Abstract
Aim:
To investigate the effects and underlying mechanisms of 118, a novel derivative of mycophenolic acid, in a murine allogeneic skin graft model.
Methods:
Skin grafts were conducted by grafting BALB/c donor tail skin into C57BL/6 skin beds (allograft) or by grafting female C57BL/6 donor tail skin into female C57BL/6 skin beds (syngraft). The mice were treated with the derivative 118 (40 mg·kg−1·d−1, po) for 13 d (3 d before and 10 d after transplantation). Skin grafts, splenocytes and graft-infiltrated lymphocytes were isolated and examined ex vivo. The effects of the derivative 118 on naive CD4+ T cell differentiation were examined in vitro.
Results:
Treatment with the derivative 118 dramatically increased the survival rate of murine allogeneic skin grafts. Flow cytometric analysis and H&E staining showed that the derivative significantly decreased inflammatory cell infiltration into the grafts. The levels of the chemokines CXCL1, CXCL2, CCL7, and CCL2 were reduced in the derivative 118-treated grafts. Additionally, the derivative 118 significantly suppressed the IL-17 levels in the grafts but did not affect the differentiation of systemic helper T cells in the murine allogeneic skin graft model. Furthermore, IL-23p19 expression was suppressed in the grafts from the derivative 118-treated group, which might be due to decreases in TLR4 and MyD88 expression. Finally, the derivative 118 did not exert direct influences on helper T cell differentiation in vitro.
Conclusion:
Treatment with the mycophenolic acid derivative 118 improves murine allogeneic skin grafts by decreasing IL-23 expression and suppressing local IL-17 secretion in the grafts, rather than directly inhibiting Th17 differentiation.
Similar content being viewed by others
Introduction
Acute allograft rejection remains a major problem in clinical transplantation1. Mycophenolate mofetil (MMF), a prodrug of mycophenolic acid (MPA), has been widely used to prevent allograft rejection2,3,4. MPA inhibits inosine monophosphate dehydrogenase (IMPDH), the rate-limiting enzyme in the de novo synthesis of guanosine nucleotides. IMPDH has 2 isoforms, IMPDH I and II. IMPDH I is nearly ubiquitously expressed, while IMPDH II is expressed mainly in activated lymphocytes. MPA selectively inhibits IMPDH II and thus preferentially inhibits the activation of lymphocytes over other cell types5,6. It has been well established over several decades that MMF suppresses T cell activation and proliferation and thus exerts immunosuppressive effects.
The IL-23/IL-17 and IL-12/IFN-γ axes are emerging as critical players in host defenses against infections and in autoimmune diseases. IL-23 has a unique p19 subunit but shares the p40 subunit with IL-12 and is secreted by macrophages and dendritic cells (DCs) very rapidly during infections or inflammatory responses. Although IL-23 is not essential for the differentiation of Th17 cells, it can maintain the Th17 phenotype and was shown to be one of the strongest factors in the direct induction of IL-17 production from Th17 cells, even in the absence of T-cell receptor engagement7. MMF has been reported to decrease atherosclerotic lesions in a murine chronic vascular inflammation model8 and to suppress granulopoiesis through the inhibition of IL-17 production in a bone marrow transplantation model9. Once produced, IL-17 acts primarily to induce chemokine expression and to recruit neutrophils; it thus initiates and accelerates inflammatory responses. MMF has been reported to effectively suppress inflammatory cell infiltration and inhibit tubular cell proliferation in rat kidney grafts10.
Neutrophil- and monocyte-recruiting chemokines, including CCR6, CXCL1, CXCL2, CCL2, and CCL7, are major factors that are upregulated by IL-1711. MMF has been reported to have strong effects on the chemokine profiles of colon, kidney and pancreatic carcinomas12. Additionally, recent reports have established that IL-17 is an essential component in cases of acute allograft rejection. Increased intragraft IL-17 levels were observed during allograft rejection in animal heart and renal allograft models13, and an IL-17R-Ig fusion protein significantly prolonged graft survival in aortic and heart allograft rodent models14. Furthermore, IL-17 mRNA and protein were elevated during acute rejection episodes in human renal and lung allograft cases15.
A derivative of MPA, 118, possesses similar in vitro immunosuppressive activities to MMF. The chemical structure of 118 is shown in Figure 1. Herein, we examined the therapeutic effects and mechanisms of 118 on murine acute skin allograft models, with a particular focus on Th17 profiles. The results showed that treatment with 118 significantly prolonged the lifespan of skin grafts. Additionally, 118 did not directly inhibit the Th17 cell polarization, but it suppressed IL-23 expression in the grafts and ultimately inhibited local IL-17 levels. In summary, the present study demonstrated that 118 had therapeutic effects in an allogeneic murine skin graft model. In addition to the classic inhibitory effects of MMF on T cell proliferation, the suppression of IL-23 expression by the innate immune system might also be a beneficial effect when using MMF and its analogues to treat transplant rejection.
Chemical structure of mycophenolic acid derivative 118.
Materials and methods
Animals
Female BALB/c (H-2d) and C57BL/6 (H-2b) mice were obtained from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences and were used at 8 to 10 weeks of age. All mice were housed in specific pathogen-free conditions (12-h light/12-h dark photoperiod, 22±1 °C, 55%±5% relative humidity). All experiments were performed according to the institutional ethical guidelines on animal care and were approved by the Institute Animal Care and Use Committee at the Shanghai Institute of Materia Medica.
Skin transplantation
For allogeneic grafts, full thickness BALB/c tail skin grafts with areas of 5 mm×5 mm were engrafted onto the backs of recipient C57BL/6 mice. For syngeneic grafts, full thickness female C57BL/6 tail skin grafts with areas of 5 mm×5 mm were engrafted onto the backs of recipient female C57BL/6 mice. The grafts were covered with Vaseline, gauze and a clinical securing bandage for 8 d. Skin graft survival was monitored on d 9, and rejection was defined as necrosis of more than 80% of the graft tissue. From d 7 onward, digital photographs were taken of each mouse, and graft survival was evaluated by two inspectors who were blinded to the particular experimental groups. There were 15 animals in each experimental group, and 118 (40 mg/kg) or vehicle control was administered orally once per day per animal.
Tissue preparation for FACS analysis
The skin grafts were carefully isolated and digested for 1 hour at 37 °C with 125 units/mL of type XI collagenase, 60 units/mL of DNase I, 60 units/mL of type I-s hyaluronidase, and 450 units/mL of type I collagenase (Sigma, St Louis, MO, USA) in PBS with 20 mmol/L HEPES. After digestion, the suspensions were filtered through 40-μm cell strainers to remove larger pieces of residual tissue. The resulting single-cell suspensions were washed, counted, and analyzed by flow cytometry on a FACSCalibur system with Flowjo software.
In vitro helper T cell differentiation
In vitro helper T cell differentiation and intracellular staining protocols were performed as follows. Briefly, splenocytes from naïve C57BL/6 mice were blocked with saturating concentrations of an anti-mCD16/CD32 mAb. After blocking, the cells were enriched by magnetic selection to remove CD8-, B220-, CD44-, and CD11b-expressing cells. The remaining cells were combined with L3T4 beads (Miltenyi Biotec) to permit positive selection on a MACS mini-separation magnetic column (MS Columns, Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the resulting cells was determined by flow cytometry analysis and was consistently >95%. The cells were plated at a density of 2×105/well and stimulated with anti-CD3 (5 μg/mL, 145-2C11, BD Bioscience, San Jose, CA, USA) and anti-CD28 (2 μg/mL, 37.51, BD Bioscience, San Jose, CA, USA) mAb. For Th1 differentiation, the cultures were supplemented with rIL-12 (10 ng/mL) and anti-IL-4 (10 μg/mL). For Th17 differentiation, the cultures were supplemented with rmIL-23 (20 ng/mL), TGF-β1 (5 ng/mL), and IL-6 (20 ng/mL). Additionally, IFN-γ and IL-4 were neutralized in the Th17 cultures with anti-IFN-γ (10 μg/mL, XMG1.2, eBioscience, San Diego, CA, USA) and anti-IL-4 (10 μg/mL, 11B11, eBioscience, San Diego, CA, USA) mAb. For Treg differentiation, purified T cells were incubated under activating conditions and polarized with 3 ng/mL rh TGFβ, 10 μg/mL anti-IFNγ, and 10 μg/mL anti-IL-4. After 96 h, the cells were restimulated for 5 h with PMA and ionomycin, CD4+ T cells were restimulated for 5 h with phorbol myristate acetate (PMA, 50 ng/mL, Sigma, St Louis, MO, USA), ionomycin (750 ng/mL, Sigma, St Louis, MO, USA), and Brefeldin A (10 μg/mL, InvitrogenCarlsbad, CA, USA). The cells were harvested for intracellular staining after the restimulation period.
Flow cytometric analysis
The cells isolated from the skin grafts were directly stained for surface markers. To detect intracellular cytokines, the cells were stimulated in vitro with 50 ng/mL phorbol myristate acetate (PMA, Sigma-Aldrich), 750 ng/mL ionomycin (Sigma-Aldrich) and Brefeldin A (10 μg/mL, Invitrogen) in a 24-well plate and were incubated at 37 °C for 4 to 5 h before staining. After surface staining, the cells were washed and resuspended in fixation/permeabilization solution and were subsequently stained intracellularly, according to the manufacturer's protocol (Foxp3 staining buffer set, eBioscience). The following reagents were used: FITC- and PE-anti-mCD4 (GK1.5), FITC- and PE-anti-mCD3 (145-2C11), PE-anti-mCD8 (2.43), FITC- and PE-anti-mCD11c (HL-3), biotinylated- and PE-anti-mCD11b (M1/70), FITC-anti-mIFN-c (XMG1.2), PE-anti-mIL-17 (TC11-18H10), and FITC-anti-mGr-1 (RB6-8C5), which were purchased from BD Pharmingen (San Diego, CA, USA); and FITC-anti-mF4/80 (BM8), PE-Cy5-anti-mouse/rat FoxP3 (FJK-16s), PE-Cy5 and FoxP3 Staining Buffer Set, which were purchased from eBioscience16,17.
Histopathologic analysis of skin grafts
Mice were sacrificed at 9 d after transplantation. The skin grafts were harvested and processed by formalin fixation and paraffin embedment, cut into approximately 5-μm sections and stained with hematoxylin and eosin (H&E). The slides were reviewed in a blinded fashion by two qualified pathologists to assess the degree of graft rejection. The modified scoring system for acute mouse skin allograft rejection was proposed by Cendales et al18. Grade 0 (nonspecific changes) is defined as a low infiltration of inflammatory cells into the epidermis. Grade 1 (mild rejection) is defined as inflammatory infiltration into the perivascular and adnexal glands, hair shafts and epidermis. Grade 2 (moderate rejection) is defined as the presence of a band-like infiltration just beneath the dermal-epidermal junction in addition to prominent inflammation. Grade 3 (severe rejection) is defined as the presence of a significant number of infiltrating inflammatory cells as well as necrotic keratinocytes that form a space between the epidermis and the dermis. In the latter grade, the grafts partially or completely detach from the host.
RNA isolation and quantitative RT-PCR
Freshly harvested graft tissue was immediately frozen. For each analysis, 100 ng of total RNA was isolated with the RNAsimple Total RNA Kit (Tiangen), according to the manufacturer's protocol, and stored at -70 °C until the time of analysis. The quantification of chemokines, IL-17A, IL-23p19, and other mRNA expression levels in the skin grafts and splenocytes were evaluated by qRT-PCR with SYBR Green PCR Reagents (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol. The relative mRNA expression levels were reported as ΔΔCt ratios and were calculated as the Ct value for the gene of interest/Ct value of the housekeeping gene. The relative mRNA expression levels of the indicated genes were quantified using a 7500 Fast Real-Time PCR System (Applied Biosystems, Table 1).
Mixed lymphocyte culture reaction
BALB/c splenocytes (3×105 cells/well, stimulator cells) were γ-irradiated with 30 Gy (Gamma cell 3000) and co-cultured with C57BL/6 splenocytes (3×105 cells/well, responder cells) in the presence of 118. After 72 h, the cells were pulsed with 1 μCi/well of [3H]-thymidine and incubated for another 24 h. The cells were harvested onto glass fiber filters, and the incorporated radioactivity was counted on a Beta Scintillation Counter.
ELISA for cytokine detection
Cytokines in the culture supernatants were assayed with mouse IFN-γ, IL-17, and IL-10 ELISA kits (all from BD Pharmingen, San Diego, CA, USA), according to the manufacturer's instructions.
Statistical analysis
To determine the graft survival rates, Kaplan-Meier graphs were constructed, and a log-rank comparison of the groups was used to calculate the P-values. For the cytokine levels, the data were presented as the means±SD, and comparisons between the values were performed with the two-tailed Student's t test. A P-value <0.05 was considered statistically significant. All experiments were repeated at least three to five times.
Results
118 prevented graft rejection in a murine allogeneic skin graft model
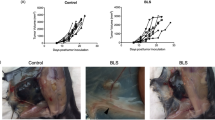
Allogeneic murine skin grafts were performed by grafting BALB/c donor tail skin into C57BL/6 skin beds. The mice were subsequently orally treated with 118 or a vehicle control for 13 consecutive days (3 d before and 10 d after the transplantation). In the vehicle-treated group, the skin grafts became hard and black and shrank gradually after transplantation, and they were almost completely rejected at 9 to 11 day after transplantation (Figure 2A, 2B). In contrast, 118 treatment significantly increased graft survival (Figure 2A). After the treatments, the skin grafts were harvested for histologic examination. As shown in Figure 1C, the syngraft group displayed no inflammatory infiltration at the 9th day after skin transplantation and thus received a grade 0 rejection score. By contrast, the allograft group displayed significant inflammatory infiltration and tissue destruction and thus received a grade 3 rejection score. In the 118-treated group, a diffuse infiltration was observed without tissue destruction, indicating grade 1 rejection. Therefore, 118 considerably suppressed inflammatory infiltration and tissue destruction in the skin grafts.
118 prevented skin graft rejection in a murine allogeneic skin graft model. Skin grafts were performed by grafting BALB/c donor tail skin into C57BL/6 skin beds (allograft) or by grafting female C57BL/6 donor tail skin into female C57BL/6 skin beds (syngraft). The syngrafted or allografted mice (n=15 per group) were orally treated with the vehicle control or 118 (po, 40 mg·kg−1·d−1) 3 d before transplantation, as described in Materials and methods. Grafts were scored as fully rejected when >80% necrosis was observed. Skin grafts were collected on d 9 after transplantation, and the sections were stained with H&E to assess inflammation. (A) Cumulative survival rate from d 1 of transplantation in the 118-treated versus allograft (vehicle) groups. (B) Appearance of a completely healed skin graft without evidence of rejection on d 7 after transplantation, acute rejection in progress on d 9 and complete acute graft rejection on d 11. (C) Representative skin sections stained with H&E (Original magnification: ×100).
118 decreased cell infiltration into grafts in a murine allogeneic skin graft model
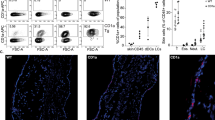
Allograft transplantation results in a rapid infiltration of innate immune cells into the graft19. We collected the grafts at different time points after transplantation and found that the numbers of infiltrating cells and the expression levels of chemokines and cytokines peaked on d 9 (data not shown). Thus, all of the samples from subsequent experiments were collected on d 9. To clarify the effects of 118 on immune cell infiltration into murine allogeneic grafts, flow cytometry was used to identify infiltrating leukocytes in the skin grafts, such as CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), neutrophils (CD11b+Gr1+), monocytes (CD11b+), DCs (CD11c+CD11b+) and macrophages (CD11b+F4/80+; Figure 3). The flow cytometric results were consistent with the histologic results and further confirmed that the percentages of skin graft-infiltrating lymphocytes, neutrophils, monocytes, DCs and macrophages were significantly lower in the 118-treated group than that in the vehicle-treated group (Figure 3).
118 inhibited inflammatory cell infiltration into the skin grafts. Skin grafts from allogeneic graft mice that received vehicle or 118 were collected on d 9 after transplantation. The numbers indicate the percentages of cells in the skin grafts. The results were expressed as the means±SEM. n=3. bP<0.05, cP<0.01 compared to the vehicle (allograft group) control (unpaired Student's t-test).
118 decreased the expression levels of chemokines and IL-17 but did not affect helper T cell differentiation in a murine allogeneic skin graft model
Because chemokine expression is important to the development of murine allogeneic skin graft rejection, chemokine mRNA expression levels were determined ex vivo by qRT-PCR. The results showed that the mRNA expression levels of CXCL1, CXCL2, CCL7, and CCL2 were decreased remarkably in the 118-treated group (Figure 4A). The levels of CCR6 mRNA, which is usually expressed by IL-17-producing Th17 cells, were less affected (Figure 4A). Next, we examined IL-17 expression in the grafts. As shown in Figure 4B, 118 treatment strongly suppressed IL-17 expression.
118 reduced chemokine and IL-17 expression in the skin grafts. Skin grafts from syngraft control mice, allograft mice and 118 treated allograft mice were harvested on d 9 and the relative mRNA levels were measured by qRT-PCR for (A) chemokines, (B) IL-17, Foxp3, and T-bet. The numbers indicate the ratios of mRNA expression in allografts to those in syngrafts and were calculated using the comparative CT method. (C) Splenocytes collected on d 9 after transplantation were stained for IFN-γ, IL-17, and Foxp3 expression and analyzed by flow cytometry to quantify the suppressive effects of 118 on the percentages of Th1, Th17, and Treg cells. The results were expressed as the means±SEM. n=3. bP<0.05, cP<0.01 allograft versus 118; (unpaired Student's t-test).
To examine the effects of 118 on the Th1, Th17, and Treg profiles in the grafts, the lymphocytes were isolated and intracellularly stained to examine the Th1, Th17, and Treg populations. As shown in Figure 4C, 118 treatment exerted only minor influences on the expression of IFN-γ, IL-17, and Foxp3 in CD4+ T cells compared to cells from the allograft group. Furthermore, the mRNA levels of T-bet and Foxp3 were not altered in the skin grafts, thus confirming that 118 treatment did not affect the infiltration of Th1 and Treg cells into the skin grafts (Figure 4B, 4C).
118 decreased IL-23p19 expression in grafts through the inhibition of TLR4 and MyD88 in a murine allogeneic skin graft model
Because 118 did not affect CCR6 expression but significantly inhibited IL-17 expression in the grafts, we examined the inducible factors that lie upstream of these proteins in Th17 cells. As shown in Figure 5A, 118 treatment did not affect RORγt expression in the grafts. As RORγt is a transcription factor that is critical to the development of Th17 cells, this result indicated that 118 treatment did not affect Th17 differentiation in the murine allogeneic skin grafts.
118 inhibited the expression of IL-23 and TLR-related genes in the skin grafts. Skin grafts or splenocytes from syngraft control mice, allograft mice and 118-treated allograft mice were harvested on d 9 and the relative mRNA levels of (A) RORγt, (B) IL-23p19, (C) MyD88, TLR4, and TLR2 were measured using qRT-PCR. The numbers indicate the ratios of mRNA expression in allografts to those in syngrafts and were calculated by the comparative CT method. The results were expressed as the means±SEM. n=3. bP<0.05, cP<0.01 (unpaired Student's t-test).
IL-23p19 does not strongly affect Th17 cell differentiation but can directly drive IL-17 production from both Th17 cells and IL-17-producing innate cells20. To determine the effects of 118 on IL-23p19 expression, we analyzed the IL-23p19 mRNA levels in both splenocytes and skin grafts. The results showed that 118 significantly decreased the levels of IL-23p19 mRNA in the skin grafts but not in the splenocytes, in comparison to the vehicle treated group (Figure 5B).
We further examined the levels of TLR2, TLR4 and MyD88 mRNA in the skin grafts, as these proteins are closely related to IL-23p19 expression. As shown in Figure 5C, the levels of TLR2 mRNA were unaffected, while the levels of TLR4 and MyD88 mRNA were significantly decreased in the 118-treated group compared with the control group.
118 inhibited allogeneic responses ex vivo but exerted fewer effects on helper T cell differentiation
An allogeneic mixed lymphocyte reaction was performed after treatment with 118. Lymphocytes from each group were collected and cocultured with irradiated naïve C57BL/6 splenocytes for 96 h. Proliferation was measured by [3H]-thymidine incorporation during the final 24 h. As shown in Figure 6A, lymphocyte proliferation was significantly lower in the 118-treated group than in the control group.
118 inhibited allogeneic responses ex vivo. (A) On d 9 after transplantation, a MLR was performed by coculturing splenocytes from syngraft control mice, allograft model and 118-treated allograft mice with irradiated naïve C57BL/6 splenocytes for 96 h. The cultures were pulsed with 1 μCi/well3H]-thymidine for the final 12 h and the activity was counted with a Beta Scintillation Counter. (B) Culture supernatants were collected at 96 h to measure IFN-γ, IL-17, and IL-10 levels by ELISA. Means±SEM. n=3. bP<0.05, cP<0.01 (unpaired Student's t-test).
Next, the supernatants from the allogeneic MLRs were collected and the IFN-γ and IL-10 levels were determined by enzyme-linked immunosorbent assays (ELISA). As shown in Figure 6B, 6C, and 6D, the IFN-γ, IL-17, and IL-10 levels were not significantly affected in supernatants from the 118-treated group when compared with those from the control group, which suggested that 118 treatment had little influence on Th1, Th17, and Treg cell differentiation or skin graft infiltration.
To further explore the direct effects of 118 on Th17 differentiation in vitro, naïve CD4+ T cells were cultured under Th17 polarizing conditions and analyzed for IFN-γ and IL-17 expression by intracellular staining. As shown in Figure 7, there were few changes in the percentages of IFN-γ+ and IL-17+CD4+ T cells, which suggested that 118 does not strongly affect CD4+ T cell differentiation under polarizing cytokine conditions in vitro.
118 did not strongly affect Th17 cell differentiation in vitro. Naïve CD4+ T cells were cultured under Th17 polarizing conditions and analyzed for IFN-γ and IL-17 expression by intracellular staining. The numbers indicate the percentages of cells. Three independent experiments were performed with similar results.
Discussion
The present study demonstrated that the oral administration of 118 effectively increased the murine allogeneic skin graft survival rate. The therapeutic effects of 118 resulted mainly from the inhibition of innate cell activity within the grafts. Treatment with 118 significantly suppressed lymphocyte, neutrophil and monocyte infiltration and decreased chemokine expression in the grafts. In particular, 118 treatment inhibited IL-23p19 expression and thus suppressed IL-17 expression in the grafts.
Both the innate and adaptive immune systems mediate acute graft rejection. Initially, the Th1 response was thought to play the pivotal pathogenic role21. However, this notion was challenged by the findings that IL-17 was critically associated with allograft rejection22,23. IL-17 is an important mediator that affects downstream inflammatory events, predominantly by recruiting neutrophils and monocytes to the grafts through the upregulation of monocyte and neutrophil-recruiting chemokines24,25. In the present study, our data revealed that 118 significantly decreased the inflammatory infiltration of the grafts. Subsequently, we found that 118 inhibited the local expression of IL-17-induced chemokines. Further results directly demonstrated that 118 suppressed IL-17 expression in the grafts. However, 118 did not influence the expression of CCR6, a chemokine that is mainly expressed by Th17 cells, in the grafts. Thus, these findings urged us to further determine the influence of 118 on the development and function of Th17 cells.
Recently, Ivanov et al26 demonstrated that the orphan nuclear receptor RORγt was a key transcription factor that mediated Th17 cell differentiation. Herein, the levels of RORγt mRNA were not affected, suggesting that 118 treatment did not suppress Th17 lineage commitment and local infiltration into the skin grafts. Previously, IL-23 was reported to possess a strong ability to directly induce IL-17 production from Th17 cells. To determine the effects of 118 treatment on IL-23 expression, we analyzed IL-23p19 mRNA levels in the splenocytes and skin grafts. The levels of IL-23p19 mRNA decreased significantly in the skin grafts but not in the splenocytes, suggesting that 118 suppressed IL-17 secretion in the skin grafts by suppressing IL-23p19 expression but not systemic Th17 differentiation.
Current publications indicate that Th1, Th17, and Treg are greatly involved in acute graft rejection, which raised the question of whether 118 could affect Th1, Th17, or Treg differentiation. We examined the percentages of Th1, Th17, and Treg lymphocytes in vivo and did not observe any differences between the treated and untreated groups. Additionally, the expression of the transcription factors associated with Th1, Th17 and Treg cells was not affected in the skin grafts, indicating that 118 did not influence systemic Th1, Th17, and Treg cell differentiation.
IL-23 is produced primarily by the innate immune cells, including macrophages and DCs, in a manner that is mainly dependent on TLR signaling27. Additionally, TLR-driven MyD88-dependent immunity is critical for skin allograft rejection through the induction of DC maturation, priming of graft-reactive T cells, and induction of T cell mediated immunity28. We further examined the levels of TLR2, TLR4, and MyD88 mRNA in the skin grafts. Our data suggested that the expression of TLR4 and MyD88, but not TLR2, was decreased in the 118-treated group. These results were consistent with the finding that TLR4 activation was required for IL-17-induced tissue inflammation29.
Finally, the results from the in vitro allogeneic MLR and differentiation experiments confirmed that 118 exerted no direct effects on helper T cell differentiation. The protein levels of IL-23 in the culture system might be too low to be detected because the cytokine secretion profiles do not always correlate with the cell proliferative activity. Although we observed a significant decrease in cell proliferation, cytokine secretion could be less affected30.
Collectively, our study demonstrates that the MPA derivative 118 is valuable and effective in the prevention of allogeneic murine skin graft rejection and that it exerts its effects by decreasing IL-23 expression and suppressing IL-17 secretion, thus ultimately abrogating inflammatory infiltration into the grafts.
Author contribution
Jian-ping ZUO, Wei TANG, and Wen-hu DUAN designed research.Fang-yuan KONG, Shi-jun HE, Ze-min LIN, Xin LI, Xiao-Hui ZHANG, Xiao-qian YANG, Feng-hua ZHU, Xian-kun TONG, and Yu ZHOU performed research.Wei CHEN contributed 118 compound. Fang-yuan KONG and Wei TANG analyzed and interpretated data. Fang-yuan KONG, Jian-ping ZUO, and Wei TANG wrote the paper.
References
Klimczak A, Siemionow M . Immune responses in transplantation: application to composite tissue allograft. Semin Plast Surg 2007; 21: 226–33.
Bardsley-Elliot A, Noble S, Foster RH . Mycophenolate mofetil — A review of its use in the management of solid organ transplantation. Biodrugs 1999; 12: 363–410.
Mathieu P, Carrier M, White M, Pellerin M, Perrault L, Pelletier G, et al. Effect of mycophenolate mofetil in heart transplantation. Can J Surg 2000; 43: 202–6.
Basara N, Blau WI . Efficacy and safety of mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant recipient. Transplant Proc 1998; 30: 4087–9.
Ransom JT . Mechanism of action of mycophenolate mofetil. Ther Drug Monit 1995; 17: 681–4.
Allison AC, Eugui EM . Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000; 47: 85–118.
Stritesky GL, Yeh N, Kaplan MH . IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol 2008; 181: 5948–55.
von Vietinghoff S, Koltsova EK, Mestas J, Diehl CJ, Witztum JL, Ley K . Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am College Cardiol 2011; 57: 2194–204.
von Vietinghoff S, Ouyang H, Ley K . Mycophenolic acid suppresses granulopoiesis by inhibition of interleukin-17 production. Kidney Int 2010; 78: 79–88.
Fuller TF, Hoff U, Rose F, Linde Y, Freise CE, Dragun D, et al. Effect of mycophenolate mofetil on rat kidney grafts with prolonged cold preservation. Kidney Int 2006; 70: 570–7.
Gorbacheva V, Fan R, Li X, Valujskikh A . Interleukin-17 promotes early allograft inflammation. Am J Pathol 2010; 177: 1265–73.
Engl T, Relja B, Natsheh I, Makarevic J, Müller I, Beecken WD, et al. Modulation of the CXC-chemokine expression profile on tumor cells by the immunosuppressive drug mycophenolate mofetil. Int J Mol Med 2005; 15: 641–7.
Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY . Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol 2002; 197: 322–32.
Li J, Simeoni E, Fleury S, Dudler J, Fiorini E, Kappenberger L, et al. Gene transfer of soluble interleukin-17 receptor prolongs cardiac allograft survival in a rat model. Eur J Cardio-Thoracic Surgery 2006; 29: 779–83.
Van Raemdonck DE, Verleden GM . The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir 2006; 27: 779–87.
Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH, et al. SM934 treated lupus-prone NZB x NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS One 2012; 7: e32424.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum 2011; 63: 2445–55.
Cendales LC, Kirk AD, Moresi JM, Ruiz P, Kleiner DE . Composite tissue allotransplantation: Classification of clinical acute skin rejection. Transplantation 2006; 81: 418–22.
Celli S, Albert ML, Bousso P . Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med 2011; 17: 744–9.
Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol 2007; 178: 4901–7.
Obata F, Yoshida K, Ohkubo M, Ikeda Y, Taoka Y, Takeuchi Y, et al. Contribution of CD4+ and CD8+ T cells and interferon-gamma to the progress of chronic rejection of kidney allografts: the Th1 response mediates both acute and chronic rejection. Transplant Immunol 2005; 14: 21–5.
Wang S, Li J . Dynamic changes in Th1, Th17, and FoxP3+ T cells in patients with acute cellular rejection after cardiac transplantation. Clin Transplant 2011; 25: E177–86.
Chung BH, Oh HJ, Piao SG, Hwang HS, Sun IO, Choi SR, et al. Clinical significance of the ratio between FOXP3 positive regulatory T cell and interleukin-17 secreting cell in renal allograft biopsies with acute T-cell-mediated rejection. Immunology 2012; 136: 344–51.
Miyamoto M, Prause O, Sjöstrand M, Laan M, Lötvall J, Lindén A . Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol 2003; 170: 4665–72.
Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol 2000; 165: 5814–21.
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126: 1121–33.
Waibler Z, Kalinke U . TLR-ligand stimulated interleukin-23 subunit expression and assembly is regulated differentially in murine plasmacytoid and myeloid dendritic cells. Mol Immunol 2007; 44: 1483–9.
Goldstein DR, Tesar BM, Akira S, Lakkis FG . Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 2003; 111: 1571–8.
Tang H, Pang S, Wang M, Xiao X, Rong Y, Wang H, et al. TLR4 activation is required for IL-17-induced multiple tissue inflammation and wasting in mice. J Immunol 2010; 185: 2563–9.
Lea NC, Orr SJ, Stoeber K, Williams GH, Lam EW, Ibrahim MA, et al. Commitment point during G0→G1 that controls entry into the cell cycle. Mol Cell Biol 2003; 23: 2351–61.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (Nos 81072652, 81273524, and 81273525).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kong, Fy., Chen, W., He, Sj. et al. Mycophenolic acid derivative 118 improves outcome of skin grafts by suppressing IL-17 production. Acta Pharmacol Sin 34, 921–929 (2013). https://doi.org/10.1038/aps.2013.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.14