Abstract
The Chinese traditional herb Tripterygium wilfordii Hook f (TwHF) has been widely used in the treatment of autoimmune and inflammatory diseases. Over the past few decades, great efforts have been made to explore modern preparations of TwHF with higher efficacy, solubility, and lower toxicity. In this study, we reviewed several examples both of naturally occurring compounds and their derivatives in TwHF, and summarized the preclinical evaluations with regard to autoimmune and inflammatory diseases. All of the candidate compounds described herein have been or are currently in clinical trials. Although some studies encountered problems, the data still provided valuable references for future studies. (5R)-5-hydroxytriptolide (LLDT-8, Leitengshu) is a novel triptolide derivative with potent immunosuppressive and anti-inflammatory activities developed at Shanghai Institute of Materia Medica. Indeed, a Phase I clinical trial for this compound has been completed in rheumatoid arthritis patients. The results will provide the basis for the further exploration of this ancient herb and encourage the research and development of valuable traditional Chinese medicine.
Similar content being viewed by others
Introduction
For thousands of years, natural products have played an important role throughout the world in treating and preventing human diseases1,2,3,4,5. The discovery and development of immunosuppressants from natural sources have an impressive record: mycophenolic acid (MPA) and cyclosporin A (CsA), the fungal metabolites isolated in 19326 and 19707, respectively; rapamycin, found in 19758; FK506, extracted from a culture filtrate of Streptomyces tsukubaensis in 19879,10; and fingolimod (FTY720), described in 199511. Although the current industry model for drug discovery does not favor natural products, the resources are so vast as to seem unlimited, and these emerging tools will provide important discoveries, leading to new medicines12. Furthermore, the drugs already in use as immunosuppressants (eg, cyclophosphamide, methotrexate, azathioprine, cyclosporin) are associated with some significant problems, including toxicity, a lack of reversibility, and increased susceptibility to viral and other infections13. Indeed, exploring new and innovative immunosuppressants from natural sources has now become a focus of intense research.
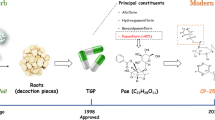
Tripterygium wilfordii Hook f (TwHF) and its extracts have been widely used in the treatment of autoimmune and inflammatory diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and dermatomyositis (DM)14,15,16,17,18, and have beneficial effects on tissue and organ transplantation19,20. In 1993, the ethyl acetate (EA) extract of TwHF was entered into a Phase I study for the treatment of RA patients21,22,23,24,25, and many clinical trials have tested triptolide for the treatment of RA and psoriasis26,27. In addition, PG490-88/F60008 (Figure 1), a water-soluble prodrug of triptolide, has been approved for entry into a Phase I clinical trial for the treatment of solid tumors28,29.
The Shanghai Institute of Materia Medica has made great efforts to discover drugs from natural products that are of clinical value and have contributed to the treatment of autoimmune diseases, such as RA, SLE, and multiple sclerosis (MS). By combining basic and applied research efforts and through the collaboration between chemistry and biology, the SIMM has developed several series of immunosuppressive drug candidates against autoimmune diseases: LLDT-8 (Figure 1), a novel triptolide analog from the Chinese traditional herb TwHF, that will enter into a Phase II clinical trial involving RA patients in China30,31,32,33,34,35,36,37,38,39,40; SM934, a water-soluble derivative of artemisinin; and periplocoside E, a pregnane glycoside identified from Periploca sepium Bge.
This review focuses on the drug candidates isolated from TwHF. Regardless of the success or failure in treatment, the experience gained through the exploration and practice is invaluable.
TwHF, a representative Chinese medicinal herb showing immunosuppressive and anti-inflammatory activities
TwHF is a deciduous climbing vine that grows up to 12 meters and has brown, angular, downy twigs. The leaves are light green, smooth adaxially, and pale gray with light-colored hairs abaxially. As a well-known Chinese medicinal herb, TwHF is distributed widely in southern China, including Fujian, Zhejiang, and Anhui Provinces. The Chinese herb Lei Gong Teng is derived from the roots of TwHF and has been used in traditional Chinese medicine for more than two thousand years. The description of TwHF has been traced to the period of the Three Kingdoms (220–280 AD) when these plants were recorded as “Mangcao” in Shennong's Chinese Materia Medica. In the ensuing thousand years, much Chinese literature, including Materia Medica of South Yunnan (dian nan ben cao) and Guidelines and details on roots and herbs (Bencao gangmu) of the Ming dynasty, Chinese herbal Iconographia Plantarum of the Qing dynasty, and Icons of Chinese medicinal plants in the 20th century, has recorded the resources, efficacy and medicinal application of TwHF. Modern research on the pharmacology of TwHF focuses on active component identification, structure modification and novel derivative discovery.
For centuries, Chinese people have used TwHF and its extracts for the treatment of autoimmune and inflammatory diseases, including RA, SLE, and DM. Contemporary researchers have attempted to standardize the TwHF extract for further development and investigate its efficacy in autoimmune diseases, and some progress had been achieved in recent years. The ethyl acetate (EA) extract of TwHF was entered into a Phase I study that included 13 patients with established RA in 199321,22,23,24,25. The EA extract of TwHF at dosages up to 570 mg/d appeared to be safe, and doses >360 mg/d were associated with clinical benefits in the patients with RA21. The dosage was normalized to previous extracts by assessing the content of triptolide and tripdiolide. A randomized, controlled, 24-week study was then conducted in 2004 in patients with active RA and 6 or more painful and swollen joints22. The results demonstrated that the treatment with a standardized extract from the peeled roots of TwHF administered (60 mg 3 times daily) over 24 weeks may be both effective and safe in treating patients with active rheumatoid arthritis.
With the recent technological developments in the isolation and structural identification of compounds, more than 100 components have been isolated from TwHF, with most of them having a potent therapeutic efficacy for a variety of autoimmune and inflammatory diseases. Among the reported active components from this herb, including triterpene, diterpene and macrocyclic alkaloids, the most noteworthy component is triptolide. Triptolide, an oxygenated diterpene, was identified as the most active component, accounting for the immunosuppressive effects of TwHF41,42. Over the past few decades, much research has been conducted on the clinical use of triptolide for the treatment of autoimmune and inflammatory diseases, such as RA, SLE, nephritis26,43,44,45,46,47,48,49,50,51,52, psoriasis27, and Crohn's disease53, and kidney transplantation54. However the strong toxicity, particularly with regard to male reproductive toxicity, limits the application of triptolide to a great extent55,56,57,58.
To identify more effective compounds with less toxicity and higher solubility, the structural modification of triptolide was studied, and derivatives were synthesized and evaluated for their biological activities. In the past few years, new water-soluble triptolide derivatives have been designed and synthesized, including PG490-88 or F60008. PG490-88 or F60008, a prodrug of triptolide, is converted to triptolide in vivo by plasma esterases following intravenous administration59,60,61,62,63,64,65,66,67. Table 1 summarizes the preclinical pharmacological study of triptolide and PG490-88/F60008 against autoimmune diseases and transplantation rejection. Although PG490-88 or F60008 has been approved for entry into a Phase I clinical trial for the treatment of solid tumors, two lethal events were observed in twenty patients, and the high inter-individual variability rendered PG490-88 or F60008 a far from optimal derivate of triptolide28,29.
(5R)-5-hydroxytriptolide (LLDT-8, Leitengshu), a novel triptolide analog in clinical trials
Great efforts have been made at the SIMM in the search for promising triptolide analogs with a low toxicity and relative high immunosuppressive activity, and a series of novel triptolide analogs have been successfully synthesized. We have identified one derivative, (5R)-5-hydroxytriptolide (LLDT-8, Leitengshu), which demonstrates potent immunosuppressive and anti-inflammatory activities31. Over the course of ten years, the biological activity of LLDT-8 has been evaluated, and the underlying mechanisms have been investigated with regard to many autoimmune and inflammatory diseases. The administration of LLDT-8 reduced the incidence and severity of collagen-induced arthritis in DBA/1 mice33. To assess the long-term effectiveness of LLDT-8, 3-month-treatment experiments were performed. The oral administration of LLDT-8 (0.125, 0.25, and 0.5 mg/kg, starting at 1 d before the booster immunization) consistently attenuated the severity of CIA compared with untreated mice. LDP, Leigongteng Duodai Pian, is the prescription drug of the extracts of TwHF used for treating RA in China. The preventive effect of LLDT-8 at a dose of 0.25 mg/kg was similar to LDP at 20 mg/kg in CIA mice. We also tested the therapeutic effect of LLDT-8 after the establishment of RA, and the inhibitory profile was persistent during the 3-month observation. LLDT-8 also exerted therapeutic effects on experimental autoimmune encephalomyelitis (EAE)36, concanavalin A-induced acute hepatitis37, graft-versus-host disease (GVHD)32, allograft rejection38, and bleomycin-induced lung fibrosis39. LLDT-8 effectively inhibited human T cell immune responses without affecting the NK cytotoxic activity, and this immunosuppressive activity was parallel to that observed for murine immunity40. The mechanism of LLDT-8 involves a variety of immune cells and molecules and includes limiting T cell function and proliferation, inhibiting macrophage activation, inducing regulatory T cell expansion, and interfering with IFN-γ-related signaling31,32,33,34,35,36,37,38,39,40. More importantly, compared to triptolide, LLDT-8 displayed a much lower toxicity both in vitro and in vivo. The CC50 value of LLDT-8 was 256.6±73.8 nmol/L, and the CC50 value of triptolide was 2.1±0.3 nmol/L in murine splenocytes. The immunosuppressive effects of LLDT-8 and triptolide were also tested in mitogen- and alloantigen-induced lymphocyte proliferation assays. The IC50 values of triptolide for inhibiting ConA-induced T lymphocyte proliferation, LPS-induced B lymphocyte proliferation, and mixed lymphocyte reaction (MLR) were 6.7±0.2, 8.6±2.8, and 2.7±0.6 nmol/L, respectively, with the IC50 values of triptolide being close to or even higher than the CC50 values. This result indicated that the activities of triptolide were largely dependent on its cytotoxicity. However, the IC50 values of LLDT-8 for inhibiting the lymphocyte proliferation caused by ConA, LPS, or MLR were lower than its CC50 values (IC50=131.7±32.4, 171.5±17.3, and 38.8±5.1 nmol/L, respectively), thus excluding the possibility that the inhibitory activities of LLDT-8 were attributable to its cytotoxicity31. When administered in mice, the lethal dose for 50% of the animal test population of LLDT-8 is 9.3 mg/kg (intraperitoneal), whereas that of triptolide is 0.86 mg/kg, with a 10-fold lower acute toxicity in vivo30. Table 2 summarizes the preclinical pharmacological study of LLDT-8 as an immunosuppressant drug candidate. Female RA patients who were over the 35 years of age and menopausal or did not have birth demands were enrolled in a tolerability and pharmacokinetic study. The tolerability and pharmacokinetic properties of LLDT-8 and its initial therapeutic efficacy were assessed and determined. According to the pharmacokinetic and pharmacodynamic results reported using experimental animals, the initial dose should be set at 0.25 mg/d. In accord with the dose escalation scheme, the highest dose could be set at 4 mg/d in the phase I clinical trial for LLDT-830. The results of the clinical trial will provide the basis for the further exploration of this novel derivate of triplide and encourage the research and development of valuable traditional Chinese medicine.
Concluding remarks
Research on TwHF has long been an intense issue. During the past few decades, several drug candidates from this herb have been or are currently in clinical trials. However, it has been demonstrated that some compounds cannot be considered the optimal derivative of triptolide. For other compounds, for example, LLDT-8, we are still awaiting the results of the clinical studies. Regardless of the results of the trials, research on this important ancient medicinal herb will continue.
Abbreviations
TwHF, Tripterygium wilfordii Hook f; TCM, traditional Chinese medicine; LLDT-8, (5R)-5-hydroxytriptolide; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; DM, dermatomyositis; EA, ethyl acetate; MPA, mycophenolic acid; CsA, cyclosporin A; FTY720, fingolimod; MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; GVHD, graft-versus-host disease.
References
Drug discovery from Nature. Susanne Grabley, Ralf Thiericke. ISBN 3-540- 64844-5 Springer-Verlag Berlin Heidelberg New York.
Paterson I, Anderson EA . The renaissance of natural products as drug candidates. Science 2005; 310: 451–3.
Koehn FE, Carter GT . The evolving role of natural products in drug discovery. Nat Rev Drug Discov 2005; 4: 206–20 .
Ganesan A . The impact of natural products upon modern drug discovery. Curr Opin Chem Biol 2008; 123: 306–17.
Newman DJ, Cragg GM, Snader KM . The influence of natural products upon drug discovery. Nat Prod Rep 2000; 17: 215–34 .
Oxford AE, Raistrick H . Studies in the biochemistry of micro-organisms: 3:5-Dihydroxyphthalic acid, a new product of the metabolism of glucose by Penicillium brevi-compactum and related species. Biochem J 1932; 26: 1902–6.
Petcher TJ, Weber H, Rüegger A . Crystal and molecular structure of an iodo-derivative of the cyclic undecapeptide cyclosporin A. Helv Chim Acta 1976; 14; 59: 1480–9.
Vézina C, Kudelski A, Sehgal SN . Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975; 28: 721–6.
Ochiai T, Nakajima K, Nagata M, Hori S, Asano T, Isono K . Studies of the induction and maintenance of long-term graft acceptance by treatment with FK506 in heterotopic cardiac allotransplantation in rats. Transplantation 1987; 44: 734–8.
Ochiai T, Nagata M, Nakajima K, Suzuki T, Sakamoto K, Enomoto K, et al. Studies of the effects of FK506 on renal allografting in the beagle dog. Transplantation 1987; 44: 729–33.
Adachi K, Kohara T, Nakao N, Aritac M, Chibac K, Mishina T, et al. Design, synthesis and structure-activity relationships of 2-substituted 2-amino-1,3-propanediols: Discovery of a novel immunosuppressant, FTY720. Bioorg Med Chem Lett 1995; 5: 853–6.
Li JW, Vederas JC . Drug discovery and natural products: end of an era or an endless frontier? Science 2009; 325: 161–5.
Allison AC . Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology 2000; 47: 63–83.
Tao X, Davis LS, Lipsky PE . Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum 1991; 34: 1274–81.
Asano K, Matsuishi J, Yu Y, Kasahara T, Hisamitsu T . Suppressive effects of Tripterygium wilfordii Hook f, a traditional Chinese medicine, on collagen arthritis in mice. Immunopharmacology 1998; 39: 117–26.
Gu WZ, Brandwein SR . Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol 1998; 20: 389–400.
Gu WZ, Banerjee S, Rauch J, Brandwein SR . Suppression of renal disease and arthritis, and prolongation of survival in MRL-lpr mice treated with an extract of Tripterygium wilfordii Hook f. Arthritis Rheum 1992; 35: 1381–6.
Gu WZ, Brandwein SR, Banerjee S . Inhibition of type II collagen induced arthritis in mice by an immunosuppressive extract of Tripterygium wilfordii Hook f. J Rheumatol 1992; 19: 682–8.
Asano K, Yu Y, Kasahara T, Hisamitsu T . Inhibition of murine chronic graft-versus-host disease by the chloroform extract of Tripterygium wilfordii Hook f. Transpl Immunol 1997; 5: 315–9.
Wang J, Xu R, Jin R, Chen Z, Fidler JM . Immunosuppressive activity of the Chinese medicinal plant Tripterygium wilfordii: II. Prolongation of hamster-to-rat cardiac xenograft survival by combination therapy with the PG27 extract and cyclosporine. Transplantation 2000; 70: 456–64.
Tao X, Cush JJ, Garret M, Lipsky PE . A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol 2001; 28: 2160–7.
Goldbach-Mansky R, Wilson M, Fleischmann R, Olsen N, Silverfield J, Kempf P, et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med 2009; 151: 229–40, W49–51.
Tao X, Younger J, Fan FZ, Wang B, Lipsky PE . Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum 2002; 46: 1735–43.
Cibere J, Deng Z, Lin Y, Ou R, He Y, Wang Z, et al. A randomized double blind, placebo controlled trial of topical Tripterygium wilfordii in rheumatoid arthritis: reanalysis using logistic regression analysis. J Rheumatol 2003; 30: 465–7.
Marks WH . Tripterygium wilfordii Hook F. versus Sulfasalazine in the treatment of rheumatoid arthritis: a well-designed clinical trial of a botanical demonstrating effectiveness. Fitoterapia 2011; 82: 85–7.
Jiang X . Clinical observations on the use of the Chinese herb Tripterygium wilfordii Hook for the treatment of nephrotic syndrome. Pediatr Nephrol 1994; 8: 343–4.
Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U . Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol 2012; 74: 424–36.
Harousseau JL, Dombret H, Pigneux A, Michallet M, Brandely M . Phase I study of F60008, a triptolide derivative, in patients with refractory or relapsing acute leukemias. 13th Congress of the European Hematology Association (EHA); 2008: 2008.
Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L, et al. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur J Cancer 2009; 45: 1764–72.
Liu J, Chen X, Zhang Y, Miao H, Liu K, Li L, et al. Derivatization of (5R)-hydroxytriptolide from benzylamine to enhance mass spectrometric detection: application to a Phase I pharmacokinetic study in humans. Anal Chim Acta 2011; 689: 69–76.
Zhou R, Zhang F, He PL, Zhou WL, Wu QL, Xu JY, et al. (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide analog mediates immunosuppressive effects in vitro and in vivo. Int Immunopharmacol 2005; 5: 1895–903.
Tang W, Yang Y, Zhang F, Li YC, Wang JX, Zhu YN, et al. Prevention of graft-versus-host disease by a novel immunosuppressant, (5R)-5-hydroxytriptolide (LLDT-8), through expansion of regulatory T cells. Int Immunopharmacol 2005; 5: 1904–13.
Zhou R, Tang W, Ren YX, He PL, Zhang F, Fu YF, et al. (5R)-5-hydroxytriptolide (LLDT-8) prevented collagen-induced arthritis in DBA/1 mice via suppressing IFN-γ production and its related signaling. J Pharmacol Exp Ther 2006; 318: 35–44.
Zhou R, Wang JX, Tang W, He PL, Yang YF, Li YC, et al. (5R)-5-hydroxytriptolide inhibits IFN-gamma-related signaling. Acta Pharmacol Sin 2006; 27: 1616–21.
Zhou R, Zheng SX, Tang W, He PL, Li XY, Yang YF, et al. Inhibition of inducible nitric-oxide synthase expression by (5R)-5-hydroxytriptolide (LLDT-8) in interferon-γ and bacterial lipopolysaccharide stimulated macrophages. J Pharmacol Exp Ther 2006; 316: 121–8.
Fu YF, Zhu YN, Ni J, Zhong XG, Tang W, Zhou R, et al. (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide derivative, prevents experimental autoimmune encephalomyelitis via inhibiting T cell activation. J Neuroimmunol 2006; 175: 142–51.
Zhou R, Tang W, Ren YX, He PL, Yang YF, Li YC, et al. Preventive effects of (5R)-5-hydroxytriptolide on concanavalin A-induced hepatitis. Eur J Pharmacol 2006; 537: 181–9.
Tang W, Yang Y, Zhou R, Li YC, Wang JX, Yang YF, et al. (5R)-5-hydroxytriptolide (LLDT-8), a potential inhibitor of chemokine, prevents allograft rejection in full MHC-mismatched mouse cardiac transplantation. Transplantation 2006; 81: 927–33.
Ren YX, Zhou R, Tang W, Wang WH, Li YC, Yang YF, et al. (5R)-5-hydroxytriptolide (LLDT-8) protects against bleomycin-induced lung fibrosis in mice. Acta Pharmacol Sin 2007; 28: 518–25.
Zhou R, Tang W, He PL, Ren YX, Yang YF, Li YC, et al. (5R)-5-hydroxytriptolide inhibits the immune response of human peripheral blood mononuclear cells. Int Immunopharmacol 2009; 9: 63–9.
Chan MA, Kohlmeier JE, Branden M, Jung M, Benedict SH . Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. Phytother Res 1999; 13: 464–7.
Gu WZ, Chen R, Brandwein S, McAlpine J, Burres N . Isolation, purification, and characterization of immunosuppressive compounds from tripterygium: triptolide and tripdiolide. Int J Immunopharmacol 1995; 17: 351–6.
Kupchan SM, Court WA . Dailey RG Jr, Gilmore CJ, Bryan RF . Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc 1972; 94: 7194–5.
Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H, et al. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol 2007; 73: 136–46.
Wang Y, Wei D, Lai Z, Le Y . Triptolide inhibits CC chemokines expressed in rat adjuvant-induced arthritis. Int Immunopharmacol 2006; 6: 1825–32.
Li R, Takazawa K, Suzuki H, Hariya A, Yamamoto T, Matsushita S, et al. Synergistic effect of triptolide and tacrolimus on rat cardiac allotransplantation. Jpn Heart J 2004; 45: 657–65.
Liu Y, Chen Y, Liu FQ, Lamb JR, Tam PK . Combined treatment with triptolide and rapamycin prolongs graft survival in a mouse model of cardiac transplantation. Transpl Int 2008; 21: 483–94.
Tao X, Davis LS, Hashimoto K, Lipsky PE . The Chinese herbal remedy, T2, inhibits mitogen-induced cytokine gene transcription by T cells, but not initial signal transduction. J Pharmacol Exp Ther 1996; 276: 316–25.
Yang SX, Gao HL, Xie SS, Zhang WR, Long ZZ . Immunosuppression of triptolide and its effect on skin allograft survival. Int J Immunopharmacol 1992; 14: 963–9.
Lu H, Hachida M, Enosawa S, Li XK, Suzuki S, Koyanagi H . Immunosuppressive effect of triptolide in vitro. Transplant Proc 1999; 31: 2056–7.
Chen BJ . Triptolide, a novel immunosuppressive and antiinflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma 2001; 42: 253–65.
Fidler JM, Ku GY, Piazza D, Xu R, Jin R, Chen Z . Immunosuppressive activity of the Chinese medicinal plant Tripterygium wilfordii. III. Suppression of graft-versus-host disease in murine allogeneic bone marrow transplantation by the PG27 extract. Transplantation 2002; 74: 445–57.
Ren J, Tao Q, Wang X, Wang Z, Li J . Efficacy of T2 in active Crohn's disease: a prospective study report. Dig Dis Sci 2007; 52: 1790–7.
Ji SM, Wang QW, Chen JS, Sha GZ, Liu ZH, Li LS . Clinical trial of Tripterygium Wilfordii Hook F in human kidney transplantation in China. Transplant Proc 2006; 38: 1274–9.
Wang J, Xu R, Jin R, Chen Z, Fidler JM . Immunosuppressive activity of the Chinese medicinal plant Tripterygium wilfordii. I. Prolongation of rat cardiac and renal allograft survival by the PG27 extract and immunosuppressive synergy in combination therapy with cyclosporine. Transplantation 2000; 70: 447–55.
Huynh PN, Hikim AP, Wang C, Stefonovic K, Lue YH, Leung A, et al. Long-term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J Androl 2000; 21: 689–99.
Hikim AP, Lue YH, Wang C, Reutrakul V, Sangsuwan R, Swerdloff RS . Posttesticular antifertility action of triptolide in the male rat: evidence for severe impairment of cauda epididymal sperm ultrastructure. J Androl 2000; 21: 431–7.
Lue Y, Sinha Hikim AP, Wang C, Leung A, Baravarian S, Reutrakul V, et al. Triptolide: A potential male contraceptive. J Androl 1998; 19: 479–86.
Chen BJ, Liu C, Cui X, Fidler JM, Chao NJ . Prevention of graft-versus-host disease by a novel immunosuppressant, PG490-88, through inhibition of alloreactive T cell expansion. Transplantation 2000; 70: 1442–7.
Crews GM, Erickson L, Pan F, Fisniku O, Jang MS, Wynn C, et al. Down-regulation of TGF-beta and VCAM-1 is associated with successful treatment of chronic rejection in rats. Transplant Proc 2005; 37: 1926–8.
Chen G, Sun H, Arp J, Garcia B, Wang X, Wise Y, et al. A synergistic effect between PG490-88 and tacrolimus prolongs renal allograft survival in monkeys. Am J Transplant 2006; 6: 714–23.
Wang X, Sun H, Chen G, Liu W, Wise Y, Yung C, et al. Immunosuppression with a combination of pg490-88 and a subtherapeutic dose of FK506 in a canine renal allograft model. Transplantation 2005; 79: 1537–44.
Fisniku O, Pan F, Wynn C, Erickson LM, Crews G, Jang MS, et al. Protective effects of PG490-88 on chronic allograft rejection by changing intragraft gene expression profiles. Transplant Proc 2005; 37: 1962–4.
Pan F, Fisniku O, Wynn C, Erickson LM, Crews G, Jang MS, et al. PG490-88, a new immunosuppressant, effectively prevents acute and chronic rejection in rat renal allografts. Transplant Proc 2005; 37: 134–6.
Leonard CT, Soccal PM, Berry GJ, Doyle RL, Theodore J, Duncan SR, et al. PG490-88, a derivative of triptolide, attenuates obliterative airway disease in a mouse heterotopic tracheal allograft model. J Heart Lung Transplant 2002; 21: 1314–8.
Chen BJ, Chen Y, Cui X, Fidler JM, Chao NJ . Mechanisms of tolerance induced by PG490-88 in a bone marrow transplantation model. Transplantation 2002; 73: 115–21.
Krishna G, Liu K, Shigemitsu H, Gao M, Raffin TA, Rosen GD . PG490-88, a derivative of triptolide, blocks bleomycin-induced lung fibrosis. Am J Pathol 2001; 158: 997–1004.
Acknowledgements
This work was supported by grants from the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” of China (No 2012ZX09102-101-006) and the Science and Technology Commission of Shanghai Municipality (STCSM) (No 08XD14053).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, W., Zuo, Jp. Immunosuppressant discovery from Tripterygium wilfordii Hook f: the novel triptolide analog (5R)-5-hydroxytriptolide (LLDT-8). Acta Pharmacol Sin 33, 1112–1118 (2012). https://doi.org/10.1038/aps.2012.108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.108
Keywords
This article is cited by
-
Tubule-specific protein nanocages potentiate targeted renal fibrosis therapy
Journal of Nanobiotechnology (2021)
-
(5R)-5-Hydroxytriptolide (LLDT-8) induces substantial epigenetic mediated immune response network changes in fibroblast-like synoviocytes from rheumatoid arthritis patients
Scientific Reports (2019)
-
CYP3A4 inducer and inhibitor strongly affect the pharmacokinetics of triptolide and its derivative in rats
Acta Pharmacologica Sinica (2018)
-
(5R)-5-hydroxytriptolide ameliorates anti-glomerular basement membrane glomerulonephritis in NZW mice by regulating Fcγ receptor signaling
Acta Pharmacologica Sinica (2018)
-
Bioactive diterpenoids from Celastraceae species
Phytochemistry Reviews (2017)