Akaike A, Hattori H . Protective effect of aminoguanidine on hypoxic-ischemic brain damage and temporal profile of brain nitric oxide in neonatal rat. Pediatr Res 2000; 47: 79–83.
Peeters-Scholte C, Koster J, Veldhuis W, van den Tweel E, Zhu C, Kops N, et al. Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia. Stroke 2002; 33: 2304–10.
Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM . The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998; 179: 194–202.
Kuypers E, Ophelders D, Jellema RK, Kunzmann S, Gavilanes AW, Kramer BW . White matter injury following fetal inflammatory response syndrome induced by chorioamnionitis and fetal sepsis: lessons from experimental ovine models. Early Hum Dev 2012; 88: 931–6.
Malaeb S, Dammann O . Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol 2009; 24: 1119–26.
Willis CL, Brooks TA, Davis TP . Chronic inflammatory pain and the neurovascular unit: a central role for glia in maintaining BBB integrity? Curr Pharm Des 2008; 14: 1625–43.
Acknowledgements
This work was supported by the NIH/NINDS (grants NS050505 and NS055215 to Louise D MCCULLOUGH), and by the American Heart Association (grant 12SDG9030000 to Fudong LIU)
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Liu, F., Mccullough, L. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin 34, 1121–1130 (2013). https://doi.org/10.1038/aps.2013.89
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.89
Keywords
This article is cited by
-
Gastrodin Regulates PI3K/AKT-Sirt3 Signaling Pathway and Proinflammatory Mediators in Activated Microglia
Molecular Neurobiology (2024)
-
LOX-1 mediates inflammatory activation of microglial cells through the p38-MAPK/NF-κB pathways under hypoxic-ischemic conditions
Cell Communication and Signaling (2023)
-
Early evolution of glial morphology and inflammatory cytokines following hypoxic-ischemic injury in the newborn piglet brain
Scientific Reports (2023)
-
Glycyrrhizic Acid Reverses Neurological Deficits by Attenuating Rac1-STAT3 Signalling-Mediated Neuroinflammation in a Mouse Model of Neonatal Hypoxic-Ischemic Encephalopathy
Revista Brasileira de Farmacognosia (2023)
-
Brain organoids for hypoxic-ischemic studies: from bench to bedside
Cellular and Molecular Life Sciences (2023)