Abstract
Aim:
To investigate the routes of elimination and excretion for triptolide recovered in rats.
Methods:
After a single oral administration of [3H]triptolide (0.8 mg/kg, 100 μCi/kg) in Sprague Dawley rats, urine and fecal samples were collected for 168 h. To study biliary excretion, bile samples were collected for 24 h through bile duct cannulation. Radioactivity was measured using a liquid scintillation analyzer, and excretion pathway analysis was performed using an HPLC/on-line radioactivity detector.
Results:
The total radioactivity recovered from the urine and feces of rats without bile duct ligation ranged from 86.6%–89.1%. Most of the radioactivity (68.6%–72.0%) was recovered in the feces within 72 h after oral administration, while the radioactivity recovered in the urine and bile was 17.1%–18.0% and 39.0%–39.4%, respectively. The HPLC/on-line radiochromatographic analysis revealed that most of the drug-related radioactivity was in the form of metabolites. In addition, significant gender differences in the quantity of these metabolites were found: monohydroxytriptolide sulfates were the major metabolites detected in the urine, feces, and bile of female rats, while only traces of these metabolites were found in male rats.
Conclusion:
Radiolabeled triptolide is mainly secreted in bile and eliminated in feces. The absorbed radioactivity is primarily eliminated in the form of metabolites, and significant gender differences are observed in the quantity of recovered metabolites, which are likely caused by the gender-specific expression of sulfotransferases.
Similar content being viewed by others
Introduction
Tripterygium wilfordii Hook F (TWHF) is a Chinese herbal medicine commonly used for the treatment of rheumatoid arthritis1,2. Triptolide (Figure 1), the major pharmacological component of TWHF3, exhibits pharmacological effects, including immunosuppressive4,5, anti-inflammatory6,7, and anti-cancer activities8. Moreover, it is the quality control standard of clinically used TWHF preparations. However, triptolide has a narrow therapeutic window, and its clinical applications are greatly limited because of its serious toxicities, including hepatotoxicity and male reproductive toxicity9,10.
Chemical structure of [3H]triptolide.
Although several studies have reported on its pharmacological activities and toxicities, only a few studies have focused on the pharmacokinetics of triptolide. It has been reported that triptolide is rapidly eliminated in rat, with a terminal half-life of approximately 20 min when administered orally11. In addition, less than 1% of the orally administered triptolide may be detected in bile, urine, and feces after 24 h.
Drug excretion studies play an important role in drug development by revealing how the body disposes of xenobiotics. Excretion results may elucidate the relationship between the pharmacokinetic results and the status of the metabolic enzymes, transporters, etc. Excretion recovery studies using radiolabeled compounds conducted in laboratory animals and humans are standard components in the development of novel drug treatments12.
The objective of the current study was to investigate the excretion recovery and pathways of [3H]triptolide and its metabolites in Sprague Dawley (SD) rats upon oral administration of 0.8 mg/kg (100 μCi/kg).
Materials and methods
Chemicals
The triptolide (purity 98%) used as a reference standard was obtained from PI & PI Technologies Inc (Guangzhou, China). Sodium borotritide (0.1 μCi with a specific activity of 10.4 μCi/mmol) was purchased from ARC Inc (Saint Louis, MO, USA). Pyridinium chlorochromate and all other chemicals were obtained from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China) as chemically pure grade or higher and were used without further purification.
Synthesis of [3H]triptolide
First, triptonide was synthesized by stirring 20 mg (0.056 mmol) of triptolide and 104 mg (0.28 mmol) of pyridinium chlorochromate in 5 mL CH2Cl2 at approximately 25 °C for 16 h. The reaction mixture was then filtered and concentrated under vacuum. After removal of the solvent, the residue was purified using a Shimadzu LC-6AD preparative HPLC system (Shimadzu Corp, Kyoto, Japan) on a YMC-pack ODS-A column (250 mm×10 mm id, 5 μm, 12 nm; YMC Co, Ltd, Kyoto, Japan) using methanol-water (60:40, v/v) as the mobile phase. The flow rate was 3.0 mL/min, and the elution profiles were monitored by absorbance measurements conducted at 218 nm. Approximately 6.8 mg (0.019 mmol) of triptonide was obtained.
Next, all obtained triptonide (6.8 mg, 0.019 mmol) and sodium borotritide (0.1 Ci) were dissolved in 5 mL tetrahydrofuran and stirred at room temperature for 4 h. The reaction solution was then purified using the HPLC system described above with methanol/water (55:45, v/v) as the mobile phase. Approximately 1.81 mg (0.005 mmol) of [3H]triptolide was obtained.
The total radioactivity was measured as 2.34 mCi by using an LS6500 liquid scintillation analyzer (Beckman, Brea, CA, USA). The purity was 99.5% as measured by using a dynamic on-line radio flow detector (AIM Research Co, Hockessin, DE, USA). The specific activity was calculated as 466 mCi/mmol.
Instrumentation for the mass balance study
The radioactivity was measured via liquid scintillation counting using a liquid scintillation analyzer (MicroBeta Trilux 1450, PerkinElmer, Boston, MA, USA).
Metabolite profiling was performed using an HPLC-accurate radioisotope quantification system, which consisted of a G1379A degasser, G1311A pump, G1367A autosampler, G1316A column oven (Agilent, Valdbronn, Germany) and a dynamic on-line radio flow detector (AIM Research Co, Hockessin, DE, USA).
Animals
SD rats weighing 180–220 g were obtained from SLRC Laboratory Animal Co, Ltd (Shanghai, China). The animals were maintained under controlled conditions according to procedures approved by the Animal Care and Use Committee of Shanghai Institute of Materia Medica (Shanghai, China). The rats were acclimated for 1 week prior to the urine and feces excretion study and 3 weeks prior to the biliary excretion study in a temperature-controlled (25 °C) room under a 12 h light/dark cycle. Food and water were provided ad libitum, except during the 12 h fasting period prior to the administration of triptolide. Food was provided 2 h after dosing.
Urine and feces excretion study
Six rats (three males and three females) were placed in the metabolism cages 24 h prior to the administration of triptolide. Food was provided in powdered form to reduce the contamination of fecal matter. After dosing with [3H]triptolide (0.8 mg/kg; 100 μCi/kg; 10 mL/kg), the rats remained in the metabolism cages for urine and feces collection. Urine and feces were collected at 4, 8, 24, 48, 72, 96, 120, 144, and 168 h after triptolide administration. The metabolism cages were rinsed with water and methanol after every urine collection, and the solvents were added to the collected urine. The feces were homogenized with water at a 1:1 ratio. After thorough mixing of each of the collected samples, the weights of the rats were recorded. At the end of this study, the rats were euthanized by CO2 inhalation.
Biliary excretion study
Each rat was anesthetized with 50 mg/kg intraperitoneal (ip) pentobarbital at a dose of 1 mL/kg. A midline incision was made in the abdomen, and the bile duct was isolated and cannulated with a PE-10 cannula. Bile was collected at 1, 2, 4, 8, 12, and 24 h after oral administration of [3H]triptolide (0.8 mg/kg; 100 μCi/kg; 10 mL/kg). All bile samples were frozen at -80 °C prior to analysis.
Sample preparation
For the excretion study, triplicate aliquots of urine (50 μL) were mixed using the StopFlow AD cocktail (AIM Research Co, Hockessin, USA) and directly quantified. Triplicate aliquots of feces homogenates (100 mg) and of bile (50 μL) were solubilized using perchloric acid/hydrogen peroxide (90:260, v/v) for 12 h at 80 °C. The supernatants were mixed with the StopFlow AD cocktail and then quantified.
For the analysis of the metabolic pathways, 0–24 h urine samples were pooled according to gender, centrifuged, and then analyzed without further purification. The 0–24 h fecal samples were homogenized, pooled by gender, and then sonicated with methanol. After centrifugation, the supernatant was evaporated to dryness using the TurboVap evaporator under a stream of nitrogen at 40 °C. The residue was reconstituted in 100 μL of methanol/water (50:50, v/v) and analyzed. For bile sample analyses, 0–24 h samples were pooled according to gender. After protein precipitation using methanol and centrifugation, the supernatant was diluted with an equal volume of water and then analyzed.
Statistical analysis
The concentrations of radioactivity in rat urine, feces, and bile were calculated using standard curves. The radioactivity and the cumulative radioactivity were determined by the volume or weight of the samples.
Results
Excretion of radioactivity
The cumulative excretion curves for radioactivity in rats are shown in Figure 2. The percentage of the radioactivity recovered was determined at 168 h after administration. The percentage of radioactivity recovered in the feces was 68.6% for male rats and 72.0% for female rats, while the radioactivity recovered in the urine was 17.1% for male rats and 18.0% for female rats. The total recovery of radioactivity eliminated in the excreta was 86.6% in male rats and 89.1% in female rats. These results indicated that virtually all the orally administered radioactivity was excreted in the urine and feces within 168 h and that fecal excretion was the major route of elimination. In addition, greater than 80% of the radioactivity recovered in feces and urine was excreted within the first 24 h after administration. The excretion rate plots of radioactivity are shown in Figure 3. The rats demonstrated a maximum excretion rate in urine from 0 to 4 h, while the maximum excretion rate in feces occurred between 8 and 24 h.
Cumulative excretion curve of 3H radioactivity in urine and feces after a single oral administration of [3H]triptolide (0.8 mg/kg) to SD rats (n=3 for each gender).
Excretion rate plots of [3H]triptolide in urine and feces after a single oral administration of [3H]triptolide (0.8 mg/kg) to SD rats (n=3 for each gender).
Biliary excretion of radioactivity
The biliary excretion of total radioactivity in bile duct-cannulated (BDC) rats accounted for 39% of the total dose measured at 24 h after dosing (Figure 4).
Cumulative excretion curve of 3H radioactivity in bile after a single oral administration of [3H]triptolide (0.8 mg/kg) to bile duct-cannulated SD rats (for male, n=3; for female, n=3 prior to 2 h, n=2 thereafter).
Excretion of the parent drug and major metabolites
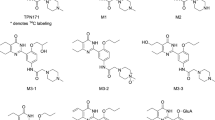
Metabolite profiling and identification were performed in our laboratory13. Figure 5 shows the proposed major metabolic sites for triptolide in rats.
Confirmed (*) and proposed (**) major metabolic sites for triptolide in rats.
A semiquantitative determination of the main metabolites was performed based on the peak areas shown on the radiochromatograms and on the excretion results.
A summary of the percentage of the doses of the parent drug and its metabolites excreted up to 24 h is shown in Table 1. Within 24 h, cumulative fecal, biliary, and urinary excretions of the parent drug in the male rat were 1.92%, 3.98%, and 3.81% of the administered dose, respectively. In the urine of female rats, only 0.0267% of the administered dose was from triptolide, while no unchanged drug was detected in the feces or bile of female rats within 24 h.
In male rats, hydroxy metabolites were the major forms of radioactivity detected in urine. Mono-, di-, and trihydroxy metabolites accounted for nearly 70% of the renal excretion of radioactivity. However, in the feces of male rats, only one monohydroxy metabolite was detected using radiochromatography. The main components in the feces of male rats were unidentified metabolites. Although there were some unidentified metabolites, hydroxy metabolites still accounted for the largest percentage of the radioactivity excreted in the bile of male rats.
In the urine of female rats, hydroxy metabolites as well as monohydroxytripotolide sulfates were the major components detected. In the feces and bile of female rats, monohydroxy metabolite sulfates contributed to the majority of the excreted radioactivity.
Discussion
Triptolide is the major pharmacologically active component of TWHF. The present study examined the excretion pathways of this clinically used compound.
The toxic doses reported in previous studies were considered when selecting the triptolide dose for this study. Previous animal studies have demonstrated a slim margin between the therapeutic and toxic concentrations of triptolide. For example, the lethal concentration of triptolide in mice was 0.86 mg/kg (ip) for 50% of the tested animal population14. Moreover, in a previous pharmacokinetic study, multiple doses from 0.6 mg/kg to 2.4 mg/kg were investigated. Based on these results, an oral dose of 0.8 mg/kg triptolide was established for the current study. During the study, one female BDC rat died 2 h after the administration of this dose.
According to observations obtained at 168 h after initial dosing, the primary route of excretion for the radioactivity was the feces. Furthermore, nearly the entire dose was excreted within the first 24 h after administration, and this result is consistent with the rapid elimination of triptolide.
In BDC rats, approximately 39% of the radioactivity administered was recovered in the bile 24 h after oral administration, suggesting that the radioactivity was mainly secreted in the bile, and then eliminated in the feces.
The HPLC/on-line radiochromatographic analysis revealed that most of the drug-related radioactivity resulted from metabolites. In male rats, hydroxy metabolites were the major metabolites detected in the urine and bile. A number of unidentified metabolites that were detected in the feces of the males were not found in their bile. These unidentified metabolites may have resulted from conversion by intestinal microflora. In female rats, similar to males, hydroxy metabolites were the major metabolites found in urine. However, the percentage of hydroxy metabolites detected in the bile of females was lower than that found in the bile of males, and monohydroxytriptolide sulfates comprised the major metabolites in the bile of the females. These sulfates were also the major components detected in the feces of female rats. These two sulfates accounted for more than 60% of the excreted fecal radioactivity, indicating that after oral administration, most of the drug was absorbed.
These metabolic pathways showed significant quantitative differences between male and female rats. First, the recovery of the parent drug was much higher in male rats than that in female rats. Second, the primary biotransformation pathways in male rats differed from those in female rats.
The difference in CYP levels between male and female rats was thought to play a primary role in triptolide toxicity because cases of triptolide toxicity in females are more serious than those in male rats. It was suggested that this discrepancy results from the gender differences observed in CYP3A2 activity and in vitro triptolide clearance15. However, our results suggested that sulfation played a more important role in the metabolism of triptolide in female rats than that in male rats. This gender difference in triptolide metabolism was likely caused by the gender-specific expression of sulfotransferases in the rat16,17,18.
In male rats, the triptolide GSH conjugate was detected as a major metabolite in bile. This metabolic pathway of triptolide further indicated its electrophilicity, which was closely associated with its toxicity.
In this study, the total recovery values for the radiolabeled drug ranged from 86.6% to 89.1%. After oral administration, most of the administered triptolide was converted into metabolites, and several were unidentified. Thus, if an excretion study had been conducted with a nonradiolabeled drug, obtaining a satisfactory result would have been challenging.
Conclusions
After a single oral administration of [3H]triptolide (0.8 mg/kg, 100 μCi/kg) in the SD rat, radioactivity was mainly secreted in bile and eliminated in feces. The majority of radioactivity was excreted in the form of metabolites. The significant gender differences observed in the quantity of metabolites were attributed to the gender-specific expression of sulfotransferases.
Author contribution
Jia LIU and Da-fang ZHONG were responsible for the study design, data analyses, and manuscript writing; Jia LIU and Xin ZHOU conducted the study; Xiao-yan CHEN was a senior advisor and provided valuable advice for this study.
References
Pan XD, Chen XC . Advances in the study of immunopharmacological effects and mechanisms of extracts of Tripterygium wilfordii Hook F in neuroimmunologic disorders. Yao Xue Xue Bao 2008; 43: 1179–85.
Tao X, Lipsky PE . The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum Dis Clin North Am 2000; 26: 29–50.
Chen BJ . Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma 2001; 42: 253–65.
Zhang G, Liu Y, Guo H, Sun Z, Zhou YH . Triptolide promotes generation of FoxP3+ T regulatory cells in rats. J Ethnopharmacol 2009; 125: 41–6.
Wang Y, Mei Y, Feng D, Xu L . Triptolide modulates T-cell inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurosci Res 2008; 86: 2441–9.
Wang Y, Jia L, Wu CY . Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand J Immunol 2008; 68: 383–90.
Gong Y, Xue B, Jiao J, Jing L, Wang X . Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and JNK in LPS-treated microglia. J Neurochem 2008; 107: 779–88.
Manzo SG, Zhou ZL, Wang YQ, Marinello J, He JX, Li YC, et al. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res 2012; 72: 5363–73.
Mei Z, Li X, Wu Q, Hu S, Yang X . The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol Res 2005; 51: 345–51.
Ni B, Jiang Z, Huang X, Xu F, Zhang R, Zhang Z, et al. Male reproductive toxicity and toxicokinetics of triptolide in rats. Arzneimittelforschung 2008; 58: 673–80.
Shao F, Wang G, Xie H, Zhu X, Sun J, A J . Pharmacokinetic study of triptolide, a constituent of immunosuppressive Chinese herb medicine, in rats. Biol Pharm Bull 2007; 30: 702–7.
Marathe PH, Shyu WC, Humphreys WG . The use of radiolabeled compounds for ADME studies in discovery and exploratory development. Curr Pharm Des 2004; 10: 2991–3008.
Liu J, Li L, Zhou X, Chen X, Huang H, Zhao S, et al. Metabolite profiling and identification of triptolide in rats. J Chromatogr B Analyt Technol Biomed Life Sci 2013; 939: 51–8.
Zhou R, Zhang F, He PL, Zhou WL, Wu QL, Xu JY, et al. (5R)-5-Hydroxytriptolide (LLDT-8), a novel triptolide analog mediates immunosuppressive effects in vitro and in vivo. Int Immunopharmacol 2005; 5: 1895–903.
Liu L, Jiang ZZ, Liu J, Huang X, Wang T, Zhang Y, et al. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicology 2010; 271: 57–63.
Zhong WZ, Zhan J, Kang P, Yamazaki S . Gender specific drug metabolism of PF-02341066 in rats — role of sulfoconjugation. Curr Drug Metab 2010; 11: 296–306.
Meerman JH, Nijland C, Mulder GJ . Sex differences in sulfation and glucuronidation of phenol, 4-nitrophenol and N-hydroxy-2-acetylaminofluorene in the rat in vivo. Biochem Pharmacol 1987; 36: 2605–8.
Singer SS, Giera D, Johnson J, Sylvester S . Enzymatic sulfation of steroids: I. The enzymatic basis for the sex difference in cortisol sulfation by rat liver preparations. Endocrinology 1976; 98: 963–74.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 30901831 and 81373479).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Zhou, X., Chen, Xy. et al. Excretion of [3H]triptolide and its metabolites in rats after oral administration. Acta Pharmacol Sin 35, 549–554 (2014). https://doi.org/10.1038/aps.2013.192
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.192
Keywords
This article is cited by
-
CYP3A4 inducer and inhibitor strongly affect the pharmacokinetics of triptolide and its derivative in rats
Acta Pharmacologica Sinica (2018)
-
Inhibition of P-glycoprotein Gene Expression and Function Enhances Triptolide-induced Hepatotoxicity in Mice
Scientific Reports (2015)