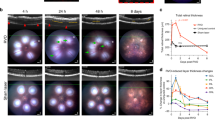
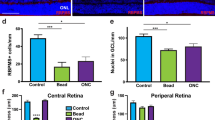
Abstract
Retinal ischemia is a very useful model to study the impact of various cell death pathways, such as apoptosis and necrosis, in the ischemic retina. However, it is important to note that the retina is formed as an outpouching of the diencephalon and is part of the central nervous system. As such, the cell death pathways initiated in response to ischemic damage in the retina reflect those found in other areas of the central nervous system undergoing similar trauma. The retina is also more accessible than other areas of the central nervous system, thus making it a simpler model to work with and study. By utilizing the retinal model, we can greatly increase our knowledge of the cell death processes initiated by ischemia which lead to degeneration in the central nervous system. This paper examines work that has been done so far to characterize various aspects of cell death in the retinal ischemia model, such as various pathways which are activated, and the role neurotrophic factors, and discusses how these are relevant to the treatment of ischemic damage in both the retina and the greater central nervous system.
Similar content being viewed by others
Introduction
Ischemia is broadly defined as the loss of blood supply to a biological tissue resulting in energy depletion and cell death, both of which are mediated by intermediate factors such as the release of excess excitatory amino acids, free-radical formation, and inflammation1. Ischemia is one of the key factors that determines the pathophysiology of many brain and retinal diseases2. As the retina is an extension of the diencephalon, retinal blood vessels share similar anatomic, physiological and embryological properties with the brain, and possess a blood-retinal barrier analogous to the blood-brain barrier3. Retinal ischemia is a common cause of visual impairment and blindness, and is a characteristic feature of various clinical retinal disorders such as ischemic optic neuropathies, obstructive arterial and venous retinopathies, carotid occlusive disorders, retinopathy of prematurity, chronic diabetic retinopathy and glaucoma4. At the cellular level, retinal ischemia consists of a self-reinforcing pro-apoptotic cascade involving neuronal depolarization, calcium influx, oxidative stress, energy depletion, and glutamatergic stimulation.
A number of animal models and analytical techniques have been used to study retinal ischemia, and an increasing number of treatments have been shown to interrupt the resulting cell death cascades and attenuate their detrimental effects; however, our knowledge remains incomplete and treatments can be improved. A more thorough understanding of the molecular mechanisms behind ischemic damage is essential to improving potential therapies, and can provide insight into the pathophysiology of other neurodegenerative conditions as well, most notably of cerebral stroke5.
The pathophysiology of retinal ischemia
Retinal ischemia occurs when the retinal circulation is insufficient to meet metabolic demands. It can be caused by general or, more commonly, by local circulatory failure. The metabolic demands of the retina are the highest of any tissue within the body, and so maintaining a consistently high blood supply is essential4. The degree of damage sustained by retinal tissue during ischemia depends on the severity and the duration of the obstruction to blood flow. The area most prominently supplied by the occluded blood vessel comprises the infarct core, while areas perfused by collateral circulation form the ischemic penumbra. Areas within the ischemic core are most severely affected by the ischemic injury, while those in the penumbra are much less affected and can retain a degree of function based on the amount of damage they have sustained. If circulation is not quickly restored to the affected area however, the penumbra is gradually incorporated into the ischemic core and becomes completely non-functional6,7. The penumbra, therefore, represents the main therapeutic target for acute ischemia therapies.
There are many pathogenic mechanisms which contribute to the cell death cascades experienced during ischemia, such as energy failure, elevation of intracellular calcium, excitotoxicity and spreading depression, generation of free radicals, blood-retinal barrier disruption, inflammation and apoptosis. The latter process is a complex cascade of cellular factors which contribute to tissue injury and impair the cellular mechanisms required to maintain ionic gradients2. Initially, the reduction in blood flow results in the depletion of substrates such as oxygen and glucose, which in turn causes the accumulation of lactate via anaerobic glycolysis. The depletion of energy results in neuronal depolarization, causing the activation of glutamate receptors, and thereby altering the ionic gradients of Na+, Ca2+, Cl−, and K+8. As the intracellular concentration of Ca2+ is increased by the dysregulation of its ionic gradient, a variety of intracellular enzymes, such as lipases, proteases and endonucleases, experience increased activity. As a result, oxygen free radicals are generated and contribute to apoptotic cell death. Oxygen free radicals are also produced by the enzymatic conversion of arachidonic acid to prostanoids and the degradation of hypoxanthine during blood reperfusion of the ischemic area9. The formation of free radicals recruits pro-inflammatory factors, such as interleukins, platelet activating factor, and tumor necrosis factor α (TNF α). As well, during ischemic conditions, mitochondrial permeability transition pores are formed, which cause the release of free radicals and pro-apoptotic molecules10. The infiltration of pro-inflammatory factors also increases the formation of free radicals. The consequences of free radicals are lipid peroxidation, membrane damage, dysregulation of cellular processes and mutations of the genome8.
The retinal blood supply
Reflecting its embryological origins, the human retina has a dual blood supply. The photoreceptors, including their cell bodies in the outer nuclear layer and the majority of the outer plexiform layer, are supplied via the choriocapillaries. These vessels are richly anastamotic and correspond to the pia-arachnoid vessels in the rest of the brain. The inner retinal layers, such as the ganglion cell layer, are nourished by the central retinal artery (CRA), which arises from the ophthalmic artery in the region of the optic foramen, and which in turn originates directly from the internal carotid artery, proximal to the origin of the middle cerebral artery2. The CRA runs alongside the optic nerve, until approximately 10 mm from the globe of the eye, at which point it enters the optic nerve. Once it has entered the optic disc, the retinal arteries divide irregularly and dichotomously. The main retinal vessels form 2 capillary plexuses: the superficial capillary network, which is found within the nerve-fiber layer, and the deep capillary network lying between the boundary of the inner nuclear layer and the outer plexiform layer. Due to the connections in bloodflow between these 2 systems, complete retinal ischemia requires occlusion of the ophthalmic artery, the principal vessel supplying all vasculature of the retina2.
Animal models of retinal ischemia
A number of in vivo and ex vivo animal models have been developed to study retinal ischemia. In order to best extrapolate the data from animal model to human clinical situation, the model which most closely resembles human retinal ischemia is preferred. An immediately limiting factor to potential model suitability is the structure of the retinal vasculature in various species. While higher primates share virtually identical retinal vascular patterns with humans, financial and ethical considerations prohibit their widespread use11. More commonly seen are small rodent models, and of these the rat is the most similar to humans. The pattern of vascular supply in the rat retina is holangiotic, as in primates and humans, and this makes it a suitable candidate as a model for study12.
Certainly, differences do exist between rat and human retinas. The principal blood supply to the rat retina is via a single posterior ciliary artery which runs along the ventromedial aspect of the optic nerve, and which divides into three branches at the optic nerve head: a central retinal artery supplying the retina, and medial and lateral long retinal arteries supplying the choroid12. Complete transection of this vessel causes severe trauma and ocular inflammation, and widespread retinal infarction; permanent occlusion of this vessel does not correspond to CRA occlusion in humans, and even a temporary occlusion in rats probably causes more widespread injury. Hence, the degree of damage done in the process of a study must be taken into account, as it may not accurately reflect the degree of damage which occurs in the human eye. Previous work has found that in order to cause reproducible, irreversible and functional ischemic injury in the Wistar rat, at least 20 min of sustained ischemia are required4,13,14.
In order to induce retinal ischemia, several methods are currently used. The high IOP (Intraocular Pressure) model of ischemia is frequently used as it is fairly simple to administer and can be used as a model to study glaucomatous injury to the eye. The basic method for this technique consists of introducing sterile fluid into the vitreous chamber of the eye. The addition of liquid into the chamber increases the pressure within the eye and compresses the vasculature passing through the optic disc and supplying the retina . Blood contained within these vessels is thereby expelled, cutting off the supply to the retinal tissue15,16. Such an intervention can be accomplished via cannulation of the eye, which is then connected to an elevated pressure liquid reservoir, which increases IOP to the level of the reservoir.
Another common method of inducing retinal ischemia is vascular ligation. Carrying out this intervention requires surgical procedures very similar to those used for optic nerve transection, whereby the investigator dissects the contents of the ocular orbit to reach the optic nerve and administer the damage17,18. At its simplest, this method involves placing a suture around the optic nerve bundle, thereby ligating the posterior ciliary vessels. Due to the close association between the optic nerve and these vessels, selective compression of the vasculature is imperfect and it commonly causes the axons of the optic nerve to be compressed and damaged. A technically more demanding version of this intervention is to separate the optic nerve and posterior ciliary vessels, thereby freeing the vasculature to be ligated independently of the optic nerve. This method produces results more appropriate to retinal ischemia without the confounding effects of optic nerve damage19.
A relatively non-invasive method of retinal vessel occlusion involves the intravenous injection of Bengal Rose, a photoresponsive dye, followed by intense retinal illumination. The principle behind this method was introduced by Watson et al in 198520, when they proposed that by inducing a photochemical reaction within the vasculature, a thrombosis could be created. This method has since been used to study ischemic injury, particularly in the brain21, as well as in the retina22,23,24. There are several advantages to this method, namely that animal preparation does not require mechanical manipulation of vasculature or parenchyma. As well, the lesion size and location can be modulated by altering the irradiating intensity, duration of light exposure, beam position and dye concentration20,25. However, this method produces variable histologic injury, which is difficult to quantify26, and it may also cause secondary damage due to neurotoxicity in addition to ischemic damage25. It is also worth noting that it is not an ideal method for the study of ischemia/reperfusion injury as the lesion created is resistant to the reintroduction of blood flow25.
Molecular analysis of retinal ischemia
The intensity of damage which occurs due to an ischemic injury is critically dependent on the duration of the insult. Several prominent molecular changes are observed within the retina over the course of such an injury, altering the protein expression patterns of the cellular population. The resulting protein signatures have many similarities with those resulting from optic nerve crush or transection injuries27,28. One of the prominent molecular changes found in retinal ischemic injury, as well as optic nerve crush and transection, is the transient increase in growth-associated factor 43 (GAP-43)29. Specifically, after ischemic injury GAP-43 was found to be increased in retinal ganglion cells (RGCs) at 3 and 7 days following reperfusion30. GAP-43 is most recognized for its expression during CNS synaptogenesis: it is a membrane-associated protein which is up-regulated in neuronal growth cones, but which is down-regulated after synaptogenesis in almost all brain regions, except for those few which preserve plasticity31. Throughout the CNS, GAP-43 expression is increased in neurons with damaged axons32. In the retina, GAP-43 is normally localized to the inner plexiform layer due to its expression by RGCs and a subset of amacrine cells33, and its increased expression in response to ischemic injury suggests structural remodeling in the inner plexiform layer of the retina in order to preserve retinal function34.
Mechanisms of cell death during retinal ischemia
Following retinal ischemia, there are two modes of cell death which occur: necrosis and apoptosis35. Both are often found playing parts in insults to the CNS, and each has discrete biochemical and histological features35. Necrosis, long considered a form of caspase-independent cell death (CID), or “accidental” cell death, is the pathological process that occurs when cells are exposed to an extreme physical or chemical insult or any other serious disruption to their normal physiology35. It is characterized by a rounding of the cell, a gain in cell volume, mitochondrial swelling, dissolution of organelles, condensation of chromatin around the nucleus, and irreparable damage to the plasma membrane both by external influences and by the release of intracellular lytic enzymes in response to the insult36,37,38. The crux of necrotic damage appears to be a compromised plasma membrane due to ATP-mediated energy depletion. The process begins with an impairment of the cell's ability to maintain homeostasis, leading to the influx of water and extracellular ions, thus drastically altering intracellular ion concentrations and severely disrupting the ionic gradient which exists across the plasma membrane35. Intracellular organelles, most notably mitochondria, become inactive, and the entire cell becomes dysfunctional. Owing to all of these disruptions, the cell eventually lyses and the cytoplasmic contents, including lysozomal enzymes, are released into the extracellular space. As a result, necrotic cell death is often associated with extensive tissue damage and inflammation35. Recent investigations into the processes of necrosis, however, have yielded evidence indicating that at least a part of the damage attributable to this process may be executed by a mechanism termed “necroptosis”39,40.
Necroptosis is a recently discovered, caspase-independent form of regulated cell death. It shares morphological features with necrosis, such as membrane and organelle swelling followed by cell lysis, and is activated by death receptors such as TNF α, FasL, and TRAIL, the very same ligands which can activate the extrinsic apoptotic pathway39,40,41. Thus, the activation of these receptors may initiate alternative death pathways42,43,44. Research indicates that the key moderators between necrosis and apoptosis are receptor-interacting protein kinase 1 (RIPK1)39,45,46, and RIPK347,48,49,50,51. The ability of RIPK1 to switch between these 2 pathways appears to rest on its serine/threonine kinase activity; its activation is essential for the activation of necroptosis, but it is dispensable for both NF-κB activation and initiation of the apoptotic pathways45. The small molecule inhibitor necrostatin-1 (Nec-1), has been shown to be a potent inhibitor of RIPK1 and of necroptosis46,52. Treatment with Nec-1 has demonstrated a reduction in infarct volume in mouse models of middle cerebral artery (MCA) occlusion, suggesting the importance of necroptosis in CNS ischemic injury39. As well, recent work has supported the impact of necroptosis in the retina as Nec-1 treatment was able to attenuate retinal thinning and RGC loss after ischemic injury40.
While necrosis is more dominant in the ischemic core, apoptosis becomes more common in the penumbra as the cells are found further away from the core2. Apoptosis is a normal process during development, and is also a defense mechanism which occurs during immune reaction or when cells are damaged; it is the other primary method by which cells die during ischemia2,53. In contrast to the uncontrolled degeneration which occurs by necrosis, apoptosis is a strictly regulated process. It plays a significant role in both acute and chronic neurodegenerative conditions, such as glaucoma, retinitis pigmentosa, cataracts, and retinoblastoma36,54. Many studies have found that following ischemia-reperfusion injury, treatment with anti-apoptotic agents is effective at preserving cellular populations throughout the retina55. There are many distinct morphological changes which are common to apoptosis: early on there is a reduction in cell volume and chromatin condensation occurs, followed by extensive plasma membrane blebbing and the detachment of cell fragments to form apoptotic bodies56,57. Macrophages or microglia then engulf the apoptotic bodies and degrade them58. In contrast with necrosis and necroptosis, there is no inflammatory reaction associated with apoptosis because the degraded cells are quickly phagocytosed and therefore do not release their contents into the extracellular space, and there is no release of pro-inflammatory cytokines59,60.
There exist 2 main apoptotic pathways: the extrinsic death receptor pathway, and the intrinsic, or mitochondrial, pathway. Both are linked, and considerable interplay occurs between them61. There is also an additional pathway which relies on the infiltration of immune cells into the tissue undergoing apoptosis. In this final case, the granzyme and perforin released by the invading immune cells degrades the cellular proteins and chromatin62. Both the extrinsic and intrinsic pathways involve an energy-dependent cascade of molecular events which result in biochemical modifications throughout the cell, such as protein cross-linking, DNA breakdown and phagocytic breakdown63.
Caspases, a family of cysteine proteases, have been found to be major regulators in the degeneration of RGCs by apoptosis64,65,66. Initially, caspases are expressed as inactive proenzymes, which are cleaved into their active form. This allows them to in turn activate other caspases downstream, and thus initiate a protease cascade. Traditionally, the primary caspases involved in the apoptotic degeneration of RGCs after axotomy have been caspase-3 and caspase-967,68,69,70,71,72. While neither caspase-3 nor caspase-9 was found to be involved in axonal degeneration73,74, it now appears that caspase-6 and caspase-8 both play prominent roles in this process, as well as in RGC apoptosis75,76. As well, caspase-2 has recently been shown to be involved in RGC apoptosis following optic nerve damage, most likely at the stage of apoptosis initiation77. Once caspases have been initiated, there appears to be an irreversible commitment to cell death58. To date, 10 major caspases have been identified and broadly categorized into initiator caspases (caspase-2, -8, -9, and -10), executioner caspases (caspase-3, -6, and -7), and inflammatory caspases (caspase-1, -4, and -5)78,79.
The extrinsic pathway of apoptosis involves transmembrane receptor-mediated interactions in order to initiate apoptosis. Most notably, it involves receptors that are part of the TNF superfamily80. Members of the TNF superfamily share a similar cysteine-rich intracellular domain, called the “death domain”, which is essential for transmitting the death signal to intracellular pathways81. So far, the majority of the research into this pathway's ligand/receptor combinations has been directed towards FasL/FasR, TNFα/TNFR1, Apo3L/DR3, Apo2L/DR4, and Apo2L/DR582,83,84,85,86.
The extrinsic phase of apoptosis is defined by the binding between these cell-surface receptors and their specific ligand. There is clustering of the receptors, and through this, cytoplasmic adapter proteins exhibiting the corresponding death domains are recruited. Among the recruited proteins are FADD, TRADD, RIP, and pro-caspase-887. The resulting structure is termed the death-inducing signaling complex (DISC), and results in the autocatalytic activation of pro-caspase-8, and the triggering of the execution phase of apoptosis88. At this point, there is also a regulator of the process, the protein c-FLIP, which will bind to FADD and caspase-8, rendering them ineffective89,90.
Activation of apoptosis by TNFα is a 2 step process. Under apoptosis-competent conditions, TNFα stimulation sequentially induces the formation of 2 protein complexes, complex I and complex IIa, which stimulate NF-κB activation and apoptosis, respectively91,92. Initially, TNFα binding to the TNF receptor 1 (TNFR1) induces the recruitment of TRADD, RIP1 and TRAF2 to the receptor's intracellular domain, thus forming complex I91. Subsequently, these RIP1 and TRADD undergo posttranslational modifications and the entire complex I dissociates from TNFR191. The addition of Fas-associated death domain (FADD) and caspase-8 forms complex IIa. The signal which stimulates the transition from complex I to complex IIa is currently unclear, however, it is known that an alternate multiprotein aggregate, Complex IIb, may form in apoptosis-deficient conditions, particularly in the presence of caspase-8 inhibitors. This contains at least one additional component: RIPK3. Interaction between RIPK1 and RIPK3 plays a critical role in mediating downstream apoptotic events93. Treatment with Nec-1 prevents recruitment of both RIPK1 and RIPK3 to Complex IIb, indicating the importance of RIPK1 in the apoptotic process47,48.
The intrinsic pathway involves a diverse array of non-receptor mediated stimuli that produce intracellular signals that act directly on intracellular targets, most notably mitochondria. This results in the opening of the mitochondrial permeability transition pore, loss of mitochondrial transmembrane potential, and the release of pro-apoptotic proteins which are normally sequestered within the mitochondria94. Among these proteins are cytochrome c, Smac/DIABLO, and HtrA2/Omi, which activate the caspase-dependent mitochondrial pathways95. Other pro-apoptotic proteins released by the mitochondria are AIF, endonuclease G, and CAD, however this only occurs once the cell is irrevocably committed to die58.
The regulation of these pro-apoptotic mitochondrial events is through members of Bcl-2 family of proteins96. This family of proteins regulates mitochondrial membrane permeability. They can be either pro-apoptotic or anti-apoptotic, and therefore have a crucial role to play as they can either enhance the signals inducing cell death, or inhibit them.
Preserved retinal function by neurotrophic factors
Neurotrophic factors are recognized as playing key roles in the development and survival of tissues within both the central and peripheral nervous system2. As well, they play an important role in countering the complex mechanisms of apoptotic neuron death in the retina97,98,99. Several investigations have been carried out to determine the efficacy of growth factors in promoting survival following ischemic injury in the retina. Recently, bFGF (basic fibroblast growth factor) has been found to support neuronal survival and promote neurite outgrowth, as well as play an essential role in the maintenance of neurons within the spinal cord and cerebral cortex100. The factor bFGF has previously been found to induce both mesodermal and neuroectodermal tissue regeneration, as well as induce the outgrowth of fibers in cultured retinal ganglion cells in cultured RGCs101, and it has also been shown to delay the degeneration of rat photoreceptors102. It has also been demonstrated that bFGF is effective at rescuing RGCs, as well as other cellular populations in the retina from ischemic injury induced by elevated intraocular pressure103. Other neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) have also been studied and have been found to protect the retina from pressure-induced ischemic injury104.
Another neurotrophic factor that has protective effects following retinal ischemia is glial cell line-derived neurotrophic factor (GDNF). In retinas subjected to ischemic injury, intraocular administration of virus encoding recombinant GDNF has yielded a preservation of retinal thickness, indicating that cellular populations were better preserved; and specifically, it was found that RGCs survived in greater numbers. In agreement with these findings, eyes receiving increased GDNF retained more functionality following ischemic injury, as measured by electroretinogram105.
Neurturin (NRTN) is a member of the GDNF family of ligands. It is one, among many, factors which interacts with members of the GDNF family of receptors (GFRαs), and activates intracellular signaling via the Ret receptor tyrosine kinase106. The Ret receptor tyrosine kinase then activates essential pro-survival intracellular signaling pathways such as the mitogen activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)-Akt, and phospholipase Cγ pathways107. It has been shown that GFL-mediated Ret signaling plays a critical role in the maintenance of multiple central and peripheral neurons108, and that within the retina, it is expressed in the ganglion cell layer and inner nuclear layer109. Through these studies, it was shown that NRTN, and the activity it induces in Ret, are important for the normal function of the retina, specifically for its actions on horizontal cells, amacrine cells, and RGCs106.
Many studies have concentrated on the efficacy of BDNF in promoting the survival of RGCs following injury to the retina or optic nerve, and in using it to develop therapeutic strategies for ocular diseases110. So far, research has indicated that, among all the neurotrophic factors, BDNF is the most effective at directing injured RGCs towards survival, a fact attributed to the high levels of expression of tyrosine receptor kinase B (TrkB; the BDNF receptor) which is expressed by these cells111,112,113. BDNF was found to be beneficial to RGCs in several injury models, including in promoting survival both in vitro and in vivo114,115, and in stimulating the growth of regenerating neurites116. Intravitreal injections of BDNF have proven to slow the loss of RGCs in a rat chronic hypertension model117, and to support the survival of retinal ganglion cells for up to 1 week following axotomy112,118. Most recently, BDNF was incorporated into rat mesenchymal stem cells (rMSCs), which were then administered to the retina following axotomy or increased intraocular pressure119. In both experimental models, levels of BDNF were successfully increased by the treatment, and surviving RGC populations were significantly larger. Whether this effect could be increased by also increasing expression of TrkB in the target cell population remains to be seen, however it has been shown that following axotomy, combined upregulation of BDNF along with TrkB does have additive effects on retinal ganglion cell survival120.
The neurotrophic factor Nerve Growth Factor (NGF) has been shown to be present normally within the eye, along with its receptors TrkA and p75. All 3 factors have, in fact, been shown to be expressed by the rat lacrimal gland tissue121,122 in vivo, and in vitro it has been shown that conjunctival cells (epithelial cells, goblet cells, immune cells and fibroblasts) all produce, store, release, and utilize NGF. The diverse activity of NGF appears to modulate the activity of these cells, and therefore affect the secretion of cytokines and other growth factors123,124,125. Due to its wide-ranging effects, NGF is believed to be implicated in a variety of ocular diseases126. As well, it has previously been shown that NGF is able to enhance RGC survival following optic nerve transection115, as well as following ischemic insult. In the latter situation, NGF was also able to promote the functional recovery of RGCs127. More recently, studies have demonstrated that in a model of elevated intraocular pressure, NGF is effective at preventing RGC death in a rat model128. While this study used elevated intraocular pressure to mimic the hallmark symptom of glaucoma, it must also be noted that increased intraocular pressure also reduces blood-flow to the retina by increasing pressure on retina vasculature. Therefore, it may be the case that some of the effects of NGF are due to its impact on ischemic injury. NGF has also been examined in conjunction with novel neuroprotective strategies to combat ischemia-induced excitotoxicity129. It was found that the neurosteroid dehydroepiandrosterone (DHEA) is able to protect RGCs from excitotoxicity, a main mechanism by which cells die as a result of ischemia4. The results also showed that DHEA works via the NGF/TrkA pathway to promote RGC survival via a cascade of events which are as yet unclear129.
Neurotrophin-3 (NT-3) is a neurotrophin which controls neuronal survival in both the peripheral and central nervous systems130. It, along with BDNF and NGF, is recognized as one of the principal neurotrophic factors in the central nervous system; the principal ligand is TrkC131. Currently, evidence points towards TrkC actually inducing apoptosis in the absence of NT-3, as the lack of its binding partner allows the intracellular domain of TrkC to become susceptible to cleavage by locally activated caspases, especially caspase-9132,133. Neurotrophins, including BDNF, GDNF and NT-3, are known to be shuttled anterogradely in RGC axons134. During ischemia, it is possible that the drop in oxygen supplied to neurons can result in a lack of ATP, which can induce a loss of the cell's ability to maintain its axonal transport system135, and this loss of pro-survival signaling by neurotrophic factors will induce apoptosis136,137. Recent work in cerebral ischemia has shown that increased levels of NT-3 improve cell survival and neurological status following transient middle cerebral artery occlusion by reducing the initial damage caused by the ischemic event138.
Neurotrophin 4 (NT-4) is also commoly known as Neurotrophin-5 (NT-5), or as NT-4/598,139. NT-4/5 forms part of the complex network of growth factors, along with BDNF, NGF, and NT-3, of retrogradely transported neurotrophins which orchestrate the generation and maintenance of neuron populations131. NT-4/5 binds selectively to the cellular receptor TrkB131,140. NT-4/5 is known to have roles in the stimulation of GAP-43 and T-α1-tubulin to induce axon regeneration139. More recent work has also shown that, along with promoting the regeneration of axons, NGF, BDNF and NT-4/5 all play a role in the formation of dendrites, and the establishment of synapses within the sympathetic ganglia141. In support of this, transgenic mice with a knockout of NT-4/5 showed a marked decrease in axon elongation during regeneration142,143. Studies of this factor have demonstrated that it does command some neuroprotective abilities, such as when it is administered to rubrospinal tract neurons following cervical axotomy139. As well, treatment with NT-4/5 has been shown to reduce infarct size in rats with middle cerebral artery occlusion144, however, unlike BDNF, it was unable to prolong survival of damaged RGCs over a prolonged period145.
Ciliary Neurotrophic Factor (CNTF) is known to play an essential, cooperative role in motoneuron survival and function146. It is expressed almost exclusively within the nervous system, however at much higher levels within the PNS than in the CNS, and in the latter is produced mostly by astrocytes147,148,149. It has demonstrated protective abilities in multiple sclerosis150, and has been previously used in a therapeutic trial for the treatment of motor neuron disease and amyotrophic lateral sclerosis151,152. CNTF, along with other neurotrophic factors such as GDNF and BDNF, has become widely recognized for its capacity to rescue RGCs following a variety of different lesions, such as ischemia, traumatic, or metabolic injury153,154,155. In addition to its anti-apoptotic effects within the retina, CNTF has been established to have regeneration-promoting properties, as it appears to stimulate neurogenesis when adenovirally delivered to injured RGCs156,157. More recently, CNTF gene transfer via adeno-associated virus (AAV) has been found to protect RGCs in the rat from a variety of acute ischemia models158. Recent work indicates that expression of CNTF by CNS astroglia may depend on the contact between astroglial cells and neurons: binding of astroglial integrin receptors would suppress CNTF expression, while loss of this contact would induce its production159.
Insulin growth factor (IGF) is found both systemically and in the CNS. Most studies on its effects have been conducted systemically, and it has been found to inhibit apoptosis in various cell types such as cardiomyocytes160,161,162. While it is acknowledged that the intracellular pathways activated by IGF may vary based on cell type and applied stress163, its activity appears to involve both the Erk164,165, and PI3K/Akt pathways166,167. Current research also suggests that the IGF-1 pathway is a promising avenue for therapeutics to improve repair after ischemia/reperfusion events in cardiac tissue163, raising the possibility of potential applications regarding retinal ischemia. Supporting this perspective is work showing that IGF contributes to retinal neovascularization following diabetic retinopathy168.
Epidermal Growth Factor (EGF) binds to EGFR to induce cellular proliferation, differentiation and survival169. The binding of EGF to EGFR has been shown to have a major impact on determining pluripotent stem cell fate within the retina170. More recent work has further examined this concept and has found that in zebrafish, heparin-binding epidermal-like growth factor (HB-EGF) is necessary and sufficient to induce the dedifferentiation of Mueller glia into multipotent progenitors capable of regenerating other cell types within the retina171. Studies on de-differentiation of Mueller glia in mammals, however, are not able to produce cells which can then re-differentiate into any cell type, only into myelinating oligodendrocytes172. Further work has also suggested that p53 plays a role in the limited ability of these “false MSCs”, halting them from fully re-entering the mitotic cycle173. However, recent studies indicate that EGF may have a function in aiding RGC survival; it has been found that Nell2, an EGF-related gene, supports RGC survival after optic nerve injury174.
Vascular endothelial growth factor (VEGF) exists as several isoforms, VEGF-A, -B, -C, and -D; the most well characterized of which is VEGF-A175. Administration of VEGF-A has been shown to enhance the formation of blood vessels following traumatic and hypoxic damage176,177.As well, previous studies have shown neuroprotective and neuroproliferative properties for VEGF-A175,178,179,180. The major anti-apoptotic pathways activated by VEGF-A are the MAPK and PI3K pathways181. It is important to note that in order to achieve this protection, VEGF-A splice variants must be expressed at biological ratios, as improper ratios result in hyper-permeable vasculature and increased edema182,183. Doing so, it has been shown that an increase of VEGF-A enhances recovery after spinal cord compression injury40 and increases RGC survival in the retina after ischemic injury175.
Excitotoxicity and ischemic injury in the retina
In the normal physiological state, neurotransmitters are only found at very low levels in the extracellular space. This is due to the restricted ion gradient maintained across the neuronal membrane, as well as the efficient and effective removal of neurotransmitters from the synaptic clefts by glia. During retinal ischemia, however, the concentration of neurotransmitters, notably glutamine, in the extracellular space increases dramatically184,185. It is also worth noticing that during reperfusion, the increased levels of neurotransmitters in the extracellular space will activate their receptors and contribute to the death of RGCs186.
While glutamate has long been recognized as the major excitatory neurotransmitter in the CNS, it has also been notable for its ability to kill neurons under certain conditions187,188. This ability has been attributed to the large presence of NMDA receptors on susceptible cells, such as RGCs. However, there has been work suggesting that excitotoxicity following ischemia does not directly affect RGCs after all189. Rather, this work suggests that amacrine cells would be the likely target for this type of degeneration, and that is the loss of these cells which indirectly leads to the degeneration of the RGC population.
Regardless of whether or not excitotoxicity is directly responsible for RGC loss during ischemic injury, it must be acknowledged that it does play a role in retinal degeneration. New work has recently proposed that it participates in a complex interplay along with oxidative stress and the disruption of mitochondrial dynamics all leading towards retinal degeneration190. Despite the complexity of this interplay leading towards cell death, recent publications have suggested possible treatments to offer neuroprotection against it. Work on opioids has shown that depending on the duration and severity of the ischemic challenge, they may counter the effects of inflammatory cytokines, such as TNF-α, and glutamate. This protection would be based on the activation of the PKC, ERK, and PI3K/Akt pro-survival pathways191.
Ultimately, the end result of glutamate excitotoxicity which would lead to cell death is the imbalance of intracellular gradients of ions such as Ca2+, K+, Na+, and Cl−192. It is commonly believed that Ca2+, particularly, is a major mediator of neuronal death193,194. During ischemic injury, the intracellular concentration of Ca2+ increases. There appear to be several mechanisms which contribute to this, including the opening of receptor-operated, and voltage-operated Ca2+ channels, efflux from intracellular stores, and a breakdown of Ca2+ buffering mechanisms195,196,197,198. One process which has been hypothesized to help in the prevention of cell death due to Ca2+ de-regulation is preconditioning. Preconditioning is accomplished by introducing small amounts of the supposed stressor to a group of cells prior to an insult199. It has been shown that preconditioning is an effective method of preventing cell death during hypoxic and ischemic insults in liver tissue200, as well as in myocardial tissue201. As well, the effects of drug-induced preconditioning against NMDA or glutamate were shown to induce neuroprotection in rat hippocampal tissue202. More recent work has followed up on the hypotheses that acetylcholine (ACh) and nicotine may be neuroprotective against excitotoxicity in the retina199,203,204. Work on the neuroprotective abilities of ACh and nicotine indicate that it hinges on their activation of nAChRs, which in turn activates the pro-survival PI3K/Akt, Bcl-2, NF-κB, and MAPK pathways205.
One of the hallmarks of the apoptotic response is cell shrinkage57,206. Potassium has received considerable attention for its role in this aspect of apoptosis as it is the most abundant and osmotically important cation within the cell207. In most mammalian cells, there is a high concentration of K+ within and high concentration of Na+ without, thus a gradient exists for the loss of intracellular K+ and the gain of extracellular Na+. This gradient is maintained by various channels and ionic transporters which cross the cell membrane, most notably the Na+/K+ ATPase208. Research has revealed that the loss of cell volume during apoptosis is largely dependent on a loss of intracellular K+209,210.
A large family of voltage-gated potassium channels (Kv) have been found in mammalian brains. They are involved in the mediation of K+ efflux upon membrane depolarization, and have been shown to play a role in mediating apoptotic cell death; of these, the most prominent appear to be Kv1.1, Kv1.3, and Kv2.1211,212,213. Inhibition of Kv1.1 and Kv1.3 by use of siRNAs was able to successfully inhibit their expression, and protect RGCs from apoptosis in optic nerve transection. As well, it was noted that Kv1.1 depletion increased levels of the antiapoptotic gene Bcl-XL, whereas depletion of Kv1.3 reduced expression of caspase-3, caspase-9 and Bad, all of which are pro-apoptotic211,212.
Kv2.1 expression has also been shown to increase following damage, but before the appearance of apoptosis208,214. Inhibition of Kv2.1 has been shown to inhibit apoptosis in vitro215, as well as in vivo216. Following cerebral ischemia, it was also found that Kv2.1 participates in promoting apoptosis217.
Conclusions
Ischemia in the CNS is undoubtedly a complex problem with many facets. It is made all the more challenging to study due to the nature of CNS tissues, which are difficult to reach and to submit to consistent injury and treatment. The retinal model of ischemic injury addresses these problems as the retina is much more accessible than other CNS tissues and yet conserves the features that characterize neuron degeneration. The retina develops from the diencephalon, and so remains part of the CNS; retinal ischemia activates the same pathways as ischemic injury in other CNS areas, and can therefore offer strong evidence regarding the pathological processes following injury. This makes the study of retinal ischemia useful for discovering the ways in which ocular diseases such as glaucoma and diabetic retinopathy affect retinal cell populations, but also for building our knowledge of the processes of ischemic damage in other CNS areas. Much has already been discovered using the model of retinal ischemia, and its continued use will only serve to increase our understanding of ischemic injury and further characterize the complex cascade of processes and factors involved.
References
van der Worp HB, van Gijn J . Clinical practice. Acute ischemic stroke. N Engl J Med 2007; 357: 572–9.
Muthaian R, Minhas G, Anand A . Pathophysiology of stroke and stroke-induced retinal ischemia: emerging role of stem cells. J Cell Physiol 2012; 227: 1269–79.
Tso MO, Jampol LM . Pathophysiology of hypertensive retinopathy. Ophthalmology 1982; 89: 1132–45.
Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J . Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004; 23: 91–147.
Baker ML, Hand PJ, Wang JJ, Wong TY . Retinal signs and stroke: revisiting the link between the eye and brain. Stroke 2008; 39: 1371–9.
Durukan A, Tatlisumak T . Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007; 87: 179–97.
McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM . Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke 2009; 40: 3864–8.
Dirnagl U, Iadecola C, Moskowitz MA . Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22: 391–7.
Lo EH, Dalkara T, Moskowitz MA . Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399–415.
Mergenthaler P, Dirnagl U, Meisel A . Pathophysiology of stroke: lessons from animal models. Metab Brain Dis 2004; 19: 151–67.
Minhas G, Morishita R, Anand A . Preclinical models to investigate retinal ischemia: advances and drawbacks. Front Neurol 2012; 3: 75.
Sugiyama K, Gu ZB, Kawase C, Yamamoto T, Kitazawa Y . Optic nerve and peripapillary choroidal microvasculature of the rat eye. Invest Ophthalmol Vis Sci 1999; 40: 3084–90.
Foulds WS, Johnson NF . Rabbit electroretinogram during recovery from induced ischaemia. Trans Ophthalmol Soc U K 1974; 94: 383–93.
Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, Ruiz-Gomez JM, Vidal-Sanz M . Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci 1996; 37: 2002–14.
Smith GG, Baird CD . Survival time of retinal cells when deprived of their blood supply by increased intraocular pressure. Am J Ophthalmol 1952; 35: 133–6.
Buchi ER, Suivaizdis I, Fu J . Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica 1991; 203: 138–47.
Magharious MM, D'Onofrio PM, Koeberle PD . Methods for experimental manipulations after optic nerve transection in the Mammalian CNS. J Vis Exp 2011; 51: e2261.
Magharious MM, D'Onofrio PM, Koeberle PD . Optic nerve transection: a model of adult neuron apoptosis in the central nervous system. J Vis Exp 2011; 51: e2241.
Gehlbach P, Purple RL . Enhancement of retinal recovery by conjugated deferoxamine after ischemia-reperfusion. Invest Ophthalmol Vis Sci 1994; 35: 669–76.
Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD . Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol 1985; 17: 497–504.
Schmidt A, Hoppen M, Strecker JK, Diederich K, Schabitz WR, Schilling M, et al. Photochemically induced ischemic stroke in rats. Exp Transl Stroke Med 2012; 4: 13.
Mosinger JL, Price MT, Bai HY, Xiao H, Wozniak DF, Olney JW . Blockade of both NMDA and non-NMDA receptors is required for optimal protection against ischemic neuronal degeneration in the in vivo adult mammalian retina. Exp Neurol 1991; 113: 10–7.
Romano C, Price M, Bai HY, Olney JW . Neuroprotectants in Honghua: glucose attenuates retinal ischemic damage. Invest Ophthalmol Vis Sci 1993; 34: 72–80.
Daugeliene L, Niwa M, Hara A, Matsuno H, Yamamoto T, Kitazawa Y, et al. Transient ischemic injury in the rat retina caused by thrombotic occlusion-thrombolytic reperfusion. Invest Ophthalmol Vis Sci 2000; 41: 2743–7.
Macrae IM . Preclinical stroke research — advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol 2011; 164: 1062–78.
Buchi ER, Lam TT, Suvaizdis I, Tso MO . Injuries induced by diffuse photodynamic action in retina and choroid of albino rats. Morphologic study of an experimental model. Retina 1994; 14: 370–8.
Hollander A, D'Onofrio PM, Magharious MM, Lysko MD, Koeberle PD . Quantitative retinal protein analysis after optic nerve transection reveals a neuroprotective role for hepatoma-derived growth factor on injured retinal ganglion cells. Invest Ophthalmol Vis Sci 2012; 53: 3973–89.
Magharious M, D'Onofrio PM, Hollander A, Zhu P, Chen J, Koeberle PD . Quantitative iTRAQ analysis of retinal ganglion cell degeneration after optic nerve crush. J Proteome Res 2011; 10: 3344–62.
Coblentz FE, Radeke MJ, Lewis GP, Fisher SK . Evidence that ganglion cells react to retinal detachment. Exp Eye Res 2003; 76: 333–42.
Ju WK, Gwon JS, Park SJ, Kim KY, Moon JI, Lee MY, et al. Growth-associated protein 43 is up-regulated in the ganglion cells of the ischemic rat retina. Neuroreport 2002; 13: 861–5.
Oestreicher AB, De Graan PN, Gispen WH, Verhaagen J, Schrama LH . B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system. Prog Neurobiol 1997; 53: 627–86.
Frey D, Laux T, Xu L, Schneider C, Caroni P . Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J Cell Biol 2000; 149: 1443–54.
Ekstrom P, Johansson K . Differentiation of ganglion cells and amacrine cells in the rat retina: correlation with expression of HuC/D and GAP-43 proteins. Brain Res Dev Brain Res 2003; 145: 1–8.
Isenmann S, Kretz A, Cellerino A . Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res 2003; 22: 483–543.
Chu D, Qiu J, Grafe M, Fabian R, Kent TA, Rassin D, et al. Delayed cell death signaling in traumatized central nervous system: hypoxia. Neurochem Res 2002; 27: 97–106.
Graham SH, Chen J . Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 2001; 21: 99–109.
Kaushik S, Pandav SS, Ram J . Neuroprotection in glaucoma. J Postgrad Med 2003; 49: 90–5.
Vanlangenakker N, Vanden Berghe T, Vandenabeele P . Many stimuli pull the necrotic trigger, an overview. Cell Death Differ 2012; 19: 75–86.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–9.
Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res 2010; 88: 1569–76.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008; 135: 1311–23.
Yuan J, Kroemer G . Alternative cell death mechanisms in development and beyond. Genes Dev 2010; 24: 2592–602.
Festjens N, Vanden Berghe T, Vandenabeele P . Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta 2006; 1757: 1371–87.
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G . Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11: 700–14.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 2000; 1: 489–95.
Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008; 4: 313–21.
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137: 1112–23.
He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009; 137: 1100–11.
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325: 332–6.
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471: 363–7.
Upton JW, Kaiser WJ, Mocarski ES . Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 2010; 7: 302–13.
Teng X, Degterev A, Jagtap P, Xing X, Choi S, Denu R, et al. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg Med Chem Lett 2005; 15: 5039–44.
Norbury CJ, Hickson ID . Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol 2001; 41: 367–401.
Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, et al. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci 1999; 19: 3809–17.
Lam TT, Abler AS, Tso MO . Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Invest Ophthalmol Vis Sci 1999; 40: 967–75.
Hacker G . The morphology of apoptosis. Cell Tissue Res 2000; 301: 5–17.
Kerr JF, Wyllie AH, Currie AR . Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–57.
Elmore S . Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35: 495–516.
Savill J, Fadok V . Corpse clearance defines the meaning of cell death. Nature 2000; 407: 784–8.
Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y . Silent cleanup of very early apoptotic cells by macrophages. J Immunol 2003; 171: 4672–9.
Igney FH, Krammer PH . Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2002; 2: 277–88.
Martinvalet D, Zhu P, Lieberman J . Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 2005; 22: 355–70.
Hengartner MO . The biochemistry of apoptosis. Nature 2000; 407: 770–6.
Baumgartner R, Meder G, Briand C, Decock A, D'Arcy A, Hassiepen U, et al. The crystal structure of caspase-6, a selective effector of axonal degeneration. Biochem J 2009; 423: 429–39.
Inoue S, Browne G, Melino G, Cohen GM . Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ 2009; 16: 1053–61.
Yi CH, Yuan J . The Jekyll and Hyde functions of caspases. Dev Cell 2009; 16: 21–34.
Kermer P, Klocker N, Labes M, Bahr M . Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci 1998; 18: 4656–62.
Kermer P, Klocker N, Bahr M . Long-term effect of inhibition of ced 3-like caspases on the survival of axotomized retinal ganglion cells in vivo. Exp Neurol 1999; 158: 202–5.
Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M . Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res 2000; 85: 144–50.
Chaudhary P, Ahmed F, Quebada P, Sharma SC . Caspase inhibitors block the retinal ganglion cell death following optic nerve transection. Brain Res Mol Brain Res 1999; 67: 36–45.
Weishaupt JH, Diem R, Kermer P, Krajewski S, Reed JC, Bahr M . Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis 2003; 13: 124–35.
Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, et al. Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci 2004; 25: 38–93.
Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 1996; 384: 368–72.
Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC . Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci 2000; 20: 1333–41.
Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M . APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 2009; 457: 981–9.
Monnier PP, D'Onofrio PM, Magharious M, Hollander AC, Tassew N, Szydlowska K, et al. Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J Neurosci 2011; 31: 10494–505.
Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis 2011; 2: e173.
Rai NK, Tripathi K, Sharma D, Shukla VK . Apoptosis: a basic physiologic process in wound healing. Int J Low Extrem Wounds 2005; 4: 138–44.
Cohen GM . Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16.
Locksley RM, Killeen N, Lenardo MJ . The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001; 104: 487–501.
Ashkenazi A, Dixit VM . Death receptors: signaling and modulation. Science 1998; 281: 1305–8.
Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 1997; 272: 32401–10.
Marsters SA, Sheridan JP, Pitti RM, Brush J, Goddard A, Ashkenazi A . Identification of a ligand for the death-domain-containing receptor Apo3. Curr Biol 1998; 8: 525–8.
Peter ME, Krammer PH . Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol 1998; 10: 545–51.
Suliman A, Lam A, Datta R, Srivastava RK . Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene 2001; 20: 2122–33.
Rubio-Moscardo F, Blesa D, Mestre C, Siebert R, Balasas T, Benito A, et al. Characterization of 8p21.3 chromosomal deletions in B-cell lymphoma: TRAIL-R1 and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood 2005; 106: 3214–22.
Wajant H . The Fas signaling pathway: more than a paradigm. Science 2002; 296: 1635–6.
Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 1995; 14: 5579–88.
Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, et al. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol 1998; 161: 3936–42.
Scaffidi C, Schmitz I, Krammer PH, Peter ME . The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 1999; 274: 1541–8.
Micheau O, Tschopp J . Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003; 114: 181–90.
Declercq W, Vanden Berghe T, Vandenabeele P . RIP kinases at the crossroads of cell death and survival. Cell 2009; 138: 229–32.
Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM . Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 2002; 277: 9505–11.
Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P . Toxic proteins released from mitochondria in cell death. Oncogene 2004; 23: 2861–74.
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G . Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 2006; 13: 1423–33.
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–56.
Huang EJ, Reichardt LF . Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001; 24: 677–736.
Lewin GR, Barde YA . Physiology of the neurotrophins. Annu Rev Neurosci 1996; 19: 289–317.
Sofroniew MV, Howe CL, Mobley WC . Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001; 24: 1217–81.
Westermann R, Grothe C, Unsicker K . Basic fibroblast growth factor (bFGF), a multifunctional growth factor for neuroectodermal cells. J Cell Sci Suppl 1990; 13: 97–117.
Baehr M, Bunge RP . Functional status influences the ability of Schwann cells to support adult rat retinal ganglion cell survival and axonal regrowth. Exp Neurol 1989; 106: 27–40.
Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM . Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 1990; 347: 83–6.
Zhang C, Takahashi K, Lam TT, Tso MO . Effects of basic fibroblast growth factor in retinal ischemia. Invest Ophthalmol Vis Sci 1994; 35: 3163–8.
Unoki K, LaVail MM . Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci 1994; 35: 907–15.
Wu WC, Lai CC, Chen SL, Sun MH, Xiao X, Chen TL, et al. GDNF gene therapy attenuates retinal ischemic injuries in rats. Mol Vis 2004; 10: 93–102.
Brantley MA Jr, Jain S, Barr EE, Johnson EM Jr, Milbrandt J . Neurturin-mediated ret activation is required for retinal function. J Neurosci 2008; 28: 4123–35.
Baloh RH, Enomoto H, Johnson EM Jr, Milbrandt J . The GDNF family ligands and receptors-implications for neural development. Curr Opin Neurobiol 2000; 10: 103–10.
Sariola H, Saarma M . Novel functions and signalling pathways for GDNF. J Cell Sci 2003; 116: 3855–62.
Harada C, Harada T, Quah HM, Maekawa F, Yoshida K, Ohno S, et al. Potential role of glial cell line-derived neurotrophic factor receptors in Muller glial cells during light-induced retinal degeneration. Neuroscience 2003; 122: 229–35.
Dawbarn D, Allen SJ . Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol 2003; 29: 211–30.
Jelsma TN, Friedman HH, Berkelaar M, Bray GM, Aguayo AJ . Different forms of the neurotrophin receptor trkB mRNA predominate in rat retina and optic nerve. J Neurobiol 1993; 24: 1207–14.
Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ . Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A 1994; 91: 1632–6.
Perez MT, Caminos E . Expression of brain-derived neurotrophic factor and of its functional receptor in neonatal and adult rat retina. Neurosci Lett 1995; 183: 96–9.
Thanos S, Vanselow J . The effect of central and peripheral neuroglia on the regeneration of the optic nerve. Fortschr Ophthalmol 1989; 86: 172–5.
Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C . Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci 1989; 9: 1263–72.
Cohen-Cory S, Fraser SE . Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature 1995; 378: 192–6.
Ko ML, Hu DN, Ritch R, Sharma SC, Chen CF . Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett 2001; 305: 139–42.
Clarke DB, Bray GM, Aguayo AJ . Prolonged administration of NT-4/5 fails to rescue most axotomized retinal ganglion cells in adult rats. Vision Res 1998; 38: 1517–24.
Park HY, Kim JH, Sun Kim H, Park CK . Stem cell-based delivery of brain-derived neurotrophic factor gene in the rat retina. Brain Res 2012; 1469: 10–23.
Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A . TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci 2002; 22: 3977–86.
Ghinelli E, Johansson J, Rios JD, Chen LL, Zoukhri D, Hodges RR, et al. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Invest Ophthalmol Vis Sci 2003; 44: 3352–7.
Muzi S, Colafrancesco V, Sornelli F, Mantelli F, Lambiase A, Aloe L . Nerve growth factor in the developing and adult lacrimal glands of rat with and without inherited retinitis pigmentosa. Cornea 2010; 29: 1163–8.
Lambiase A, Micera A, Sgrulletta R, Bonini S . Nerve growth factor and the immune system: old and new concepts in the cross-talk between immune and resident cells during pathophysiological conditions. Curr Opin Allergy Clin Immunol 2004; 4: 425–30.
Lambiase A, Micera A, Pellegrini G, Merlo D, Rama P, De Luca M, et al. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Invest Ophthalmol Vis Sci 2009; 50: 4622–30.
Micera A, Lambiase A, Puxeddu I, Aloe L, Stampachiacchiere B, Levi-Schaffer F, et al. Nerve growth factor effect on human primary fibroblastic-keratocytes: possible mechanism during corneal healing. Exp Eye Res 2006; 83: 747–57.
Lambiase A, Mantelli F, Sacchetti M, Rossi S, Aloe L, Bonini S . Clinical applications of NGF in ocular diseases. Arch Ital Biol 2011; 149: 283–92.
Siliprandi R, Canella R, Carmignoto G . Nerve growth factor promotes functional recovery of retinal ganglion cells after ischemia. Invest Ophthalmol Vis Sci 1993; 34: 3232–45.
Colafrancesco V, Coassin M, Rossi S, Aloe L . Effect of eye NGF administration on two animal models of retinal ganglion cells degeneration. Ann Ist Super Sanita 2011; 47: 284–9.
Kokona D, Charalampopoulos I, Pediaditakis I, Gravanis A, Thermos K . The neurosteroid dehydroepiandrosterone (DHEA) protects the retina from AMPA-induced excitotoxicity: NGF TrkA receptor involvement. Neuropharmacology 2012; 62: 2106–17.
Frade JM, Bovolenta P, Rodriguez-Tebar A . Neurotrophins and other growth factors in the generation of retinal neurons. Microsc Res Tech 1999; 45: 243–51.
Ichim G, Tauszig-Delamasure S, Mehlen P . Neurotrophins and cell death. Exp Cell Res 2012; 318: 1221–8.
Tauszig-Delamasure S, Yu LY, Cabrera JR, Bouzas-Rodriguez J, Mermet-Bouvier C, Guix C, et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci U S A 2007; 104: 13361–6.
Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L, et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature 2010; 467: 59–63.
von Bartheld CS, Wang X, Butowt R . Anterograde axonal transport, transcytosis, and recycling of neurotrophic factors: the concept of trophic currencies in neural networks. Mol Neurobiol 2001; 24: 1–28.
Su KG, Banker G, Bourdette D, Forte M . Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Curr Neurol Neurosci Rep 2009; 9: 411–7.
Caleo M, Medini P, von Bartheld CS, Maffei L . Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J Neurosci 2003; 23: 287–96.
Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, et al. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci 1998; 18: 2808–21.
Zhang J, Shi Q, Yang P, Xu X, Chen X, Qi C, et al. Neuroprotection of neurotrophin-3 against focal cerebral ischemia/reperfusion injury is regulated by hypoxia-responsive element in rats. Neuroscience 2012; 222: 1–9.
Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W . BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci 1997; 17: 9583–95.
Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A . Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron 1991; 7: 857–66.
Glebova NO, Ginty DD . Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci 2004; 24: 743–51.
English AW, Meador W, Carrasco DI . Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci 2005; 21: 2624–34.
English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A . Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol 2007; 67: 158–72.
Chan KM, Lam DT, Pong K, Widmer HR, Hefti F . Neurotrophin-4/5 treatment reduces infarct size in rats with middle cerebral artery occlusion. Neurochem Res 1996; 21: 763–7.
Zhi Y, Lu Q, Zhang CW, Yip HK, So KF, Cui Q . Different optic nerve injury sites result in different responses of retinal ganglion cells to brain-derived neurotrophic factor but not neurotrophin-4/5. Brain Res 2005; 1047: 224–32.
Holtmann B, Wiese S, Samsam M, Grohmann K, Pennica D, Martini R, et al. Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J Neurosci 2005; 25: 1778–87.
Ip NY . The neurotrophins and neuropoietic cytokines: two families of growth factors acting on neural and hematopoietic cells. Ann N Y Acad Sci 1998; 840: 97–106.
Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 1989; 342: 920–3.
Park CK, Ju WK, Hofmann HD, Kirsch M, Ki Kang J, Chun MH, et al. Differential regulation of ciliary neurotrophic factor and its receptor in the rat hippocampus following transient global ischemia. Brain Res 2000; 861: 345–53.
Linker RA, Maurer M, Gaupp S, Martini R, Holtmann B, Giess R, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med 2002; 8: 620–4.
A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology 1996; 46: 1244–9.
Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, Goodpasture JC, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol 1996; 39: 256–60.
Weise J, Isenmann S, Klocker N, Kugler S, Hirsch S, Gravel C, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis 2000; 7: 212–23.
Isenmann S, Klocker N, Gravel C, Bahr M . Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur J Neurosci 1998; 10: 2751–6.
Klocker N, Braunling F, Isenmann S, Bahr M . In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport 1997; 8: 3439–42.
Cui Q, Yip HK, Zhao RC, So KF, Harvey AR . Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 2003; 22: 49–61.
Watanabe M, Tokita Y, Kato M, Fukuda Y . Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience 2003; 116: 733–42.
MacLaren RE, Buch PK, Smith AJ, Balaggan KS, MacNeil A, Taylor JS, et al. CNTF gene transfer protects ganglion cells in rat retinae undergoing focal injury and branch vessel occlusion. Exp Eye Res 2006; 83: 1118–27.
Kang SS, Keasey MP, Cai J, Hagg T . Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice. J Neurosci 2012; 32: 9277–87.
Wang L, Ma W, Markovich R, Lee WL, Wang PH . Insulin-like growth factor I modulates induction of apoptotic signaling in H9C2 cardiac muscle cells. Endocrinology 1998; 139: 1354–60.
Wang L, Ma W, Markovich R, Chen JW, Wang PH . Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res 1998; 83: 516–22.
Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 2010; 120: 254–65.
Vivar R, Humeres C, Varela M, Ayala P, Guzman N, Olmedo I, et al. Cardiac fibroblast death by ischemia/reperfusion is partially inhibited by IGF-1 through both PI3K/Akt and MEK-ERK pathways. Exp Mol Pathol 2012; 93: 1–7.
Pang L, Sawada T, Decker SJ, Saltiel AR . Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem 1995; 270: 13585–8.
Parrizas M, Saltiel AR, LeRoith D . Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem 1997; 272: 154–61.
Kulik G, Klippel A, Weber MJ . Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol 1997; 17: 1595–606.
Yao R, Cooper GM . Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 1995; 267: 2003–6.
Bronson SK, Reiter CE, Gardner TW . An eye on insulin. J Clin Invest 2003; 111: 1817–9.
Herbst RS . Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004; 59: 21–6.
Lillien L . Changes in retinal cell fate induced by overexpression of EGF receptor. Nature 1995; 377: 158–62.
Wan J, Ramachandran R, Goldman D . HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell 2012; 22: 334–47.
Czekaj M, Haas J, Gebhardt M, Muller-Reichert T, Humphries P, Farrar J, et al. In vitro expanded stem cells from the developing retina fail to generate photoreceptors but differentiate into myelinating oligodendrocytes. PLoS One 2012; 7: e41798.
Ueki Y, Karl MO, Sudar S, Pollak J, Taylor RJ, Loeffler K, et al. P53 is required for the developmental restriction in Muller glial proliferation in mouse retina. Glia 2012; 60: 1579–89.
Munemasa Y, Chang CS, Kwong JM, Kyung H, Kitaoka Y, Caprioli J, et al. The neuronal EGF-related gene Nell2 interacts with Macf1 and supports survival of retinal ganglion cells after optic nerve injury. PLoS One 2012; 7: e34810.
D'Onofrio PM, Thayapararajah M, Lysko MD, Magharious M, Spratt SK, Lee G, et al. Gene therapy for traumatic central nervous system injury and stroke using an engineered zinc finger protein that upregulates VEGF-A. J Neurotrauma 2011; 28: 1863–79.
Borlongan CV, Hess DC . New hope for stroke patients: mobilization of endogenous stem cells. CMAJ 2006; 174: 954–5.
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003; 111: 1843–51.
Greenberg DA, Jin K . From angiogenesis to neuropathology. Nature 2005; 438: 954–9.
Jin KL, Mao XO, Greenberg DA . Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A 2000; 97: 10242–7.
Sondell M, Lundborg G, Kanje M . Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 1999; 19: 5731–40.
Lapish CC, Belardetti F, Ashby DM, Ahn S, Butts KA, So K, et al. A preclinical assessment of d.l-govadine as a potential antipsychotic and cognitive enhancer. Int J Neuropsychopharmacol 2011: 1–15.
Tengel T, Alcocer MJ, Schleucher J, Larsson G . Complete assignment and secondary structure of the Brazil nut allergen Ber e 1. J Biomol NMR 2005; 32: 336.
Wang D, Guo M, Liang Z, Fan J, Zhu Z, Zang J, et al. Crystal structure of human vacuolar protein sorting protein 29 reveals a phosphodiesterase/nuclease-like fold and two protein-protein interaction sites. J Biol Chem 2005; 280: 22962–7.
Louzada-Junior P, Dias JJ, Santos WF, Lachat JJ, Bradford HF, Coutinho-Netto J . Glutamate release in experimental ischaemia of the retina: an approach using microdialysis. J Neurochem 1992; 59: 358–63.
Neal MJ, Cunningham JR, Hutson PH, Hogg J . Effects of ischaemia on neurotransmitter release from the isolated retina. J Neurochem 1994; 62: 1025–33.
Brandstatter JH, Hartveit E, Sassoe-Pognetto M, Wassle H . Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci 1994; 6: 1100–12.
Choi DW . Excitotoxic cell death. J Neurobiol 1992; 23: 1261–76.
Rothman SM, Olney JW . Excitotoxicity and the NMDA receptor--still lethal after eight years. Trends Neurosci 1995; 18: 57–8.
Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA . Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci 2004; 26: 544–57.
Nguyen D, Alavi MV, Kim KY, Kang T, Scott RT, Noh YH, et al. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis 2011; 2: e240.
Husain S, Abdul Y, Potter DE . Non-analgesic effects of opioids: neuroprotection in the retina. Curr Pharm Des 2012 Jun 28. [Epub ahead of print]
Hansen AJ . Effect of anoxia on ion distribution in the brain. Physiol Rev 1985; 65: 101–48.
Nicotera P, Orrenius S . The role of calcium in apoptosis. Cell Calcium 1998; 23: 173–80.
Lipton P . Ischemic cell death in brain neurons. Physiol Rev 1999; 79: 1431–568.
Garcia ML, Usachev YM, Thayer SA, Strehler EE, Windebank AJ . Plasma membrane calcium ATPase plays a role in reducing Ca(2+)-mediated cytotoxicity in PC12 cells. J Neurosci Res 2001; 64: 661–9.
Limbrick DD Jr, Pal S, DeLorenzo RJ . Hippocampal neurons exhibit both persistent Ca2+ influx and impairment of Ca2+ sequestration/extrusion mechanisms following excitotoxic glutamate exposure. Brain Res 2001; 894: 56–67.
Rintoul GL, Raymond LA, Baimbridge KG . Calcium buffering and protection from excitotoxic cell death by exogenous calbindin-D28k in HEK 293 cells. Cell Calcium 2001; 29: 277–87.
Wang C, Nguyen HN, Maguire JL, Perry DC . Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab 2002; 22: 206–14.
Brandt SK, Weatherly ME, Ware L, Linn DM, Linn CL . Calcium preconditioning triggers neuroprotection in retinal ganglion cells. Neuroscience 2011; 172: 387–97.
Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, et al. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl 2006; 12: 628–35.
Webster KA, Discher DJ, Bishopric NH . Cardioprotection in an in vitro model of hypoxic preconditioning. J Mol Cell Cardiol 1995; 27: 453–8.
Youssef FF, Addae JI, Stone TW . NMDA-induced preconditioning attenuates synaptic plasticity in the rat hippocampus. Brain Res 2006; 1073–1074: 183–9.
Wehrwein E, Thompson SA, Coulibaly SF, Linn DM, Linn CL . Acetylcholine protection of adult pig retinal ganglion cells from glutamate-induced excitotoxicity. Invest Ophthalmol Vis Sci 2004; 45: 1531–43.
Thompson SA, Smith O, Linn DM, Linn CL . Acetylcholine neuroprotection against glutamate-induced excitotoxicity in adult pig retinal ganglion cells is partially mediated through alpha4 nAChRs. Exp Eye Res 2006; 83: 1135–45.
Asomugha CO, Linn DM, Linn CL . ACh receptors link two signaling pathways to neuroprotection against glutamate-induced excitotoxicity in isolated RGCs. J Neurochem 2010; 112: 214–26.
Wyllie AH . Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980; 284: 555–6.
Franco R, Bortner CD, Cidlowski JA . Potential roles of electrogenic ion transport and plasma membrane depolarization in apoptosis. J Membr Biol 2006; 209: 43–58.
Bortner CD, Cidlowski JA . Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys 2007; 462: 176–88.
Bortner CD, Hughes FM Jr, Cidlowski JA . A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem 1997; 272: 32436–42.
Hughes FM Jr, Bortner CD, Purdy GD, Cidlowski JA . Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem 1997; 272: 30567–76.
Koeberle PD, Schlichter LC . Targeting K(V) channels rescues retinal ganglion cells in vivo directly and by reducing inflammation. Channels (Austin) 2010; 4: 337–46.
Koeberle PD, Wang Y, Schlichter LC . Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ 2010; 17: 134–44.
Yuan H, Wang WP, Feng N, Wang L, Wang XL . Donepezil attenuated oxygen-glucose deprivation insult by blocking Kv2.1 potassium channels. Eur J Pharmacol 2011; 657: 76–83.
Shen QJ, Zhao YM, Cao DX, Wang XL . Contribution of Kv channel subunits to glutamate-induced apoptosis in cultured rat hippocampal neurons. J Neurosci Res 2009; 87: 3153–60.
Zaks-Makhina E, Kim Y, Aizenman E, Levitan ES . Novel neuroprotective K+ channel inhibitor identified by high-throughput screening in yeast. Mol Pharmacol 2004; 65: 214–9.
Cotella D, Hernandez-Enriquez B, Wu X, Li R, Pan Z, Leveille J, et al. Toxic role of K+ channel oxidation in mammalian brain. J Neurosci 2012; 32: 4133–44.
Dallas ML, Boyle JP, Milligan CJ, Sayer R, Kerrigan TL, McKinstry C, et al. Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J 2011; 25: 1519–30.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D'Onofrio, P., Koeberle, P. What can we learn about stroke from retinal ischemia models?. Acta Pharmacol Sin 34, 91–103 (2013). https://doi.org/10.1038/aps.2012.165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.165
Keywords
This article is cited by
-
TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice
Cell Death & Disease (2020)
-
Endothelial activation of caspase-9 promotes neurovascular injury in retinal vein occlusion
Nature Communications (2020)
-
Upper Miocene–Pliocene provenance evolution of the Central Canyon in northwestern South China Sea
Marine Geophysical Research (2019)
-
FAF1 mediates necrosis through JNK1-mediated mitochondrial dysfunction leading to retinal degeneration in the ganglion cell layer upon ischemic insult
Cell Communication and Signaling (2018)
-
Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses
Cell Death & Disease (2018)