Abstract
Aim:
To explore whether the synthetic cannabinoid receptor agonist WIN55,212-2 could protect oligodendrocyte precursor cells (OPCs) in stroke penumbra, thereby providing neuroprotection following permanent focal cerebral ischemia in rats.
Methods:
Adult male SD rats were subjected to permanent middle cerebral artery occlusion (p-MCAO). The animals were administered WIN55,212-2 at 2 h, and sacrificed at 24 h after the ischemic insult. The infarct volumes and brain swelling were assessed. The expression of cannabinoid receptor type 1 (CB1) in the stroke penumbra was examined using Western blot assay. The pathological changes and proliferation of neural glial antigen 2-positive OPCs (NG2+ cells) in the stroke penumbra were studied using immunohistochemistry staining.
Results:
p-MCAO significantly increased the expression of CB1 within the stroke penumbra with the highest level appearing at 2 h following the ischemic insult. Administration of WIN55,212-2 (9 mg/kg, iv) significantly attenuated the brain swelling, and reduced the infarct volume as well as the number of tau-immunoreactive NG2+ cells (tau-1+/NG2+ cells) in the stroke penumbra. Moreover, WIN55,212-2 significantly promoted the proliferation of NG2+ cells in the stroke penumbra and in the ipsilateral subventricular zone at 24 h following the ischemic insult. Administration of the selective CB1 antagonist rimonabant (1 mg/kg, iv) partially blocked the effects caused by WIN55,212-2.
Conclusion:
Tau-1 is expressed in NG2+ cells following permanent focal cerebral ischemic injury. Treatment with WIN55,212-2 reduces the number of tau-1+/NG2+ cells and promotes NG2+ cell proliferation in the stroke penumbra, which are mediated partially via CB1 and may contribute to its neuroprotective effects.
Similar content being viewed by others
Introduction
Considerable evidence demonstrates that the preservation of only gray matter neurons following stroke may be insufficient for recovery of the neurological function, suggesting the importance of protecting glial cells in the white matter as well1. Following occlusion of the middle cerebral artery for 30 min, rats exhibit a swelling of oligodendrocytes2, and in neonatal hypoxic-ischemic rats, oligodendrocyte precursor cells (OPCs) die rapidly3. Remyelination is performed by OPCs, and the promotion of OPC survival may contribute to the development of reparative strategies for demyelinating diseases4. Therefore, preclinical studies of drugs with therapeutic potential in acute stroke should include an assessment of oligodendrocyte and OPC survival.
The quantification of oligodendrocytes that are immunoreactive for the microtubule-associated protein tau can be used to assess potential therapeutic interventions for pathologies in which oligodendrocytes have been exposed to ischemia5,6. As OPCs contain tau7, a remaining question is whether tau-immunoreactivity is increased in OPCs following cerebral ischemia. Notably, OPC membranes contain the chondroitin sulfate proteoglycan neural glial antigen 2 (NG2)8, and NG2-positive cells are present in the rat brain during development and in adulthood9. To determine whether OPCs express tau-1 following permanent middle cerebral artery occlusion (p-MCAO), we performed immunohistochemistry to co-label tau-1 and the OPC marker NG2 following 24 h p-MCAO injury.
Cannabinoid receptor type 1 (CB1) immunoreactivity is up-regulated following transient MCAO injury10, and numerous studies have suggested that endocannabinoids, acting through CB1, may be promising neuroprotective agents in several degenerative brain conditions. Whether a p-MCAO injury has effects on the expression of CB1 remains controversial. In this study, we identified the expression of CB1 in penumbral areas following p-MCAO injury and explored the hypothesis that treatment with the synthetic cannabinoid agonist WIN55,212-2, at a time when CB1 is highly expressed, could reduce the number of tau-immunoreactive NG2-positive cells and promote their proliferation, thereby providing neuroprotection.
Materials and methods
Chemicals and reagents
We purchased the non-selective cannabinoid receptor agonist R(+)-WIN55,212-2 mesylate (C27H26N2O3·CH4SO3, (R)-(+)-[2, 3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) from Cayman Chemical (Ann Arbor, Michigan, USA, purity ≥98%). Rimonabant hydrochloride [C22H21Cl3N4O·HCl, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide monohydrochloride], a selective CB1 antagonist, was purchased from Axon Medchem (Groningen, Netherlands, purity: 99%).
Animals
One hundred and twelve adult male Sprague-Dawley (SD) rats weighing 250–300 g were purchased from Zhejiang Laboratory Animals Center (Hangzhou, China) and kept under standard housing conditions at a temperature between 20 °C and 23 °C with a 12 h light-dark cycle at a relative humidity of 50%. All animal tests and experimental procedures were approved by the Administration Committee of Experimental Animals in Jiangsu Province and the Ethics Committee of China Pharmaceutical University.
Induction of permanent focal cerebral ischemia
Permanent focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) according to the method of Koizumi et al11. To block the origin of the middle cerebral artery, a monofilament nylon suture (approximate diameter: 0.26 mm) was prepared by rounding the tip by heating and coating with poly-l-lysine (Sigma). The nylon suture was introduced through the right external carotid artery into the internal carotid artery and advanced approximately 18–20 mm intracranially from the common carotid artery bifurcation. Body temperature was maintained at 37.0 °C with a temperature control system. As recommended by Longa et al12, the neurological evaluation was carried out as follows: score 0, no apparent neurological deficits; score 1, contralateral forelimb flexion; score 2, decreased resistance to lateral push; score 3, spontaneous movement in all directions and contralateral circling when pulled by the tail; score 4, no spontaneous locomotion and depressed levels of consciousness.
Animal treatment
First, we observed the time course of CB1 protein expression following p-MCAO in male SD rats. A total of 42 SD rats were randomly assigned to 7 experimental groups (n=6 per group). After assessing the model13, 28 rats were used for the experiment (n=4 per group). Protein expression was evaluated at 1, 2, 3, 4, 5, and 6 h following p-MCAO insult, by using Western blot. Next, we examined whether post-treatment with the synthetic cannabinoid agonist WIN55,212-2 has neuroprotective roles, such as decreasing the infarct volume, brain swelling and neurological deficits, while CB1 is highly expressed. A total of 40 male SD rats were randomly assigned to five groups receiving one of the following treatments: sham; vehicle; WIN55,212-2 (1 mg/kg); WIN55,212-2 (9 mg/kg); WIN55,212-2 (9 mg/kg) combined with rimonabant (1 mg/kg). After model assessment13, 30 male SD rats were used in the experiment (n=6). The rats in the first two groups were intravenously administered a vehicle solution at 2 h following p-MCAO. The rats in the WIN55,212-2 (1 mg/kg) and WIN55,212-2 (9 mg/kg) groups received an intravenous administration of WIN55,212-2 at 2 h following p-MCAO. The rats in the last group were intravenously administered rimonabant (1 mg/kg) at 1.5 h following p-MCAO, and WIN55,212-2 (9 mg/kg) was injected 30 min later. All of the animals were humanely sacrificed 24 h following p-MCAO, and the brain samples were isolated to determine cerebral infarct volume and swelling. Finally, to investigate the pathology and proliferation of NG2-positive cells, immunofluorescence staining was performed. A total of 30 male SD rats were randomly assigned to five groups. The grouping and methods of drug administration were identical to the second experiment. After model assessment13, 20 male SD rats were used in the experiment (n=4). The S-phase marker 5-bromo-2′-deoxyuridine (BrdU) (Sigma) was dissolved in saline and administered to SD rats at a dose of 100 mg/kg ip at 10 and 20 h following p-MCAO to label dividing cells14.
Assessment of cerebral infarct volume and brain swelling
To analyze the degree of cerebral infarction, 2,3,5-triphenyl-tetrazolium chloride (TTC) staining was assessed using methods previously published by Leker et al15. Infarct volumes are expressed as a percentage of the contralateral hemisphere as measured by image analysis software15. Brain swelling was calculated according to the following formula: (infarct volume+undamaged ipsilateral−contralateral volume)×100/contralateral volume (%)16.
Western blots
The protocol used to isolate brain samples from the penumbra and normal contralateral areas was published previously by Kramer et al17. Thirty-microgram protein samples were size-fractionated by 10% SDS-PAGE and immunoblotted with a polyclonal rabbit anti-CB1 antibody (1:500, GeneTex) and an HRP-conjugated secondary antibody (1:1000, Chemicon). β-Actin, detected by a mouse monoclonal anti-β-actin antibody (1:1000, Sigma), served as a loading control.
Immunohistochemistry staining
The method used for immunofluorescence staining has been published previously by Wojtowicz et al14. The following primary antibodies were used: polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) (1:200, Chemicon), polyclonal rabbit anti-NG2 (1:300, Chemicon), monoclonal mouse anti-tau-1 (1:600, Chemicon), monoclonal mouse anti-Ki67 (1:300, Novocastra Laboratories), and monoclonal mouse anti-BrdU (1:300, Chemicon). Following incubation with the primary antibody, sections were incubated for 90 min with Cy2- and Cy3-conjugated goat anti-rabbit and anti-mouse secondary antibodies (1:300, Invitrogen) in blocking solution at room temperature. Finally, to detect nuclei, sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) in potassium phosphate buffered saline for 10 min at room temperature. The GFAP+ region was used to delineate the penumbra surrounding the core of the infarct (Supplementary Figure 1)18. The number of cells in the penumbra and subventricular zone (SVZ) that were positive for various markers was quantified using digitized confocal images, captured from a minimum of five serial coronal sections spaced 250 μm apart, corresponding to the Bregma coordinates -0.8 mm through 2.5 mm19. The data are presented as the total number of positive cells within the specific region analyzed.
Statistical analysis
Except for the neurological deficit scores, all values are expressed as the mean±SD, and significant differences between the groups were determined by a two-way analysis of variance (ANOVA). The neurological deficit scores were expressed as the medians, and significant differences between the groups were determined by a non-parametric Mann-Whitney test. P-values<0.05 were considered an indication of statistical significance.
Results
Determination of CB1 expression in p-MCAO rats
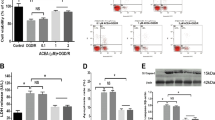
Following 2 h of p-MCAO, the level of CB1 protein expressed within the penumbra, standardized to β-actin protein levels, was significantly increased compared to 1 h following p-MCAO (Figure 1B, 1C, n=4, P<0.01). No significant changes in CB1 protein expression in the contralateral control area were observed in any group (Figure 1A, 1C, n=4, P>0.05).
WIN55,212-2 protects against p-MCAO injury partially through CB1
We next explored whether treatment with WIN55,212-2 2 h following p-MCAO, during which time CB1 was highly expressed, has neuroprotective roles, decreasing the infarct volume, brain swelling and neurological deficits in the p-MCAO model. There are not only two doses including 3 mg/kg, 1 mg/kg used previously in the pre-treatment of cerebral ischemic reperfusion20, but the dose of 9 mg/kg also have been designed in order to study the dose-dependent effect of p-MCAO model in the preliminary experiments. The infarct volume (% of the contralateral hemisphere) in the vehicle animals was 32.1%±1.8%. The WIN55,212-2 (9 mg/kg) and WIN55,212-2 (3 mg/kg) groups both had significantly smaller infarct volumes than the vehicle group (Supplementary Figure 2A, n=4, P<0.05). However, there was no significant difference in the infarct volume between the WIN55,212-2 (1 mg/kg) and vehicle groups (Supplementary Figure 2A, n=4, P>0.05). Consequently, we chose 9 and 1 mg/kg of WIN55,212-2 as the drug treatment doses in the formal experiment.
In the formal experiment, the ischemic infarctions were white following 24 h p-MCAO, and TTC staining revealed regular inclusion of the neocortex and basal ganglia. The results showed that the infarct volume (% of the contralateral hemisphere) in vehicle animals was 31.2%±3.1% and that the volume in the WIN55,212-2 (9 mg/kg) group was significantly lower (reduced by 61.6% compared with the vehicle animals; 12.5%±2.3% infarct volume) (Figure 2A, n=6, P<0.01). The percentage of brain swelling was significantly different between rats in the vehicle group (11.3%±1.2%) and the WIN55,212-2 (9 mg/kg) group (3.3%±1.1%) (Figure 2B, n=6, P<0.01). Compared to the vehicle group, WIN55,212-2 (9 mg/kg) treatment significantly improved the neurological score 24 h after p-MCAO insult (Figure 2C, n=6, P<0.01).
WIN55,212-2 protects against p-MCAO insult partially through CB1. (A, B) Brain infarct volume and brain swelling 24 h following p-MCAO. (C) The effects of WIN55,212-2 on neurological score (assessed 24 h following p-MCAO). The bars represent brain infarct volume and brain swelling are expressed as the mean±SD. The neurological deficit scores are expressed as the medians. n=6. bP<0.05, cP<0.01 vs the sham group. fP<0.01 vs the vehicle group.
To determine whether the neuroprotective effects of WIN55,212-2 were mediated by CB1, we formed preliminary experimental groups that received iv injections of rimonabant (1 mg/kg), rimonabant (2 mg/kg), WIN55,212-2 (9 mg/kg) combined with rimonabant (1 mg/kg), or WIN55,212-2 (9 mg/kg) combined with rimonabant (2 mg/kg). The administration of rimonabant alone led to infarct volumes of 29.1%±5.6% and 30.2%±3.1% (% of the contralateral hemisphere) for the doses of 1 and 2 mg/kg, respectively, which did not have any influence on infarct volumes compared with the vehicle group (Supplementary Figure 2A, n=4, P>0.05). Compared with the WIN55,212-2 (9 mg/kg) group, the neuroprotective effects were partially antagonized by the combined treatment with rimonabant (1 or 2 mg/kg) (Supplementary Figure 2A, n=4, P<0.01). There was no significant difference in infarct volume between the rimonabant 1 mg/kg and 2 mg/kg alone/combined treatment (Supplementary Figure 2A, n=4, P>0.05). Therefore, we chose 1 mg/kg rimonabant for the combined treatment with WIN55,212-2 (9 mg/kg) in the formal experiment. We found in the formal experiment that compared to the WIN55,212-2 (9 mg/kg) group, all neuroprotective effects were partially antagonized by the combined treatment with rimonabant (1 mg/kg) (Figure 2, n=6, P<0.01).
NG2-positive cells are immunoreactive for tau-1 following p-MCAO, and WIN55,212-2 reduces the number of NG2+/tau-1+ cells by acting partially through CB1
Twenty four hours following p-MCAO insult, NG2, and tau-1 co-expressing cells were detectable in the penumbral area. Compared to the sham group, we found that the numbers of tau-1+/DAPI+ and tau-1+/NG2+ cells (% of DAPI+ cells) in the penumbra were significantly higher in the vehicle group 24 h following p-MCAO (Figure 3Af, 3Ag, 3Ba, 3Bb, n=4, P<0.01). Compared to the vehicle group, WIN55,212-2 (9 mg/kg) treatment was able to decrease the numbers of tau-1+/DAPI+, tau-1+/NG2+, and tau-1+/NG2− cells (% of DAPI+ cells) (Figure 3Ag, 3Ai, 3Ba, 3Bb, n=4, P<0.01); however, the low dosage of WIN55,212-2 (1 mg/kg) did not have these effects (Figure 3Ag, 3Ah, 3Ba, 3Bb, n=4, P>0.05). There was no significant difference in the number of NG2+/tau-1+ (% of DAPI+ cells) between the rimonabant (1 mg/kg) and vehicle groups (Supplementary Figure 2Ba, 2Bd, 2C, n=4, P>0.05). Following the combination treatment with rimonabant, the numbers of both NG2+/tau-1+ and NG2−/tau-1+ cells (% of DAPI+ cells) were significantly higher than in the group treated with WIN55,212-2 (9 mg/kg) alone (Figure 3Ai, 3Aj, 3Bb, n=4, P<0.01). In the contralateral control area, there was no significant alteration in the number of tau-1+/DAPI+ cells (% of DAPI+ cells) compared to the sham group (Figure 3Aa–3Ae, 3Ba, 3Bb, n=4, P>0.05).
Tau-1 is expressed in NG2-positive cells following p-MCAO, and WIN55, 212-2 reduces the number of NG2+/tau-1+ cells, partially by acting through CB1. (Ag, Ai, Ba, Bb) Compared to the vehicle group, the numbers of DAPI+/tau-1+ and NG2+/tau-1+ cells (% of DAPI+ cells) were significantly lower in the WIN55, 212-2 (9 mg/kg) group 24 h following p-MCAO. (Ai, Aj, Bb) Compared to the WIN55, 212-2 (9 mg/kg) group, the numbers of NG2+/tau-1+ and NG2−/tau-1+ cells (% of DAPI+ cells) were significantly higher following rimonabant co-treatment. (Aa–Ae, Ba, Bb) In the contralateral control area, the numbers of tau-1+/DAPI+ and tau-1+/NG2+ cells (% of DAPI+ cells) were not significantly different from those of the sham group. The results are expressed as the mean±SD. n=4. bP<0.05, cP<0.01 vs the vehicle group. fP<0.01 vs the sham group.
WIN55,212-2 selectively promotes the proliferation of NG2-positive cells in the penumbra partially through CB1
One concern when using BrdU immunohistochemistry is that cells undergoing DNA repair are detected in addition to cells undergoing cell division21. In this study, we first used another proliferation marker, Ki67, which labels cells in all of the phases of the cell cycle except for G014. Compared to the vehicle group, the numbers of Ki67+/DAPI+ and Ki67+/NG2+ cells (% of DAPI+ cells) in the penumbra were significantly increased in the WIN55,212-2 (9 mg/kg) group (Figure 4Ag, 4Ai, 4Ba, 4Bb, n=4, P<0.01), and no significant differences were observed in the WIN55,212-2 (1 mg/kg) group (Figure 4Ag, 4Ah, 4Ba, 4Bb, n=4, P>0.05). There was no significant change in the numbers of Ki67+/NG2+ and BrdU+/NG2+ (% of DAPI+ cells) cells between the rimonabant (1 mg/kg) and vehicle groups (Supplementary Figure 2Bb, 2Bc, 2Be, 2Bf, 2C, n=4, P>0.05). Following rimonabant treatment, the number of NG2+/Ki67+ cells (% of DAPI+ cells) was significantly lower than that of the group treated with WIN55,212-2 (9 mg/kg) alone, whereas the number of NG2−/Ki67+ cells (% of DAPI+ cells) was significantly higher (Figure 4Ai, 4Aj; 4Bb, n=4, P<0.01, P<0.05). In the contralateral control area, the number of DAPI+/Ki67+ cells (% of DAPI+ cells) was not significantly different between the sham and WIN55,212-2 (9 mg/kg) groups (Figure 4Aa, 4Ad, 4Ba, n=4, P>0.05). To identify the source of the new NG2-positive cells generated following WIN55,212-2 treatment, we analyzed the population of rapidly proliferating cells by BrdU labeling. Only a few NG2+ cells (0.52%±0.1%) were BrdU+ in the vehicle group 24 h following p-MCAO (Figure 5Ab, 5B). Interestingly, compared to the vehicle group, the numbers of BrdU+/NG2+ cells (% of DAPI+ cells) in the WIN55,212-2 (9 mg/kg) and WIN55,212-2 (1 mg/kg) groups were significantly higher (Figure 5Ab–5Ad, 5B, n=4, P<0.01). However, following rimonabant treatment, the number of BrdU+/NG2+ cells (% of DAPI+ cells) was significantly lower than in the group treated with WIN55,212-2 (9 mg/kg) alone (Figure 5Ad, 5Ae, 5B, n=4, P<0.01).
WIN55,212-2 selectively increases the proliferation of penumbral NG2-positive cells partially through CB1. (Ag, Ai, Ba, Bb) Compared to the vehicle group, the numbers of DAPI+/Ki67+ and NG2+/Ki67+ cells (% of DAPI+ cells) were significantly increased in the WIN55, 212-2 (9 mg/kg) group 24 h following p-MCAO. (Ad, Ae, Ai, Aj, Bb). Following rimonabant co-treatment, the number of Ki67+/NG2+ cells (% of DAPI+ cells) was significantly decreased, while the percentage of Ki67+/NG2− cells was significantly increased compared to the group treated with WIN55, 212-2 (9 mg/kg) alone. The results are expressed as the mean±SD. n=4. bP<0.05, cP<0.01 vs vehicle group. eP<0.05, fP<0.01 vs sham group.
The identification of BrdU-positive cells in the penumbral areas following administration of WIN55, 212-2. (Aa, Ab, Ad, B) Compared to the vehicle and sham group, the numbers of NG2+/BrdU+ cells (% of DAPI+ cells) were significantly increased in the WIN55, 212-2 (9 mg/kg) group 24 h following p-MCAO. (Ac, Ad, B) Compared to the WIN55, 212-2 (9 mg/kg) group, the numbers of NG2+/BrdU+ cells (% of DAPI+ cells) in WIN55, 212-2 (1 mg/kg) group were significantly lower. (Ad, Ae, B) Following rimonabant co-treatment, the number of NG2+/BrdU+ cells (% of DAPI+ cells) was significantly lower than in the group treated with WIN55,212-2 (9 mg/kg) alone. Scale bars: 50 μm. The results are expressed as the mean±SD. n=4. bP<0.05, cP<0.01 vs the vehicle group. eP<0.05, fP<0.01 vs the sham group.
WIN55,212-2 increases the proliferation of ipsilateral SVZ NG2-positive progenitor cells partially through CB1
In addition to the penumbral areas, the SVZ is a source of post-natal glial precursors that can migrate to nearby areas affected by infarction and then differentiate into oligodendrocytes22. Quantitative data analysis showed that, in the ipsilateral SVZ, the number of Ki67+/DAPI+ cells (% of DAPI+ cells) was significantly higher in the WIN55,212-2 (9 mg/kg) group than in the vehicle and sham group (Supplementary Figure 3Aa, 3Ab, 3Ad, 3Ba, n=4, P<0.01). Compared to the vehicle group, the number of NG2+/Ki67+ cells (% of DAPI+ cells) was significantly higher in the WIN55,212-2 (9 mg/kg) and WIN55,212-2 (1 mg/kg) groups (Supplementary Figure 3Ab1, 3Ac1, 3Ad1, 3Bb, n=4, P<0.01). Following rimonabant co-treatment, the numbers of Ki67+/DAPI+ and NG2+/Ki67+ cells (% of DAPI+ cells) were significantly lower than the group treated with WIN55,212-2 (9 mg/kg) alone (Supplementary Figure 3Ad1, 3Ae1, 3Ba, 3Bb, n=4, P<0.01). Following WIN55,212-2 treatment, the numbers of BrdU+/NG2+ cells (% of DAPI+ cells) in the WIN55,212-2 (9 mg/kg) and WIN55,212-2 (1 mg/kg) groups were significantly higher than those in the vehicle group (Supplementary Figure 4Ab–4Ad, 4B, n=4, P<0.01). However, following rimonabant co-treatment, the number of BrdU+/NG2+ cells (% of DAPI+ cells) was significantly lower than that in the group treated with WIN55,212-2 (9 mg/kg) alone (Supplementary Figure 4Ad, 4Ae, 4B, n=4, P<0.01).
Discussion
In this study, we used a permanent focal cerebral ischemia model in adult rats to investigate the neuroprotective effects of WIN55,212-2 on NG2-positive cells, and our results highlighted the following: (1) tau-1 is expressed in NG2-positive cells in the penumbra following 24 h p-MCAO insult; (2) post-treatment with WIN55,212-2 (9 mg/kg) when CB1 is highly expressed significantly decreases the cerebral swelling, cerebral infarction volume and the number of tau-immunoreactive NG2-positive cells within the stroke penumbra; and (3) WIN55,212-2 selectively increases the proliferation of NG2-positive cells following 24 h p-MCAO insult. In addition, by using the selective CB1 antagonist rimonabant, we identified a partial role of CB1 in mediating the effects mentioned above.
Previous studies have reported that the activation of CB1 is a neuroprotective strategy in models of cerebral ischemia10. However, whether a p-MCAO injury affects the expression of CB1 remains controversial. In this study, we identified high levels of CB1 expression in the penumbral area following 2 h of p-MCAO. Of note, the infarct volume reached its maximum at 2 h after p-MCAO insult23. Based on this information, we speculated that the exogenous cannabinoid agonist WIN55,212-2 should be administered prior to 2 h post-p-MCAO, while the ischemic injury is still progressing and the level of CB1 protein is increasing. Following preliminary dose-response experiments, we chose 9 and 1 mg/kg of WIN55,212-2 as the formal experimental doses. We found that treatment with WIN55,212-2 (9 mg/kg) 2 h following insult significantly decreased the cerebral swelling and cerebral infarction observed in p-MCAO rats. We also used the selective CB1 antagonist rimonabant to verify the above results. Rimonabant is an extensively studied CB1 inverse agonist that antagonized the effects of WIN 55,212-2 in a dose-dependent manner. McMahon reported that intravenous administration of 0.32 and 1.0 mg/kg SR 141716A (rimonabant) in rhesus monkeys increased the ED50 of subsequent WIN55,212-2 doses to 2.9- and 4.3-fold, respectively24. Based on these data, we selected 1 mg/kg and 2 mg/kg iv doses in our preliminary experiments. We found that the administration of rimonabant (1 or 2 mg/kg iv) alone did not have any influence on infarct volumes compared with the vehicle group and that the neuroprotective effects of WIN55,212-2 on p-MCAO were partially reversed by rimonabant, suggesting that rimonabant (either 1 or 2 mg/kg iv) could be used for antagonism of the CB1-mediated effects in this model. As there was no significant change in infarct volume between the rimonabant 1 mg/kg and 2 mg/kg alone/combined treatments, we chose 1 mg/kg of rimonabant for the combined treatment with WIN55,212-2 in the formal experiment. Rimonabant partially abrogated the neuroprotective effects of WIN55,212-2 on cerebral swelling, cerebral infarction and neurological score, indicating that CB1 was most likely involved in mediating the effects mentioned above, although we could not completely exclude the possibility that WIN55,212-2 acts through CB2 in this model.
Many studies have reported that myelin repair can occur in penumbral areas in the MCAO model25, leading us to investigate the effects of WIN55,212-2 on NG2-positive cells. It has been shown elsewhere that, among glial cells, only damaged oligodendrocytes express the dephosphorylated form of the tau protein (tau-1) following acute neural disorders including stroke5,6. Zehr et al26 have reported that over-expression of human tau is associated with the apoptosis of oligodendrocytes. Therefore, quantitation of tau-1 immunoreactive oligodendrocytes can be used to assess ischemic oligodendrocyte pathology. In this study, we found that tau-1 was extensively expressed in NG2-positive cells in the penumbra following 24 h of p-MCAO insult, and compared with the sham group, the number of tau-1+/NG2+ cells was significantly increased in the vehicle group. WIN55,212-2 treatment (9 mg/kg) significantly decreased the number of tau-1+/NG2+ cells in the penumbra, and rimonabant reversed the neuroprotective effects of WIN55,212-2, further demonstrating the involvement of CB1 in the mediation of the effects mentioned above. Furthermore, low numbers of tau-1+/DAPI+ cells were observed in the contralateral control area. It is known that tau protein is abundant in neurons, and most tau in the developing branches neurons is more highly dephosphorylated at the site recognized by the tau-1 antibody than is tau in the somatodendritic compartment27,28. In this study, we used adult SD rats, which might have low numbers of axons that could express tau-1. In addition, the number of neurons is certainly not equal in different brain regions. Azevedo et al reported that the ratio of non-neuronal to neuronal cells in the white matter is 15.4129. In this study, the ischemic penumbra was mainly defined at the white matter, prompting us to choose the contralateral white matter as the control area. The number of tau-1+/NG2- cells in our study was partially consistent with prior research.
Beyond the fact that oligodendrocyte and OPCs extensively expressed tau-1 after p-MCAO insult, promotion of tau dephosphorylation in neurons could be one of the possible reasons why the number of tau-1 positive cells significantly increased in the penumbra. Previous studies have reported that oxidative stress promotes tau dephosphorylation at the tau-1 epitope in neuronal cells by activating PP1 and PP-2A30,31. Our findings partially agree with reports that the number of tau-1+/NG2- cells are significantly increased in the penumbra areas, indicating that the p-MCAO insult may also promote tau dephosphorylation in neuronal cells. Because hyperphosphorylation of tau is known to affect cell apoptosis32, promotion of tau dephosphorylation in neurons could indicate an initial cellular response against oxidative insults30. However, the high levels of tau dephosphorylated at the tau-1 epitope are associated with greater vulnerability to apoptosis induced by hydrogen peroxide, with mechanisms involving a failed dephosphorylation/activation of Bcl-233. Therefore, the phosphorylation and dephosphorylation levels of tau proteins could not be used to accurately identify the survival of neurons. WIN55,212-2 treatment decreased the number of tau-1+/NG2− cells, which might have some relationship with the survival of neurons. However, further study is required to determine the effects of WIN55,212-2 on neurons.
To study whether WIN55,212-2 could promote NG2-positive proliferation, we used both Ki67 and BrdU staining in the penumbra and ipsilateral SVZ. We found that WIN55,212-2 treatment (9 mg/kg) significantly increased the numbers of Ki67+/NG2+ and BrdU+/NG2+ cells in the penumbra. Interestingly, the vehicle-treated rat brains showed spontaneous proliferation; however, the majority of these BrdU+ cells did not express NG2. Following rimonabant co-treatment, the numbers of NG2+/Ki67+ and NG2+/BrdU+ cells were significantly decreased, while the percentage of NG2-/Ki67+ cells was significantly increased. These results indicate that WIN55,212-2 may selectively increase the proliferation of NG2-positive cells partially via CB1. One previous study suggested that the levels of CB1 mRNA and protein in OPCs appear to be increased relative to other types of glial cells34, and this is one likely mechanism to explain the selective effects observed here. In this study, WIN55,212-2 was administered 2 h after p-MCAO, when low levels of CB1 protein were observed in the contralateral cerebral hemisphere, and the number of NG2+/Ki67+ cells in the WIN55,212-2 (9 mg/kg) group was significantly increased. It is therefore conceivable that the capacity of WIN55,212-2 to promote proliferation in NG2-positive cells is related to the level of CB1 expression.
In addition to the classic cannabinoid receptors, novel receptors capable of binding cannabinoids, such as the transient receptor potential vanilloid 1 (TRPV 1), have recently been identified. In particular, TRPV 1 is expressed in the sensory neurons of the dorsal root ganglion and has been demonstrated to play a critical role in the induction of thermal hyperalgesia in inflammatory pain models35,36. WIN55,212-2 can evoke anti-hyperalgesia by promoting dephosphorylation of TRPV 1 at Thr144 and Thr370 in sensory neurons37. Although there were no reports of WIN55,212-2 directly protecting OPCs or increasing oligodendrogliogenesis through TRPV 1, we cannot ignore the possible involvement of TRPV 1 in the mediation of the indirectly neuroprotective effects of cannabinoids in stroke. Muzzi et al reported that rinvanil, a TRPV 1 agonist, could induce mild hypothermia in promising candidates for hypothermic treatment of stroke38. To prevent the potential hypothermic effects of WIN55,212-2 in this study, we used a heat lamp to maintain consistent temperatures for each rat. It is of great interest and value to investigate whether the hypothermic effects of WIN55,212-2 are mediated through TRPV 1 receptors, which might be further studied by our subsequent research.
In conclusion, we determined that tau-1 is expressed in NG2-positive cells following p-MCAO injury and that WIN55,212-2 protects the NG2-positive cells in the penumbra by reducing the co-expression of tau-1 and promoting proliferation. By using the selective CB1 antagonist rimonabant, we demonstrated that the neuroprotective mechanism of WIN55,212-2 on NG2-positive cells is, in part, mediated through CB1.
Author contribution
Jing SUN, Hong LIAO, Lu-yong ZHANG, and Shu SONG designed the research plan; Jing SUN, Yin-quan FANG, Tao CHEN, Hong REN, Jing-jing GUO, and Jun YAN performed the research; Yin-quan FANG and Tao CHEN analyzed the data; and Jing SUN and Hong LIAO wrote the paper.
References
Arai K, Lo EH . Oligovascular signaling in white matter stroke. Biol Pharm Bull 2009; 32: 1639–44.
Leonardo P, Garcia JH, Gutierrez JA . Cerebral white matter is highly vulnerable to ischemia. Stroke 1996; 27: 1641–6.
Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS . Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int J Devl Neurosci 2001; 19: 197–208.
Mekhail M, Almazan G, Tabrizian M . Oligodendrocyte-protection and remyelination post-spinal cord injuries: a review. Prog Neurobiol 2012; 96: 322–39.
Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM . Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke 2001; 32: 2149–54.
Guimarães JS, Freire MA, Lima RR, Picanço-Diniz CW, Pereira A, Gomes-Leal W . Minocycline treatment reduces white matter damage after excitotoxic striatal injury. Brain Res 2010; 1329: 182–93.
Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J . Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J Neurosci 2002; 22: 698–707.
Nishiyama A, Komitova M, Suzuki R, Zhu X . Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 2009; 1: 9–22.
Mangin JM, Gallo V . The curious case of NG2 cells: transient trend or game changer? ASN Neuro 2011; 3: e00052.
Hillard CJ . Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr Pharm Des 2008; 23: 2347–61.
Koizumi J, Yoshida Y, Nakazawa T, Ooneda G . Experimental studies of ischemic brain edema: 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn Stroke J 1986; 8: 1–8.
Longa EZ, Weinstein PR, Carlson S, Cummins R . Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91.
Ulrich D and Group, Members on the MCAO-SOP. Standard operating procedures (SOP) in experimental stroke research: SOP for middle cerebral artery occlusion in the mouse. Available from Nature Precedings <http://hdl.handle.net/10101/npre.2009.3492.1> (2009).
Wojtowicz JM, Kee N . BrdU assay for neurogenesis in rodents. Nat Protoc 2006; 3: 1399–405.
Leker RR, Gai N, Mechoulam R, Ovadia H . Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU–210. Stroke 2003; 34: 2000–6.
Oida Y, Hamanaka J, Hyakkoku K, Shimazawa M, Kudo T, Imaizumi K, et al. Post-treatment of a BiP inducer prevents cell death after middle cerebral artery occlusion in mice. Neurosci Lett 2010; 484: 43–6.
Kramer M, Dang J, Baertling F, Denecke B, Clarner T, Kirsch C, et al. TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. J Neurosci Methods 2010; 187: 84–9.
Benjamin DH, Theo DP, Gary KS . Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke 2005; 36: 2718–24.
Solbrig MV, Fan Y, Hermanowicz N, Morgese MG, Giuffrida A . A synthetic cannabinoid agonist promotes oligodendrogliogenesis during viral encephalitis in rats. Exp Neurol 2010; 226: 231–41.
Hu B, Wang Q, Chen Y, Du J, Zhu X, Lu Y, et al. Neuroprotective effect of WIN55,212-2 pretreatment against focal cerebral ischemia through activation of extracellular signal-regulated kinases in rats. Eur J Pharmacol 2010; 645: 102–7.
Taupin P . BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev 2007; 1: 198–214.
Zhang L, Michael C, Ruilan Z, Lei W, Jing Z, Ying W . Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PloS One 2010; 6: e11016.
Anke P, Nadine J, Otto WW, Christiane F . Identification of ischemic regions in a rat model of stroke. PLoS One 2009; 4: e4764.
McMahon LR . Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 2006; 319: 1211–8.
Ken A, Eng HL . Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med 2009; 1: 6.
Zehr C, Lewis J, McGowan E, Crook J, Lin WL, Godwin K, et al. Apoptosis in oligodendrocytes is associated with axonal degeneration in P301L tau mice. Neurobiol Dis 2004; 3: 553–62.
Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF . Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci 2004; 7: 1059–69.
Mandell JW, Banker GA . A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci 1996; 16: 5727–40.
Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009; 513: 532–41.
Zambrano CA, Egaña JT, Núñez MT, Maccioni RB, González-Billault C . Oxidative stress promotes tau dephosphorylation in neuronal cells: the roles of cdk5 and PP1. Free Radic Biol Med 2004; 36: 1393–402.
Liu R, Pei JJ, Wang XC, Zhou XW, Tian Q, Winblad B, et al. Acute anoxia induces tau dephosphorylation in rat brain slices and its possible underlying mechanisms. J Neurochem 2005; 94: 1225–34.
Idan-Feldman A, Ostritsky R, Gozes I . Tau and caspase 3 as targets for neuroprotection. Int J Alzheimers Dis 2012; doi:10.1155/2012/493670.
Liu XA, Liao K, Liu R, Wang HH, Zhang Y, Zhang Q, et al. Tau dephosphorylation potentiates apoptosis by mechanisms involving a failed dephosphorylation/activation of Bcl-2. J Alzheimers Dis 2010; 19: 953–62.
Molina-Holgado E, Vela JM, Arévalo-Martín A, Almazán G, Molina-Holgado F, Borrell J . Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci 2002; 22: 9742–53.
Xu X, Wang P, Zou X, Li D, Fang L, Gong K, et al. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV 1 in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol 2010; 222: 93–107.
Ho KW, Ward NJ, Calkins DJ . TRPV1: a stress response protein in the central nervous system. Am J Neurodegener Dis 2012; 30: 1–14.
Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM . Cannabinoid WIN 55, 212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem 2006; 281: 32879–90.
Muzzi M, Felici R, Cavone L, Gerace E, Minassi A, Appendino G, et al. Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab 2012; 32: 978–82.
Acknowledgements
This research was supported in part by the National Natural Science Foundation of China (No 81070967) and the Natural Science Foundation of Jiangsu Province (No BK2009296). We wish to thank Su-juan YUAN in Yancheng City No 1 People's Hospital for technological assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary figure is available at the Acta Pharmacologica Sinica website.
Supplementary information
Supplementary Fig. 1
Define the penumbral areas (DOC 654 kb)
Supplementary Fig. 2
Dose-response preliminary finding experiments (DOC 250 kb)
Supplementary Fig. 3
WIN55, 212-2 increases the proliferation of ipsilateral SVZ NG2-positive progenitor cells partially through CB1 (DOC 4605 kb)
Supplementary Fig. 4
Identification of BrdU-positive cells in ipsilateral SVZ following administration of WIN55, 212-2 (DOC 889 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Fang, Yq., Ren, H. et al. WIN55,212-2 protects oligodendrocyte precursor cells in stroke penumbra following permanent focal cerebral ischemia in rats. Acta Pharmacol Sin 34, 119–128 (2013). https://doi.org/10.1038/aps.2012.141
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.141
Keywords
This article is cited by
-
Promoting Oligodendrocyte Differentiation from Human Induced Pluripotent Stem Cells by Activating Endocannabinoid Signaling for Treating Spinal Cord Injury
Stem Cell Reviews and Reports (2022)
-
Modulatory Activity of the Endocannabinoid System in the Development and Proliferation of Cells in the CNS
Neurotoxicity Research (2022)
-
G-Protein-Coupled Receptors and Ischemic Stroke: a Focus on Molecular Function and Therapeutic Potential
Molecular Neurobiology (2021)
-
Minocycline Attenuates Neonatal Germinal-Matrix-Hemorrhage-Induced Neuroinflammation and Brain Edema by Activating Cannabinoid Receptor 2
Molecular Neurobiology (2016)
-
Cannabinoids in Experimental Stroke: A Systematic Review and Meta-Analysis
Journal of Cerebral Blood Flow & Metabolism (2015)