Abstract
Aim:
To examine the effects of β3-adrenoceptor (β3-AR) activation on atherosclerotic plaque development in ApoE−/− mice.
Methods:
Thirty six week-old male ApoE−/− mice on a high-fat diet were treated with atorvastatin (10 mg·kg-1·d-1, po), BRL37344 (β3-AR agonist, 1.65 or 3.30 μg/kg, ip, twice a week) or SR52390A (β3-AR antagonist, 50 μg/kg, ip, twice a week) for 12 weeks. Wild-type C57BL/6J mice receiving a normal diet were taken as healthy controls. At the end of the treatments, serum levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), non-high density lipoprotein cholesterol (nHDL-C), glucose and insulin were measured. The thoracic aortas were dissected out, the area of atherosclerotic plaques and extent of fibrosis in the plaques were examined using HE and Masson's trichome staining, respectively.
Results:
Compared to wild-type mice, ApoE−/− mice fed on a high-fat diet exhibited prominent hyperlipidemia and insulin resistance, associated with large area of atherosclerotic plaques and great extent of fibrosis in aortas. Atorvastatin significantly decreased the serum levels of TC and nHDL-C, and reduced the plaque area and collagen content in aortas. BRL37344 significantly decreased the serum levels of TG, TC, nHDL-C, glucose and insulin, and increased HDL-C and the insulin sensitivity, and dose-dependently reduced the plaque area and collagen content in aortas. SR52390A treatment did not affect any parameters studied.
Conclusion:
The β3-AR agonist impedes the progression of atherosclerosis in ApoE−/− mice, through improvement of the lipid and glucose profiles.
Similar content being viewed by others
Introduction
β receptors were classified as β1- and β2-adrenoceptors (β-ARs) until the early 1980s, when a third member of the β-ARs was cloned from adipose tissue1. β3-AR activation leads to lipolysis in white adipose tissue and thermogenesis in brown adipose tissue2. β3-AR is a G protein-coupled receptor that activates adenylyl cyclase, cAMP/protein kinase A (PKA) and the PI3-kinase pathway3.
In the ob/ob mouse model for obesity, β3-AR expression and function in white and brown adipose tissues are markedly reduced4. β3-AR agonists could improve glycemic control and insulin sensitivity in addition to reducing plasma triglycerides (TG), free fatty acids and body weight in obese diabetic animals5,6,7.
Abnormal lipid and glucose metabolism plays a major role in the pathogenesis of atherosclerosis8. Because activation of β3-AR improves lipid and glucose metabolism in obese and diabetic rodent models5,6,7, we propose that chronic β3-AR activation could attenuate atherosclerosis. The current study examined the potential effects of a representative β3-AR agonist and antagonist on the formation and fibrosis of atherosclerotic plaques in ApoE-deficient (ApoE−/−) mice. The concentrations of plasma lipids and glucose were also monitored in addition to insulin sensitivity.
Materials and methods
Reagents
BRL37344 (sodium-4-[-2[-2-hydroxy-2-(-3-chloro-phenyl) ethylamine]propyl] phenoxyacetate) and SR52390A (3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate) were purchased from Sigma (St Louis, MO, USA). Atorvastatin was obtained from Pfizer (Dalian, China). An ultra-sensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit was purchased from Crystal Chem (Downers Grove, PA, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). The reverse transcription-polymerase chain reaction (RT-PCR) kit (A3500) was obtained from Promega (Madison, WI, USA). All primers were synthesized by Invitrogen (Shanghai, China). The SYBR Green real-time PCR kit was purchased from Katara Bio (Shiga, Nagano, Japan).
Mice
Homozygous ApoE−/−9 and C57BL/6J mice were provided by Beijing Vital River Laboratory Animal Technology (Certificate No: SCXK 2006-0008). The mice were housed in a specific pathogen-free facility under a 12/12 h light-dark cycle at 23 °C and were used after at least one week of adaptation. ApoE−/− mice were given a high-fat diet containing 0.15% (w/w) cholesterol and 21% (w/w) fat starting from 10 weeks of age. C57BL/6J mice received normal chow throughout the experiment. All mice had unrestricted access to tap water. Animal care and use were carried out in accordance with the Chinese standards (GB14925-2001).
Experimental protocols
Starting from 36 weeks of age, ApoE−/− mice randomly received atorvastatin (10 mg/kg by daily gavage), the β3-AR agonist BRL37344 [1.65 or 3.30 μg/kg by intraperitoneal (ip)injection, twice a week], the β3-AR antagonist SR52390A (50 μg/kg by ip injection, twice a week), or a saline vehicle of the same volume (ip injection, twice a week) for 12 weeks (n=10 per treatment condition). Wild-type (WT) C57BL/6J mice that received a normal diet and were treated with vehicle were included as healthy controls (n=10). Body weight was monitored at 10, 20, 30, 36, 42 and 48 weeks of age.
Serum lipids, glucose and insulin
Blood samples were collected 6 h after fasting using a retro-orbital approach. Serum glucose, TG, total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were examined with a CX7 analyzer (Beckman Coulter, Fullerton, CA, USA). Non-high density lipoprotein cholesterol (nHDL-C) was determined using Friedewald's formula10,11. The serum insulin concentration was determined by ELISA.
Morphometric analysis of atherosclerotic plaques
A section of the thoracic aorta between the distal end of the aortic arch and the diaphragm was dissected after anesthetization with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). Tissue samples were immersed in 10% formaldehyde, dehydrated and embedded in optimum cutting temperature compound (OCT). Cross-sections (6 μm) were prepared every 100 μm. Eight sections were prepared from each animal subject for hematoxylin and eosin (H&E) or Masson's trichrome staining12. Images were captured using a light microscope (DP 70, Olympus, Tokyo, Japan) and analyzed using an image analysis program (Image-Pro Plus 3.0) by an observer who was blinded to the treatment condition. The assessment of the lumen and lesion areas was performed using a method that was described by Paigen and colleagues13. The extent of the fibrosis is presented as the percent area stained divided by the total plaque area.
Transmission electron microscopy
The midsection of the thoracic aorta was fixed in 2.5% glutaraldehyde followed by osmium tetroxide treatment, dehydrated in a graded ethanol series and embedded for cross-sectioning to identify monocytes that adhered to the endothelial wall. Smooth muscle cells (SMCs) and elastic fiber layers were observed with a transmission electron microscope. Two animals from each group were examined (12 tissue samples×5 sections for a total of 60 sections).
Quantitative real-time PCR
RNA extraction, reverse transcription-PCR and quantitative real-time PCR of β3-AR mRNA in epididymal white adipose tissue were performed as previously described14 using a SYBR Green real-time PCR kit. The PCR primer sequences were as follows: forward 5′-TCGACATGTTCCTCCACAAA-3′ and reverse 5′-GATGGTCCAAGATGGTGCTT-3′ (NCBI reference sequence: NM_013462.3). The reaction was performed as follows: 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. GAPDH mRNA was used as a control (NCBI reference sequence: NM_008084.2) and amplified with the following primers: forward 5′-ACCCAGAAGACTGTGGATGG-3′ and reverse 5′-ACATTGGGGGTAGGAACAC-3′. The results were normalized against GAPDH and expressed as fold changes using the 2–ΔΔCT method15. The experiment was performed in triplicate.
Body fat
Whole body fat was measured in conscious mice using a quantitative magnetic resonance method (Echo MRI 3-in-1; Echo Medical Systems, Houston, TX, USA)16.
Statistical analysis
The results are expressed as the mean±SD. The data were evaluated by one-way analysis of variance (ANOVA) followed by the Bonferroni t-test (equal variances) or Dunn's multiple comparison test (unequal variances) for pair-wise comparisons. The statistical significance was set at P<0.05.
Results
Body weight and fat
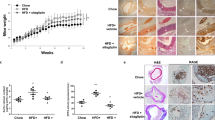
Across the treatment conditions, the mice had similar body weights prior to the experiment at 10 weeks of age. Immediately prior to the drug treatment (36 weeks of age), ApoE−/− mice receiving vehicle were significantly heavier than the WT control mice (P=0.038). At the completion of the experiment (48 weeks of age), ApoE−/− mice receiving vehicle were significantly lighter than the WT controls (P=0.01) (Figure 1A). Atorvastatin treatment increased the body weight (P=0.032 vs ApoE−/− vehicle control at 48 weeks of age) (Figure 1B), whereas the β3-AR agonist decreased the body weight (P<0.001 vs ApoE−/− vehicle control at 42 weeks of age). However, at a later time point (48 weeks), the weights of ApoE−/− mice receiving BRL37344 were similar to those of the ApoE−/− control mice. The β3-AR antagonist increased the body weight of mice at 42 and 48 weeks of age (P<0.01 vs ApoE−/− vehicle control).
Body weight and fat mass at different ages. (A) Body weights in the ApoE−/− control and WT groups were measured from 10 to 48 weeks of age. (B) The change in body weight with different interventions from 36 to 48 weeks of age (n=10 per group). (C) The fat mass was measured in ApoE−/− and WT mice at 36, 42, and 48 weeks of age (n=6 per group). Mean±SD. bP<0.05 compared with WT mice. eP<0.05 compared with ApoE− /− control mice. hP<0.05 compared with the atorvastatin treatment group.
ApoE−/− mice initially had a higher body fat content (P<0.001 vs WT control at 36 weeks of age). During the next 12 weeks, however, the fat mass of ApoE−/− mice gradually declined (Figure 1C). Atorvastatin did not affect the fat mass of ApoE−/− mice (P=0.019). The β3-AR agonist decreased the fat mass (P<0.01 vs ApoE−/− vehicle control), and the β3-AR antagonist increased the fat mass (P<0.01 vs the ApoE−/− vehicle control).
Lipid analysis
ApoE−/− mice developed severe hypercholesterolemia and hypertriglyceridemia, showing a 27.7-fold increase in the very low-density lipoprotein/low-density lipoprotein cholesterol (VLDL/LDL-C) ratio and a 62% reduction in the HDL-C/TC ratio compared with WT controls of the same age (Table 1). Atorvastatin reduced serum TC by 45.5% and the VLDL/LDL-C ratio by 46.9% but did not alter HDL cholesterol or TG. At 1.65 and 3.30 μg/kg, the β3-AR agonist decreased plasma TG by 41% and 51% (vs ApoE−/− mice receiving vehicle), TC by 18% and 23%, and VLDL/LDL-C by 26% and 34%, respectively; additionally, the plasma HDL-C was increased by 30% and 52%, and the HDL-C/TC ratio was increased by 9.7% and 115.7%, respectively. A posthoc analysis indicated that the higher dose of 3.30 μg/kg had a stronger effect (P<0.05 vs 1.65 μg/kg). The β3-AR antagonist did not affect serum lipids (P>0.05 for all) (Table 1).
Serum glucose and insulin
ApoE−/− mice given a high-fat diet displayed a significant increase in serum glucose and insulin. BRL37344 decreased serum glucose and insulin (P<0.01 vs ApoE−/− vehicle control) (Table 2). BRL37344 also increased the insulin sensitivity index (ISI=Ln[1/(Ins×Glu)]); however, the effects were not dose-dependent. At both doses, BRL37344 reduced glucose, insulin and the ISI to levels that were even lower than those achieved with atorvastatin (P<0.01). The β3-AR antagonist SR52390A did not affect serum glucose or insulin levels (P>0.05 vs ApoE−/− mice receiving vehicle).
Effects on atherosclerotic lesions
At 48 weeks of age, apparent atherosclerotic lesions were observed in the ApoE−/− mice but not in the WT controls (Figure 2A and 2B). Atorvastatin markedly decreased the atherosclerotic plaque area and increased the lumen area (P<0.01 vs ApoE−/− vehicle control) (Figure 2C). BRL37344 dose-dependently decreased the atherosclerotic area to a level comparable to that achieved with atorvastatin (Figure 2D and 2E). SR52390A treatment did not produce any effects (Figure 2F) (Table 3).
Morphology of the atherosclerotic lesions in WT and ApoE−/− mice. Cryosections of the thoracic aorta area were obtained from WT mice (A), ApoE−/− control mice (B), atorvastatin-treated mice (C), low-dose BRL37344-treated mice (D), high-dose BRL37344-treated mice (E) and SR52390A-treated mice (F) prior to staining with hematoxylin and eosin (H&E). Scale bars=200 μm.
The ApoE−/− control mice displayed prominent fibrous caps on their atherosclerotic lesions (Figure 3B). Fibrous caps were not apparent in mice receiving atorvastatin or BRL37344 at either dose (Figure 3C–3E; P<0.01 for all). SR52390A had no effect (Figure 3F) (Table 3).
Morphology of the fibrous plaques of WT mice and ApoE−/− mice. Cryosections of the thoracic aorta area were obtained from WT mice (A), ApoE−/− control mice (B), atorvastatin-treated mice (C), low-dose BRL37344-treated mice (E), high-dose BRL37344-treated mice (E) and SR52390A-treated mice (F) prior to staining with Masson's trichrome. The fibrous plaques with fibrous caps contained smooth muscle cells in B and F (red arrows). Scale bars=100 μm.
Transmission electron microscopy revealed normal SMCs in the WT control mice (Figure 4A). The ApoE−/− control mice had fewer swollen SMCs with rarefaction of the nuclear chromosome; obscured, swollen mitochondria; lack of cristae; and fiber hyperplasia (Figure 4B). Atorvastatin decreased these pathological changes (Figure 4C). In mice receiving BRL37344, there were fewer normal SMCs and a large number of swollen SMCs with mild fiber hyperplasia (regardless of dose) (Figure 4D and 4E). In mice receiving SR52390A, there were also fewer swollen SMCs (Figure 4F).
Transmission electron microscopy images of thoracic aortas obtained from each group. The morphology of SMCs (white arrow) was normal (without fiber hyperplasia) in the WT group (A); Fewer swollen SMCs were noted with obvious fiber hyperplasia (red arrow) in the control and SR52390A-treated groups (B and F); the morphology of thoracic aortas was essentially normal in the atorvastatin group (C); there were many swollen SMCs with mild fiber hyperplasia in mice treated with low and high doses of BRL37344 (D and E).
β3-adrenoceptor mRNA expression in white adipose tissue
β3-AR mRNA in epididymal white adipose tissue was decreased in ApoE−/− mice compared with WT controls (P<0.01) (Figure 5). BRL37344 increased β3-AR mRNA; however, the effects were not dose-dependent. Neither atorvastatin nor the β3-AR antagonist affected the β3-AR mRNA level.
Discussion
Atherosclerotic disease is the leading cause of morbidity and mortality in both developed and developing countries. It is associated with a metabolic and vascular cluster of diseases. Major risk factors for atherosclerosis include hyperlipidemia, glucose intolerance, hypertension, smoking and aging. Hyperlipidemia is defined as an increased flux of free fatty acids (FFA); increased TG, TC, and nHDL-C levels; and a reduced HDL-C level. Previous studies have shown that early events in atherogenesis include the accumulation of lipoprotein aggregates in the subendothelial space and vessel damage caused by apoB-containing low- and very-low-density lipoproteins17,18. Statins improve cholesterol metabolism by reducing nHDL-C and prevent the progression of atherosclerotic disease19. HDL removes excess cholesterol from cells, transporting it to the liver for excretion20. A low HDL-C level is commonly found in subjects with elevated serum TG and VLDL, a profile that is associated with a high risk of coronary heart disease (CHD)21.
Recent studies have shown that the β3-AR agonists can alleviate disorders of glucose and lipid metabolism, increase insulin sensitivity and minimize inflammation5,6. It is known that the metabolism of lipids and glucose, insulin resistance and inflammation play major roles in atherosclerotic progression. However, the effects of β3-AR on atherosclerotic disease are not known. In this study, β3-AR agonists or antagonist was given to the old ApoE−/− mice on high-fat diet for 12 weeks and their effects on atherosclerotic development were observed22,23.
Compared with WT control mice, ApoE−/− mice were significantly heavier before BRL37344 treatment, and BRL37344 treatment decreased body weight. We speculated that ApoE deficiency prevented the development of obesity, with decreased fat accumulation in the liver and adipose tissues. These results are consistent with the reports by Kypreos et al24. Atorvastatin did not reduce body weight or fat mass in ApoE−/− mice, suggesting that atorvastatin indirectly affected food intake and fat synthesis. The β3-AR agonist BRL37344 reduced body weight and fat mass in ApoE−/− mice. In contrast, the β3-AR antagonist SR52390A increased body weight and fat mass. These findings are consistent with the hypothesis that β3-AR activation could reduce food intake and accelerate lipolysis in ApoE−/− mice on a high-fat diet7,25.
The aged ApoE−/− mice developed prominent dyslipidemia (eg, high serum TG, TC and nHDL-C levels and a low HDL-C/TC ratio) as well as atherosclerotic lesions when given a high-fat diet. These results are consistent with reports by Kawashima et al16 and Zadelaar et al26. The β3-AR agonist reduced the levels of TG, TC, and nHDL-C and markedly raised the HDL-C level and the HDL-C/TC ratio. Such findings are consistent with previous studies showing that chronic β3-AR activation could increase the oxidative capacity of adipocytes within white adipose tissue by upregulating the expression of uncoupling protein (UCP) 127,28,29,30. β3-AR activation could also reduce the levels of nHDL-C and chylomicron in addition to raising the HDL-C level via the secretion of hormone-sensitive lipase and adiponectin and the upregulation of adiponectin receptor expression6,31,32,33. In the present study, atorvastatin did not improve TG or HDL-C levels; however, it reduced TC and nHDL-C levels in ApoE−/− mice. Atorvastatin could inhibit the synthesis of cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) in the liver, and by doing so, it could regulate TG and HDL-C metabolism. The current study suggested that the β3-AR agonist is superior relative to atorvastatin for increasing HDL-C and HDL-C/TC while decreasing TG.
In our experiments, ApoE−/− mice on a high-fat diet displayed hyperglycemia, hyperinsulinemia and insulin resistance. In agreement with previous studies in obesity/diabetes animal models5,6,34, the stimulation of β3-AR improved glycemic control, as evidenced by reduced hyperinsulinemia and hyperglycemia and elevated ISI in ApoE−/− mice. Mechanistically, the β3-AR agonist could upregulate insulin receptors and glucose transporters in the peripheral tissues, promoting glucose uptake and insulin-independent utilization in adipose tissue and skeletal muscle and reduced glycogen synthesis in the liver35. β3-AR activation has been reported to enhance insulin action in the liver and adipose tissue by mediating the insulin-like growth factor I (IGF-I)/insulin receptor signaling pathways of skeletal muscle7. Although there was a slight decrease in the insulin level in our study, atorvastatin did not reduce the glucose level or the ISI. These results suggest that atorvastatin is not sufficient for glycemic control in ApoE−/− mice. Atorvastatin inhibits the synthesis of fats by inhibiting the expression of sterol regulatory element binding protein-1 (SREBP-1) in the liver; however, it has less influence on glucose metabolism36. Indeed, atorvastatin has been shown to decrease the expression of the glucose transporter GLUT-4 in adipocytes and impair glucose tolerance37.
Quantitative real-time PCR confirmed β3-AR mRNA expression in white adipose tissue; this expression was downregulated in the ApoE−/− control mice and by SR52390A treatment. The β3-AR mRNA level was increased by BRL37344 and atorvastatin. These results are consistent with previous studies on obese/diabetic mice4,6,38. The basis of these findings could be the inhibitory effects of insulin on β3-AR expression39. Previous studies have shown that the β3-AR agonist CL-316,234 increases β3-AR mRNA levels and improves glucose metabolism. Similar results were obtained in this study with ApoE−/− mice. Upregulation of β-AR may further increase the effect of the β3-AR agonist on plasma lipids, glucose and anti-insulin resistance6. Neither atorvastatin nor SR52390A increased the β3-AR mRNA level in the white adipose tissue of ApoE−/− mice. These results could partially explain the failure of both drugs to improve glycemic control.
Our study demonstrated that the β3-AR agonist could reduce the atherosclerotic plaque area and collagen content and increase the lumen area in the thoracic aorta of ApoE−/− mice. The observed changes in HDL-C could activate the liver orphan receptor (LXR), accelerate reverse cholesterol transport (RCT), and prevent the progression of atherosclerosis40. Additionally, β3-AR agonists can increase the protein levels of adiponectin and adiponectin receptors, which contribute to increases in the expression of apolipoprotein A-1 (apoA-I) and ATP-binding cassette transporter A1 (ABCA1) in liver tissue6,41. ApoA-1 and ABCA1 transport excess peripheral cholesterol to the liver, thus preventing cholesterol accumulation and atherosclerotic plaque formation42,43.
In conclusion, this study demonstrated that β3-AR activation could impede the progression of atherosclerosis, possibly through improving lipid and glucose metabolism. At a high dose, the β3-AR agonist BRL37344 was similar to atorvastatin with respect to its ability to reduce atherosclerosis.
Author contribution
Yan-fang LI designed the research, Zhao-hong WANG and Yan-qing GUO performed the research, Yan-qing GUO analyzed the data, and Zhao-hong WANG wrote the paper.
References
Tan S, Curtis-Prior PB . Characterization of the beta-adrenoceptor of the adipose cell of the rat. Int J Obes 1983; 7: 409–14.
Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, et al. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem 1997; 272: 17686–93.
Chernogubova E, Cannon B, Bengtsson T . Norepinephrine increases glucose transport in brown adipocytes via beta3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology 2004; 145: 269–80.
Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW . Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol Endocrinol 1994; 8: 518–27.
Kato H, Ohue M, Kato K, Nomura A, Toyosawa K, Furutani Y, et al. Mechanism of amelioration of insulin resistance by beta3-adrenoceptor agonist AJ-9677 in the KK-Ay/Ta diabetic obese mouse model. Diabetes 2001; 50: 113–22.
Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y . The effects of beta(3)-adrenoceptor agonist CL-316,243 on adiponectin, adiponectin receptors and tumor necrosis factor–alpha expressions in adipose tissues of obese diabetic KKAy mice. Eur J Pharmacol 2008; 584: 202–6.
Kim H, Pennisi PA, Gavrilova O, Pack S, Jou W, Setser-Portas J, et al. Effect of adipocyte beta3-adrenergic receptor activation on the type 2 diabetic MKR mice. Am J Physiol Endocrinol Metab 2006; 290: E1227–36.
Corella D, Ordovas JM . The metabolic syndrome: a crossroad for genotype-phenotype associations in atherosclerosis. Curr Atheroscler Rep 2004; 6: 186–96.
Zhang SH, Reddick RL, Piedrahita JA, Maeda N . Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992; 258: 468–71.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Johnson J, Carson K, Williams H, Karanam S, Newby A, Angelini G, et al. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation 2005; 111: 1422–30.
Gervais M, Pons S, Nicoletti A, Cosson C, Giudicelli JF, Richer C . Fluvastatin prevents renal dysfunction and vascular NO deficit in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2003; 23: 183–9.
Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA . Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 1987; 68: 231–40.
Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y . Beta-adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-alpha expression in adipocytes. Eur J Pharmacol 2007; 569: 155–62.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–8.
Kawashima Y, Chen J, Sun H, Lann D, Hajjar RJ, Yakar S, et al. Apolipoprotein E deficiency abrogates insulin resistance in a mouse model of type 2 diabetes mellitus. Diabetologia 2009; 52: 1434–41.
Tamminen M, Mottino G, Qiao JH, Breslow JL, Frank JS . Ultrastructure of early lipid accumulation in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 1999; 19: 847–53.
Tanaka A . Postprandial hyperlipidemia and atherosclerosis. J Atheroscler Thromb 2004; 11: 322–9.
Carneiro AV, Costa J, Borges M . Statins for primary and secondary prevention of coronary heart disease. A scientific review. Rev Port Cardiol 2004; 23: 95–122.
Brewer HB Jr, Remaley AT, Neufeld EB, Basso F, Joyce C . Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol 2004; 24: 1755–60.
Assmann G, Schulte H, von Eckardstein A, Huang Y . High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996; 124: S11–20.
Quinn R . Comparing rat's to human's age: how old is my rat in people years? Nutrition 2005; 21: 775–7.
Jawień J, Nastałek P, Korbut R . Mouse models of experimental atherosclerosis. J Physiol Pharmacol 2004; 55: 503–17.
Kypreos KE, Karagiannides I, Fotiadou EH, Karavia EA, Brinkmeier MS, Giakoumi SM, et al. Mechanisms of obesity and related pathologies: role of apolipoprotein E in the development of obesity. FEBS J 2009; 276: 5720–8.
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L . Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002; 51: 271–5.
Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 2007; 27: 1706–21.
Margareto J, Larrarte E, Marti A, Martinez JA . Up-regulation of a thermogenesis-related gene (UCP1) and down-regulation of PPARgamma and aP2 genes in adipose tissue: possible features of the antiobesity effects of a beta3-adrenergic agonist. Biochem Pharmacol 2001; 61: 1471–8.
Tchivileva IE, Tan KS, Gambarian M, Nackley AG, Medvedev AV, Romanov S, et al. Signaling pathways mediating beta3-adrenergic receptor-induced production of interleukin-6 in adipocytes. Mol Immunol 2009; 46: 2256–66.
Granneman JG, Burnazi M, Zhu Z, Schwamb LA . White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab 2003; 285: E1230–6.
Cao W, Medvedev AV, Daniel KW, Collins S . Beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem 2001; 276: 27077–82.
Mottillo EP, Shen XJ, Granneman JG . Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab 2007; 293: E1188–97.
Robidoux J, Kumar N, Daniel KW, Moukdar F, Cyr M, Medvedev AV, et al. Maximal beta3-adrenergic regulation of lipolysis involves Src and epidermal growth factor receptor-dependent ERK1/2 activation. J Biol Chem 2006; 281: 37794–802.
Kolovou GD, Anagnostopoulou KK, Cokkinos DV . Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad Med J 2005; 81: 358–66.
Oana F, Takeda H, Matsuzawa A, Akahane S, Isaji M, Akahane M . Adiponectin receptor 2 expression in liver and insulin resistance in db/db mice given a beta3-adrenoceptor agonist. Eur J Pharmacol 2005; 518: 71–6.
Coman OA, Păunescu H, Ghiţă I, Coman L, Bădărăru A, Fulga I . Beta 3 adrenergic receptors: molecular, histological, functional and pharmacological approaches. Rom J Morphol Embryol 2009; 50: 169–79.
Suzuki M, Kakuta H, Takahashi A, Shimano H, Tada-Iida K, Yokoo T, et al. Effects of atorvastatin on glucose metabolism and insulin resistance in KK/Ay mice. J Atheroscler Thromb 2005; 12: 77–84.
Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T . Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49: 1881–92.
Gettys TW, Watson PM, Seger L, Padgett M, Taylor IL . Adrenalectomy after weaning restores beta3-adrenergic receptor expression in white adipocytes from C57BL/6J-ob/ob mice. Endocrinology 1997; 138: 2697–704.
Fève B, Elhadri K, Quignard-Boulangé A, Pairault J . Transcriptional down-regulation by insulin of the beta 3-adrenergic receptor expression in 3T3-F442A adipocytes: a mechanism for repressing the cAMP signaling pathway. Proc Natl Acad Sci U S A 1994; 91: 5677–81.
Baranowski M . Biological role of liver X receptors. J Physiol Pharmacol 2008; 59: 31–55.
Oku H, Matsuura F, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, et al. Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett 2007; 581: 5029–33.
Wang H, Peng DQ . New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis 2011; 10: 176.
Capurso C, Capurso A . From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol 2012; 57: 91–7.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No 81270380).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Zh., Li, Yf. & Guo, Yq. β3-Adrenoceptor activation attenuates atherosclerotic plaque formation in ApoE−/− mice through lowering blood lipids and glucose. Acta Pharmacol Sin 34, 1156–1163 (2013). https://doi.org/10.1038/aps.2013.70
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.70
Keywords
This article is cited by
-
Brown adipose tissue and its therapeutic application
Science Bulletin (2016)