Abstract
Aim:
To investigate the effects of the PPAR-γ agonist rosiglitazone on acute lung injury induced by the herbicide paraquat (PQ) and the underlying mechanisms of action.
Methods:
Male Sprague-Dawley rats were injected with PQ (20 mg/kg, ip). Rosiglitazone (3 or 10 mg/kg, ip) was administered 1 h before PQ exposure. Peripheral blood was collected at 4, 8, 24 and 72 h after PQ exposure for measuring the levels of MDA, TNF-α and IL-1β, and the SOD activity. Lung tissues were collected at 72 h after PQ exposure to determine the wet-to-dry (W/D) ratios and lung injury scores, as well as the protein levels of NF-κBp65, PPAR-γ, Nrf2, IκBα and pIκBα.
Results:
At 72 h after PQ exposure, the untreated rats showed a 100% cumulative mortality, whereas no death was observed in rosiglitazone-pretreated rats. Moreover, rosiglitazone pretreatment dose-dependently attenuated PQ-induced lung edema and lung histopathological changes. The pretreatment significantly reduced the levels of TNF-α, IL-1β and MDA, increased SOD activity in the peripheral blood of PQ-treated rats. The pretreatment also efficiently activated PPAR-γ, induced Nrf2 expression and inhibited NF-κB activation in the lung tissues of PQ-treated rats. Furthermore, the pretreatment dose-dependently inhibited IκB-α degradation and phosphorylation, thus inhibiting NF-κB activation.
Conclusion:
Pretreatment with rosiglitazone protects rats against PQ-induced acute lung injury by activating PPAR-γ, inducing Nrf2 expression and inhibiting NF-κB activation.
Similar content being viewed by others
Introduction
Paraquat (PQ) is one of the most widely used herbicides in the world and is highly toxic to humans and animals1,2,3 The lung is a major target organ during PQ poisoning, which is characterized by edema, hemorrhage, interstitial inflammation, and bronchial epithelial cell proliferation4. Respiratory failure as a result of lung injury is the most common cause of death from PQ. Redox reactions and lipid peroxidation of cellular membranes are the main mechanism underlying the toxic effects of PQ5. One type of redox reaction, the inflammatory reaction, has been reported to be the main mechanism underlying PQ-induced acute lung injury6. Several drugs have been analyzed as potential treatments for PQ-induced lung toxicity, but a specific antidote has not yet been found1,7.
Rosiglitazone is a member of the thiazolidinedione (TDZ) family and is a ligand for peroxisome proliferator-activated receptor-γ (PPAR-γ). Rosiglitazone is used clinically for the treatment of type 2 diabetic patients due to its insulin-sensitizing effects. Recent studies have indicated that rosiglitazone inhibits hyperoxia-8, endotoxin-9, and carrageenan-induced10 acute lung injury. However, whether rosiglitazone can inhibit PQ-induced acute lung injury remains unknown. Recent studies have indicated that PPAR-γ agonists antagonize signal transducing kinases or transcriptional regulators, such as activator protein-1 (AP-1), nuclear factor of activated T cells, and NF-κB11,12. Moreover, accumulating evidence has indicated that PPAR-γ agonists induce antioxidant and defense genes encoding glutathione S-transferase-α (GST-α), heme oxygenase-1 (HO-1), and CD36 through Nrf2 regulation13,14,15. Whether rosiglitazone exerts its effects on PQ-induced acute lung injury by activating Nrf2 and suppressing NF-κB requires further elucidation. In the present study, we investigated whether rosiglitazone had protective effect on PQ-induced acute lung injury in a rodent model. In particular, we sought to further explore the underlying biological mechanisms. Our results may lead to the use of rosiglitazone as a treatment for PQ-induced acute lung injury.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 200–250 g were purchased from the Experimental Animal Center of China Medical University. Rats were housed in cages in a temperature- (20–25 °C) and humidity-controlled (40%–70%) environment with a daily light-dark cycle. The rats had access to food and water ad libitum. All animal experiments were conducted in accordance with the Institutional Animal Ethics Committee and Animal Care Guidelines of China Medical University governing the use of experimental animals.
Reagents
PQ and rosiglitazone were purchased from Sigma-Aldrich (St Louis, MO, USA). Rabbit anti-PPAR-γ, anti-p65, anti-IκBα, anti-pIκBα, and anti-β-actin monoclonal antibodies were purchased from Cell Signaling Technology Inc (Beverly, MA, USA). Rabbit anti-Nrf2 monoclonal antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). A mouse anti-TBP monoclonal antibody was purchased from Abcam (Cambridge, MA, USA). The ELISA kits for TNF-α and IL-1β were purchased from R&D, America. The superoxide dismutase (SOD) and malondialdehyde (MDA) determination kits were purchased from Jiancheng Bioengineering Institute of Nanjing (Nanjing, China). The purity of all of the chemical reagents was of analytical grade or higher.
Experimental protocols
After adapting to the environment, the rats were intraperitoneally administered PQ at doses of 10, 20, 30, 40, and 50 mg/kg. Cumulative PQ poisoning rates and mortalities were observed at different time points (4, 8, 24, 72, and 168 h) after PQ administration. A dose of 20 mg/kg was chosen after preliminary poisoning rate and mortality studies were conducted with different doses of PQ. In the following experiments, the rats were randomly distributed into four groups: a control group (n=10), a PQ-treated group (n=10), a rosiglitazone (3 mg/kg) + PQ-treated group (n=10), and a rosiglitazone (10 mg/kg)+PQ-treated group (n=10). Rosiglitazone was intraperitoneally administered at doses of 3 and 10 mg/kg. After 1 h, PQ was intraperitoneally administered at a dose of 20 mg/kg. The peripheral blood was then collected through the orbital veins at 4, 8, 24, and 72 h, and centrifuged at 4000 r/min for 15 min. The supernatants were collected to measure SOD activity, and MDA, TNF-α and IL-1β levels. Throughout the study period, each animal was observed carefully for clinical signs of PQ-related toxicity. The lungs of the rats were harvested 72 h after PQ administration to detect NF-κBp65, PPAR-γ and Nrf2 expression using Western blotting analyses and histological examinations. IκBα and pIκBα were also detected using Western blotting analyses.
Wet-to-dry (W/D) lung weight ratios
The rats were killed via exsanguination 72 h after PQ administration. The whole lungs were removed. Each lung was dried, weighed, and then placed in an oven at 80 °C for 48 h to obtain the “dry” weight. The ratio of the wet weight of the wet lung to the dry one of the lung was calculated to assess tissue edema16.
Histopathologic evaluations
The rats were killed 72 h after PQ administration. The lungs were then removed and stored in 4% paraformaldehyde for 48 h at 4 °C. Hematoxylin and eosin staining was carried out according to the regular staining method, and the slides were evaluated using a semiquantitative scoring method. Lung injury was graded in a blinded fashion from 0 (normal) to 4 (severe) for interstitial inflammation, neutrophil infiltration, congestion, and edema. The total lung injury score was calculated by adding up the individual scores from each category16.
MDA levels and SOD activity assays
MDA levels and SOD activity in the peripheral blood were spectrophotometrically assayed using assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Both detective procedures were performed according to the manufacturer's recommended instructions.
TNF-α and IL-1β ELISA assays
The levels of TNF-α and IL-1β in the peripheral blood were measured via ELISA assays using commercially available kits according to the manufacturer's recommended instructions. The levels of TNF-α and IL-1β in the samples were calculated based on a standard curve. The detection ranges of the TNF-α and IL-1β ELISA assays were 12.5–800 pg/mL and 31.25–2000 pg/mL, respectively. Samples that had a concentration that exceeded the limit of the standard curve were measured after dilution.
Immunohistochemistry
The lung tissues were fixed in 4% paraformaldehyde for 48 h at 4 °C and processed for paraffin embedding. Paraffin-embedded blocks were cut into 4 μm thick sections and mounted onto slides. The sections were pretreated at 60 °C for 1 h, then dewaxed in xylene, hydrated, and washed in 0.01 mol/L of citrate buffer. After inhibiting endogenous peroxidase using 3% H2O2 in methanol, the sections were incubated with anti-NF-κBp65, anti-PPAR-γ and anti-Nrf2 polyclonal antibodies overnight at 4 °C. The sections were then thoroughly washed with a phosphate-buffered saline (PBS) solution, after which point the corresponding secondary antibodies were applied and incubated at room temperature for 30 min. Reaction products were visualized following incubation with diaminobenzidine (DAB) and then counterstained with hematoxylin. Negative controls were generated by omitting the primary antibodies.
Western blotting
Proteins were extracted from the lungs using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology, China). The extracts were boiled for 5 min with loading buffer, and the proteins were then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels and transferred onto polyvinylidene difluoride sheets. The membranes were washed with PBST and 5% skim milk for 1 h at room temperature. Following three washes with PBST, the membranes were incubated with primary antibodies overnight at room temperature. After three additional washes, secondary antibodies were added and the membranes were incubated for 1 h at room temperature. After three final washes, the blots were developed using Beyo ECL Plus reagent (Beyotime Biotechnology, Haimen, China), exposed to film for 1 min, and placed in developing and fixing solutions for 1 min each.
Statistical analyses
The data are expressed as the mean±SD. Statistical analyses were carried out using SPSS 16.0. One-way ANOVA followed by the Student-Newman-Keuls test was used to compare the results that were obtained in the different treatment groups. Differences were considered to be statistically significant when P<0.05.
Results
Cumulative poisoning rates and mortality in rats following PQ exposure
As shown in Figures 1 and 2, the cumulative poisoning rate was low when rats were treated with PQ at a dose of 10 mg/kg. Although the cumulative poisoning rate was satisfactory, the cumulative mortality was too high when rats were treated with 30, 40, or 50 mg/kg of PQ. The cumulative poisoning rate was satisfactory and mortality was low when rats were treated with PQ at a dose of 20 mg/kg. Therefore, we selected 20 mg/kg as the final dosage and 72 h as the observation time. All of the rats in our study displayed the typical symptoms of ALI. No deaths were observed at this dosage and time.
Cumulative poisoning rate of rats exposed to paraquat (PQ) at different doses and different time points. Rats were intraperitoneally administered of PQ at the doses of 10, 20, 30, 40, and 50 mg/kg. Cumulative poisoning rate was observed at different time points (4, 8, 24, 72, and 168 h) after the administration of PQ.
Cumulative mortality of rats exposed to paraquat (PQ) at different doses and different time points. Rats were intraperitoneally administered PQ at the doses of 10, 20, 30, 40, and 50 mg/kg. Cumulative mortality was observed at different time points (4, 8, 24, 72, and 168 h) after the administration of PQ.
Effects of rosiglitazone on the clinical signs of PQ poisoning in rats
No deaths were observed for the duration of the experimental period, but clinical signs of toxicity, including polypnea, cyanosis, crouch, decreased activity, diarrhea and anorexia were observed in some of the rats that had been exposed to PQ. The rats from the groups that had been pretreated with rosiglitazone displayed few symptoms, which suggested that rosiglitazone treatment could attenuate PQ-induced symptoms.
Effects of rosiglitazone on W/D lung ratios in rats with PQ-induced acute lung injury
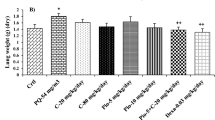
W/D lung ratios were evaluated 72 h after PQ administration. As shown in Figures 3A and 3B, lung edema and the W/D lung ratios in the PQ-treated group were significantly higher than those in the control group (P<0.05). Rosiglitazone treatment at 3 or 10 mg/kg decreased lung edema and the W/D lung ratios in rats with PQ-induced lung injury (P<0.05).
Effects of rosiglitazone on lung W/D ratio and histopathological changes in lung tissues of paraquat (PQ)-induced acute lung injury rats. Rosiglitazone was intraperitoneally administered 1 h before intraperitoneal administration of PQ. The lung W/D ratio (A, B) and lung histological evaluation (C, D, HE staining, 100×) was determined after PQ administration for 72 h. RSGL: rosiglitazone at the low dose of 3 mg/kg, RSGH: rosiglitazone at the high dose of 10 mg/kg. The values presented are the mean±SD (n=6). bP<0.05 vs PQ group.
Effects of rosiglitazone on histopathological changes in the lung tissues of rats with PQ-induced acute lung injury
The effects of rosiglitazone on histopathological changes in the lung tissue were determined by histochemical staining with H&E 72 h after PQ administration. As shown in Figures 3C and 3D, the lungs of rats that were exposed to PQ displayed significant inflammatory alterations that were characterized by lung edema, alveolar hemorrhage, inflammatory cell infiltration, and destruction of epithelial and endothelial cell structure. Treatment with rosiglitazone relieved many of the symptoms of PQ-induced acute lung injury that were outlined above.
Effects of rosiglitazone on MDA levels and SOD activity in the peripheral blood of rats with PQ-induced acute lung injury
MDA levels in the peripheral blood were significantly higher in the PQ group than those in the control group (P<0.05), as shown in Figure 4A. The increased levels were significantly inhibited by treatment with rosiglitazone (10 mg/kg) at 24 and 72 h after PQ administration (P<0.05). As shown in Figure 4B, PQ administration resulted in significant decreases in SOD activity in the peripheral blood (P<0.05). However, treatment with rosiglitazone counteracted these effects at 8, 24, and 72 h after PQ administration.
Effects of rosiglitazone on the cytokine production, SOD activity and MDA level, in peripheral blood of PQ-induced acute lung injury rats. Rosiglitazone was intraperitoneally administered 1 h before intraperitoneal administration of PQ. Peripheral blood was collected after PQ administration for 4, 8, 24, and 72 h to analyze MDA level and SOD activity (A, B), levels of IL-1β and TNF-α (C, D). RSGL: rosiglitazone at the dose of 3 mg/kg, RSGH: rosiglitazone at the dose of 10 mg/kg. The values presented are the mean±SD (n=6). bP<0.05 vs PQ group.
Effects of rosiglitazone on cytokine production in the peripheral blood of rats with PQ-induced acute lung injury
We used ELISAs to analyze the effects of rosiglitazone on the production of TNF-α and IL-1β in the peripheral blood at 4, 8, 24, and 72 h after PQ administration. As shown in Figure 4C and 4D, treatment with PQ alone caused significant increases in IL-1β and TNF-α compared to the control group (P<0.05). Rosiglitazone markedly reduced the production of TNF-α at 8, 24, and 72 h after PQ administration and markedly reduced the production of IL-1β at 24 and 72 h after PQ administration compared with the PQ-treated group (P<0.05).
Effects of rosiglitazone on PPAR-γ expression in the lung tissues of rats with PQ-induced acute lung injury
PPAR-γ localization in the lung tissues is illustrated in Figure 5C. In the control group, PPAR-γ positive signals were weakly observed in the lung tissues. At 72 h after PQ administration, PPAR-γ positive signals were further decreased in the lung tissues. Notably, rosiglitazone pretreatment significantly increased PPAR-γ positive signals in the lung tissues. The results of the Western blotting analyses are illustrated in Figure 5A. The PQ-treated group revealed decreased PPAR-γ expression as compared to the control group. Rosiglitazone pretreatment significantly increased PPAR-γ expression in the lung tissues (P<0.05).
Effects of rosiglitazone on Nrf2, PPAR-γ, and NF-κB signal transduction in lung tissues of paraquat (PQ)-induced acute lung injury rats. Rosiglitazone was intraperitoneally administered 1 h before intraperitoneal administration of PQ. Lung tissue was collected after PQ administration for 72 h, to determine the expression of Nrf2, PPAR-γ, and NF-κBp65 by immunohistochemistry (C, ×400) and Western blotting (A), IκB-α and pIκB-α by Western blotting (B). RSGL: rosiglitazone at the dose of 3 mg/kg, RSGH: rosiglitazone at the dose of 10 mg/kg. The values presented are the mean±SD (n=5). bP<0.05 vs PQ group.
Effects of rosiglitazone on Nrf2 expression in the lung tissues of rats with PQ-induced acute lung injury
Nrf2 localization in the lung tissues is illustrated in Figure 5C. In the control group, Nrf2 positive signals were weakly detected in the lung tissues. At 72 h after PQ administration, Nrf2 positive signals were observed to be subtly increased in the lung tissues. Notably, Nrf2 expression in the lung tissues was significantly increased in the groups that had been pretreated with rosiglitazone. The results of the Western blotting analyses are illustrated in Figure 5A. The PQ-treated group displayed increased Nrf2 expression compared to the control group. Rosiglitazone pretreatment further increased Nrf2 expression in the lung tissues (P<0.05).
Effects of rosiglitazone on NF-κB signal transduction in the lung tissues of rats with PQ-induced acute lung injury
As shown in Figure 5A and 5B, the PQ-treated group displayed significant IκBα degradation and phosphorylation compared to the control group. In contrast, IκBα degradation and phosphorylation in the rosiglitazone-pretreated groups were significantly reduced compared to the PQ-treated group (P<0.05). The nuclear extracts of PQ-treated rats displayed increases in the levels of the p65 subunit of NF-κB. In contrast, rosiglitazone treatment significantly decreased the levels of the p65 subunit of NF-κB in the nuclear extracts (P<0.05).
Discussion
The main target organ of PQ poisoning is the lungs. Edema is a representative symptom of PQ-induced acute lung injury. To quantify the magnitude of pulmonary edema, we detected the W/D ratios of sampled lung tissues. Our experiments suggested that treatment with rosiglitazone significantly inhibited lung edema, because the W/D ratio in the rosiglitazone-treated group was significantly lower than that in the PQ-treated group. We also observed PQ-induced pathological alterations, including alveolar edema, hemorrhage, inflammatory cell infiltration, and diffuse alveolar collapse that was accompanied by wall thickening. However, pretreatment with rosiglitazone was very effective at preventing PQ-induced lung tissue damage in rats.
Free radicals are known to play a crucial role in PQ-induced lung intoxication17. PQ-induced toxicity has been reported to be related to redox cycling of an iron-PQ complex, which in turn catalyzes the formation of reactive oxygen species (ROS) and ultimately progresses to lipid peroxidation18. In the present study, we found that MDA levels were significantly increased, while SOD activity was significantly decreased, in the peripheral blood of rats after PQ exposure. However, rosiglitazone treatment significantly increased SOD activity and decreased MDA levels in the peripheral blood.
Nrf2 is a transcription factor that induces antioxidant defenses and gene expression by binding to cis-acting antioxidant response elements (AREs)19 and is essential for tissue protection against various oxidants and xenobiotics20. The protective role of the Nrf2-ARE pathway has been demonstrated in oxidant-mediated inflammatory lung injury (for example, hyperoxia toxicity)21,22,23,24,25,26,27,28,29,30. In the present study, we demonstrated that PQ treatment alone induced the expression of Nrf2 in the lung tissues. In addition, we demonstrated that rosiglitazone could further increase Nrf2 expression. Therefore, the potent anti-oxidant effects of rosiglitazone may involve Nrf2 activation. Although the cellular targets and molecular mechanisms leading to rosiglitazone-induced Nrf2 activation remain to be elucidated, our study has shed some light on them.
Several important inflammatory and chemotactic cytokines, such as TNF-α and IL-1β, are involved in the inflammatory process during acute lung injury. These cytokines, as well as other pro-inflammatory compounds, initiate, amplify, and perpetuate the inflammatory response during acute lung injury. TNF-α is the primary endogenous mediator of the inflammatory process. TNF-α may play a role in the initiation or progression of multiple organ failure during endotoxic shock, and it has also been shown to be a particularly important mediator of acute lung injury31. IL-1β plays a key role in the development of acute lung injury, and can inhibit fluid transport across the distal lung epithelium32 causing surfactant abnormalities33 and increasing protein permeability across the alveolar-capillary barrier34. In this experiment, we found that the levels of TNF-α and IL-1β were significantly increased in the peripheral blood of rats after PQ exposure. However, when the rats were pretreated with rosiglitazone, the levels of TNF-α and IL-1β in the peripheral blood of PQ-poisoned rats were significantly decreased.
The NF-κB transcription factor plays a critical role in a number of different cellular processes. NF-κB is normally sequestered in the cytoplasm by a family of inhibitory proteins known as IκBs. A wide variety of stimuli, which have been extensively studied over the past two decades, can cause IκBα phosphorylation, a process that is followed by its ubiquitination and subsequent degradation. The loss of IκBα results in the release of the free NF-κB subunit p65, which translocates from the cytoplasm to the nucleus where p65 activates the expression of pro-inflammatory mediators (TNF-α, IL-1β, iNOS, COX2, etc)35. NF-κB plays an important role in lung injury in PQ-poisoned rats36. Our results demonstrated that PQ induced IκBα degradation and phosphorylation, which increased the levels of the p65 subunit of NF-κB in the nuclear extracts of lung tissues. In contrast, rosiglitazone treatment inhibited the degradation and phosphorylation of IκBα and decreased the levels of the p65 subunit of NF-κB in the nuclear extracts of the lung tissues. Therefore, the potent anti-inflammatory effects of rosiglitazone may be suggested to involve IκBα activation, which precipitates the translocation of the p65 subunit from the cytoplasm to the nucleus. However, the cellular targets and molecular mechanisms that lead to rosiglitazone-mediated IκBα activation remain to be elucidated. Li et al37 found that activation of Nrf2-antioxidant signaling attenuated the NF-κB inflammatory response. In our study, rosiglitazone activated Nrf2, which suggested that rosiglitazone may activate IκBα and attenuate the NF-κB-induced inflammatory response via activation of Nrf2. However, this hypothesis remains to be further elucidated.
PPAR-γ, a member of the nuclear receptor family that plays an important role in lipid and carbohydrate homeostasis, regulates the expression of several genes, including anti-inflammatory and antioxidant genes13,14,15. To further investigate the mechanisms underlying the effects of rosiglitazone on PQ-induced acute lung injury, we detected PPAR-γ expression in the lungs of PQ-poisoned rats using immunohistochemistry and Western blotting. The results of these analyses revealed that the PPAR-γ expression in PQ-poisoned rats was lower than that in control rats. However, rosiglitazone treatment activated PPAR-γ and increased its expression, which suggested that rosiglitazone may exert an inhibitory effect on NF-κB and an activating effect on Nrf2 via PPAR-γ activation. This hypothesis requires further verification. For example, Nrf2 and NF-κB expression may need to be detected after silencing or blocking PPAR-γ.
In summary, the results of the present study revealed that rosiglitazone had a protective effect against PQ-induced acute lung injury. Pretreatment with rosiglitazone attenuated pulmonary histological injuries and lung edema, decreased the levels of inflammatory cytokines in the peripheral blood, and reduced the concentrations of MDA and enhanced SOD activity in the serum. These data strongly suggested that rosiglitazone has potent anti-inflammatory and antioxidant activities and may offer a novel strategy for the modulation of PQ-induced acute lung injury. Furthermore, we found that rosiglitazone inhibited NF-κB activation by decreasing the phosphorylation and degradation of IκBα. Rosiglitazone also activated Nrf2 and PPAR-γ, which may partially explain its underlying mechanism of action. We hypothesize that rosiglitazone may activate PPAR-γ and subsequently induce Nrf2 expression and inhibit NF-κB activation. However, further in vitro investigation is required before conclusions can be drawn. More comprehensive studies are still required before this drug could be used clinically to treat PQ-induced acute lung injury.
Author contribution
Min ZHAO designed the research. Zhen-ning LIU conducted the research. Qiang ZHENG, Hong-yu ZHAO, Wei-jian HOU, and Shu-ling BAI provided helpful advice regarding the research.
References
Suntres ZE . Role of antioxidants in paraquat toxicity. Toxicology 2002; 180: 65–77.
Parvez S, Raisuddin S . Effects of paraquat on the freshwater fish Channa punctata: non-enzymatic antioxidants as biomarkers of exposure. Arch Environ Contam Toxicol 2006; 50: 392–7.
Neves FF, Sousa RB, Pazin-Filho A, Cupo P, Elias Júnior J, Nogueira-Barbosa MH . Severe paraquat poisoning: clinical and radiological findings in a survivor. J Bras Pneumol 2010; 36: 513–6.
Venkatesan N . Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci 2000; 66: 21–8.
Yasaka T, Okudaira K, Fujito H, Matsumoto J, Ohya I, Miyamoto Y . Further studies of lipid peroxidation in human paraquat poisoning. Arch Intern Med 1986; 146: 681–5.
Orito K, Suzuki Y, Matsuda H, Shirai M, Akahori F . Chymase is activated in the pulmonary inflammation and fibrosis induced by paraquat in hamsters. Tohoku J Exp Med 2004; 203: 287–94.
Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remiao F, Bastos ML, Carvalho F . Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008; 38: 13–71.
Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, et al. Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol 2010; 299: 672–80.
Liu D, Zeng BX, Zhang SH, Wang YL, Zeng L, Geng ZL, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Crit Care Med 2005; 33: 2309–16.
Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol 2004; 483: 79–93.
Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory esponse. J Clin Invest 1999; 104: 383–9.
Mao JW, Tang HY, Wang YD . Influence of rosiglitazone on the expression of PPARγ, nf-κb, and tnf-α in rat model of ulcerative colitis. Gastroenterol Res Pract 2012; 2012: 845672.
Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 2004; 94: 609–16.
Kim EH, Surh YJ . 15-deoxy-delta12, 14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors. Biochem Pharmacol 2006; 72: 1516–28.
Park EY, Cho IJ, Kim SG . Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid x receptor heterodimer. Cancer Res 2004; 64: 3701–7.
Yin H, Jin XB, Gong Q, Yang H, Hu YY, Gong FL, et al. Fructose-1,6-diphosphate attenuates acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol 2008; 8: 1842–7.
Kim JH, Gil HW, Yang JO, Lee EY, Hong SY . Effect of glutathione administration on serum levels of reactive oxygen metabolites in patients with paraquat intoxication: a pilot study. Korean J Intern Med 2010; 25: 282–7.
Gil HW, Oh MH, Woo KM, Lee EY, Oh MH, Hong SY . Relationship between pulmonary surfactant protein and lipid peroxidation in lung injury due to paraquat intoxication in rats. Korean J Intern Med 2007; 22: 67–72.
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small MAF heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997; 236: 313–22.
Kensler TW, Wakabayashi N, Biswal S . Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007; 47: 89–116.
Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of Nrf2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 2002; 26: 175–82.
Cho HY, Reddy SP, Yamamoto M, Kleeberger SR . The transcription factor Nrf2 protects against pulmonary fibrosis. FASEB J 2004;18: 1258–60.
Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M . Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol 2001; 173: 154–60.
Chan K, Kan YW . Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A 1999; 96: 12731–6.
Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, et al. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol 2005; 175: 6968–75.
Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004; 114: 1248–59.
Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 2005; 202: 47–59.
Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 2006; 116: 984–95.
Reddy NM, Kleeberger SR, Cho HY, Yamamoto M, Kensler TW, Biswal S, et al. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol 2007; 37: 3–8.
Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, et al. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med 2009; 179: 138–50.
Zhang HQ, Wang HD, Lu DX, Qi RB, Wang YP, Yan YX, et al. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock 2008; 29: 617–22.
Roux JH, Kawakatsu B, Gartland M, Pespeni D, Sheppard MA . Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 2005; 280: 18579–89.
Hybertson BM, Lee YM, Cho HG, Cho OJ, Repine JE . Alveolar type II cell abnormalities and peroxide formation in lungs of rats given IL-1 intratracheally. Inflammation 2000; 24: 289–303.
Lee YM, Hybertson BM, Cho HG, Terada LS, Cho O, Repine AJ, et al. Platelet-activating factor contributes to acute lung leak in rats given interleukin-1 intratracheally. Am J Physiol Lung Cell Mol Physiol 2000; 279: 75–80.
Hawiger J . Innate immunity and inflammation: a transcriptional paradigm. Immunol Res 2001; 23: 99–109.
Tong F, Tian YP, Huo SH, Hu L, Su JL, Chen H, et al. Changes of p38MAPK and nuclear factor-kappa B in lung tissue of acute paraquat poisoned rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2007; 25: 518–22.
Wenge Li, Tin Oo Khor, Changjiang Xu, Guoxiang Shen, Woo-Sik Jeong, Siwang Yu, et al. Activation of Nrf2-antioxidant signaling attenuates NF-κB-inflammatory response and elicits apoptosis. Biochem Pharmacol 2008; 76: 1485–9.
Acknowledgements
This study was supported by the National Natural Science Foundation of China, Grant No 81171793 /H1503.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Zn., Zhao, M., Zheng, Q. et al. Inhibitory effects of rosiglitazone on paraquat-induced acute lung injury in rats. Acta Pharmacol Sin 34, 1317–1324 (2013). https://doi.org/10.1038/aps.2013.65
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.65
Keywords
This article is cited by
-
Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation
Cell Death & Disease (2019)
-
JNK Inhibitor SP600125 Attenuates Paraquat-Induced Acute Lung Injury: an In Vivo and In Vitro Study
Inflammation (2017)
-
NLRP3 inflammasome activation regulated by NF-κB and DAPK contributed to paraquat-induced acute kidney injury
Immunologic Research (2017)
-
Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes
Acta Pharmacologica Sinica (2016)
-
Rosiglitazone, a Peroxisome Proliferator-Activated Receptor (PPAR)-γ Agonist, Attenuates Inflammation Via NF-κB Inhibition in Lipopolysaccharide-Induced Peritonitis
Inflammation (2015)