Abstract
Aim:
To assess a novel hormone replacement therapy (HRT) paradigm using raloxifene, aspirin combined with estrogen in rabbit model of menopause.
Methods:
Female New Zealand white rabbits were ovariectomized or sham-operated. The ovariectomized rabbits were divided into 7 groups: estradiol valerate (E2), raloxifene, aspirin, E2 /raloxifene, E2/aspirin, E2 /raloxifene/aspirin and vehicle. Two weeks after the operation, the rabbits were administered the above drugs for 12 weeks. Then, the mammary glands were examined histologically, uterus was weighted, and blood sample was collected for analyzing the levels of estrogen, serum lipids and monocyte chemoattractant protein (MCP)-1, and platelet aggregation. The aortic tissue was examined morphometrically.
Results:
Compared with E2 0.1 mg·kg−1·d−1 treatment alone, the pairing of raloxifene 10 mg·kg−1·d−1 with E2 significantly decreased the extent of mammary gland branches and ducts (5.53%±1.23% vs 15.4%±2.17%, P<0.01), as well as the uterine weight (2.16±0.35 g vs 4.91±0.75 g, P<0.01). However, E2/raloxifene or E2 alone treatment significantly stimulated platelet aggregation relative to vehicle group. Addition of aspirin 5 mg·kg−1·d−1 reduced platelet aggregation to almost the same level as the vehicle group. E2 treatment exerted a positive effect on serum lipids and MCP-1, and a regression in aortic intimal plaque size compared to the vehicle. Raloxifene reinforced the positive effects of E2.
Conclusion:
The combination of raloxifene, aspirin and E2 exhibits positive lipid, MCP-1 and atherosclerotic responses with minimal stimulation of breast and uterine tissues as well as platelet aggregation in a rabbit model of the menopause.
Similar content being viewed by others
Introduction
The beneficial effects of estrogen are well described and include reductions in low density lipoprotein cholesterol (LDL-C) and inflammatory cytokines, such as monocyte chemoattractant protein-1 (MCP-1), an increase in high density lipoprotein cholesterol (HDL-C), and enhanced vascular function, amongst others1, 2, 3. In animal models, estrogen has been reported to reduce atherosclerosis4 and a clinical study also demonstrated a lower calcified plaque burden in the coronary arteries of postmenopausal women aged 50 to 59 years old assigned to estrogen therapy than in those assigned to placebo5. However, estrogen therapy has also been reported to be linked with an increased risk of tissue-specific side effects, including uterine endometrial hyperplasia, which may result in uterine cancer, and proliferative effects on mammary tissue, which may result in an increased risk of breast cancer6, 7. The ideal hormone replacement therapy (HRT) should reproduce the beneficial effects of estrogen without producing these adverse responses. This concept has led to the development of HRT with combined estrogen and progestin, with progestin serving to reverse the endometrial hyperplasia induced by estrogen. However, findings from randomized placebo-controlled trials in postmenopausal women do not support any cardiovascular benefits of estrogen plus progestin, and demonstrate an increase in the risk of breast cancer8, 9. Although the role of progestin remains poorly defined, preclinical and clinical studies have suggested that the co-administration of progestin may oppose the cardioprotective effects of estrogen10, 11, 12. Furthermore, progestin has no anti-estrogenic effect on mammary tissue and, as a result, women on combined estrogen-progestin treatment had an increase in the relative risk for breast cancer8, 9.
Continued efforts to provide efficacious HRT with improved safety and tolerability for postmenopausal women have generated interest in the development of selective estrogen receptor (ER) modulators (SERMs). Estrogen typically exhibits an ER agonist effect in all tissues, whereas SERMs demonstrate mixed functional activity (ER agonist/antagonist) depending on the target tissue13. To date, no SERM alone has been able to achieve an ideal balance of ER agonist and antagonist activity for an optimal postmenopausal therapy. However, it may be possible to achieve optimal results based on the blended tissue-selective activities of a SERM and estrogen in a novel approach.
The SERM chosen in this study to test our hypothesis was raloxifene, as it has been confirmed to have estrogen agonist effects on lipids and estrogen antagonist effects on the breast and uterus14, 15. It is also the only SERM being specifically studied for its effects on coronary heart disease events in a prospective randomized controlled trial, which demonstrated that the incidence of coronary events was significantly lower in postmenopausal women <60 years of age assigned to raloxifene compared with those assigned to the placebo16. Although raloxifene has been reported to be associated with an increased incidence of vasomotor symptoms17, 18, a previous study confirmed that combined use of 17β-estradiol with raloxifene was able to decrease the frequency of vasomotor symptoms in comparison with raloxifene treatment alone19. Based on these observations, the pairing of raloxifene with estrogen may represent an attractive therapeutic option.
However, estrogen has been reported to increase the risk of venous thrombosis and arterial thrombotic events, such as stroke20, 21, 22. Aspirin, one of the most widely used blood-thinning agents in the world, is used long-term in low doses to prevent heart attacks, strokes and blood clot formation in people at high risk for these events. In animal tests, low-dose aspirin has been shown to have a preventive effect on thrombus formulation23 and in a clinical trial, it has been shown to prevent deep venous thrombosis and pulmonary embolism in patients postoperatively24.
Therefore, based on the available evidence, it may be postulated that combined use of raloxifene and aspirin with estrogen might serve as a new treatment paradigm for HRT. To determine whether this regimen would result in a physiological profile that was distinct from that of estrogen alone, we evaluated breast, uterine, platelet aggregation, lipid, MCP-1 and atherosclerotic lesion responses in a rabbit model of the menopause.
Materials and methods
Eighty healthy and sexually mature (age, 3 months; weight, 2.25±0.20 kg) female New Zealand White rabbits (Agricultural Sciences Institute Products; Ji-nan, China) were used in the present study. The rabbits were housed individually in standard rabbit cages with a room temperature of 20±2 °C and 12-h light cycle. After 2 weeks of acclimation, bilateral ovariectomies were performed in 70 rabbits under general anesthesia (sodium pentobarbital 30 mg/kg, intravenously). All rabbits subsequently received a 1.5% cholesterol diet for 12 weeks. The rabbits were randomized into seven groups of 10 rabbits: (A) a placebo-control group receiving vehicle control (saline: 2% Tween 80, 0.5% methylcellulose; OVX+Veh group); (B) estradiol valerate (E2, Delpharm Lille SAS, Lys-lez-Lannoy, France) 0.1 mg·kg−1·d−1 (OVX+E2 group); (C) raloxifene hydrochloride (Lilly SA, Madrid, Spain) 10 mg·kg−1·d−1 (OVX+Ral group); (D) aspirin (Huanghai Company, Shandong, China) 5 mg·kg−1·d−1 (OVX+ASA group); (E) raloxifene paired with E2 (OVX+E2+Ral group); (F) aspirin paired with E2 (OVX+E2+ASA group); and (G) raloxifene and aspirin in combination with E2 (OVX+E2+Ral+ASA group). Compounds were administered orally in a saline vehicle to the rabbits. The dose of E2 (0.1 mg·kg−1·d−1) selected was the dose commonly used for research, which has been shown not to cause serious disorders in the major organs of cholesterol-fed rabbits25. The dose of raloxifene (10 mg·kg−1·d−1) was chosen based on previous pharmacokinetic data that showed this dosage generates a plasma raloxifene concentration comparable with that observed in clinical settings and which reduces aortic accumulation of cholesterol in rabbits26. Furthermore, it has been reported that, at a dose of 10 mg·kg−1·d−1, raloxifene is able to counteract the effects of 0.1 mg·kg−1·d−1 of E2 and maintain the uterine weight at levels that are indistinguishable from those of vehicle-treated controls in animal tests27. Aspirin was used at an antithrombotic dosage (5 mg·kg−1·d−1). One group of 10 rabbits was sham-operated, and also received a 1.5% cholesterol diet but no hormone treatment (sham group). The study protocol was approved by the Animal Care Committee of Shandong University, China and was performed according to the Guidelines for the Use of Experimental Animals by the Ministry of Health, China.
At the end of study, the rabbits were sacrificed after 12 h of fasting. Breast tissue specimens were fixed in 10% neutral buffered formalin, routinely processed, paraffin embedded, sectioned and stained with hematoxylin and eosin. The percentage of the extent of gland branches and ducts in the mammary gland was determined using a computer-based quantitative color image analysis system Ipp6.0 (Media Cybernetics, Bethesda, MD, USA). The acquisition of images and analysis of gland branches and ducts were performed in a blinded fashion. Uteri were excised and weighed after removal of associated fat and luminal fluids.
Platelet-rich plasma (PRP) was separated from acid citrate dextrose-anticoagulated blood. Purity of PRP was validated by Coulter counter (Qilu Hospital Hematology Laboratory, Shandong, China), yielding <0.1% of leukocyte or red blood cell contamination. The concentration of PRP was adjusted to 5×105/μL. Platelet aggregation to adenosine diphosphate (ADP, 10 μmol/L) was performed in PRP by a turbidimetric method using a whole-blood aggregometer in optical mode (Model No 560-CA, Chrono-log, Havertown, PA, USA).
Serum collected from the rabbits underwent E2 measurement using enzyme-linked immunoassay (ELISA). The ELISA kits were purchased from DRG International Inc (Mountainside, NJ, USA) and the experiments were performed following manufacturer's protocol. The laboratory personnel that performed this assay were blinded with respect to any information concerning the study groups. All samples were run in duplicate and the mean values for each sample were used in the analysis. For quality control purposes, positive controls containing known amounts of E2 were included in each batch. This also ensured that the assay values did not dramatically shift over time. The sensitivity was 1.4 pg/mL, the intra-assay co-efficient of variation and inter-assay coefficient of variation for all assays were <5%.
The biochemical evaluation of serum lipids was carried out in the same laboratory as the other tests in this study. The laboratory adhered to the criteria of the World Health Organization Lipid Reference Laboratories. All biochemical examinations (serum total cholesterol, HDL-C and triglycerides) were carried out using a chromatographic enzymatic method in a Technicon automatic analyzer HITACHI-7180 (Hitachi High-Technologies Corporation, Tokyo, Japan). Serum for the measurement of these lipids was harvested immediately after admission onto the study. LDL-C was calculated using the Friedwald formula: LDL-C=total cholesterol–HDL-cholesterol –(1/5) triglycerides28. The estimation of all lipids was rigorously quality-controlled by a consultant biochemist (KL), and frequently checked with the values of another reference laboratory. Inter-assay and intra-assay variabilities of estimations were kept at less than 5%.
For the measurement of serum MCP-1, a quantitative sandwich enzyme immunoassay from R&D systems (Abingdon, UK) was used, where a monoclonal antibody specific for MCP-1 had been pre-coated onto a microplate. Briefly, assay diluent RD1A plus standard or sample was added to each well and left to incubate for 2 h at room temperature. The plates were washed four times to eliminate any unbound substances. Then, a conjugate (polyclonal antibody conjugated to horseradish peroxidase) was added to each well for detection of the cytokine. After a 2-h incubation at room temperature, the plates were washed four times and substrate solution was added to each well. A 20 min incubation at room temperature allowed color development in proportion to the amount of cytokine bound in the initial step. Finally, a stop solution was added to each well and the intensity of the coloring measured. The sensitivity was 5.0 pg/mL, the intra- and interassay variability was 4.8% and 5.8%, respectively.
The aorta was flushed with 250 mL of cold (4 °C) perfusion fixation (4% paraformaldehyde in phosphate buffered saline) at 100 mmHg, then removed from the aortic valve to the iliac bifurcation and carefully cleaned of adhering fat and connective tissue. The aorta was freed from the adventitia, opened longitudinally, pinned flat and fixed in 4% paraformaldehyde for 24 h, then rinsed with water, stained with Oil Red O solution for 4 h and rinsed with 70% alcohol. The percentage of aorta staining positively with Oil Red O was determined. Quantification was performed by capturing images of the aortas with a digital camera and analyzed using the Ipp6.0computer-based quantitative color image analysis system. A standard size of the aortic segment was used for all rabbits studied. The acquisition of images and analysis of lesions were always performed in a blinded fashion.
Data were expressed as mean±SEM. Inter-group treatment comparisons were performed with one-way ANOVA with the Dunnett test. All analyses were performed using SPSS 13.0 software (SPSS, Inc, Chicago, IL, USA). A P-value of 0.05 or less was considered statistically significant (two-sided).
Results
In the OVX rabbit model, an estrogenic stimulatory response was found with E2 treatment in breast tissue. In the OVX+E2 group, the alveoli and ducts were dilated to a variable extent, and the percentage of the extent of gland branches and ducts in the mammary glands increased significantly compared to the OVX+Veh group (15.4%±2.17% vs 4.88%±1.15%, P<0.01). No estrogenic responses were found in the OVX+Ral group, when raloxifene was co-administered with E2 at a dose of 10 mg·kg−1·d−1 (OVX+E2+Ral group), breast stimulation induced by E2 was reduced to vehicle control levels (5.53%±1.23% vs 4.88%±1.15%, P>0.05). No effect on breast tissue was found with aspirin compared to the OVX+Veh group (Figure 1).
Breast response of E2/raloxifene/aspirin in mature OVX rabbits. After 12 weeks of treatment, breast tissue responses to E2 alone, raloxifene alone, aspirin alone, E2 plus raloxifene, aspirin and E2, aspirin paired with E2 and combined raloxifene were analyzed (n=10 animals per treatment group). (A) In OVX rabbits, alveoli and ducts embedded in a loose fibrous stroma with varying amount of fat were variably dilated in the OVX+E2 group, a regression of the parenchymal lobuloalveolar structures and a replacement of the loose fibrous tissue by fat and dense fibrous tissue were found in the OVX+E2+Ral group. HE stain, Bar=200 μm. (B) The percentage of the extent of gland branches and ducts in the mammary glands. Mean±SEM. n=10. cP<0.01 relative to sham group; fP<0.01 relative to vehicle control; iP<0.01 relative to OVX+E2 group. Veh, vehicle; E2, estradiol valerate; Ral, raloxifene; ASA, aspirin.
In the OVX rabbit model, 12-week treatment with E2 at 0.1 mg·kg−1·d−1 (OVX+E2 group) demonstrated a significant increase in uterine weight, which is a surrogate measure for an estrogenic stimulatory response (P<0.01 vs OVX+Veh group). Associated morphological changes were also observed (data not shown). Specifically, E2 resulted in an increase in luminal epithelial hypertrophy compared with vehicle control. No significant increase in uterine weight was found in OVX+Ral group compared to the OVX+Veh group (2.12±0.4 g vs 2.07±0.34 g, P>0.05), and when raloxifene was co-administered with E2 (OVX+E2+Ral group), the increase in uterine weight induced by E2 was reduced to the levels of the vehicle control group (2.16±0.35 g vs 2.07±0.34 g, P>0.05) (Figure 2).
Uterine response of E2/raloxifene/aspirin in mature OVX rabbits. After 12 weeks of treatment, uterine wet weights (g) in response to E2 alone, raloxifene alone, aspirin alone, raloxifene paired with E2, aspirin and E2, aspirin paired with E2 and combined raloxifene were determined. Compared with sham-operated rabbits, ovariectomy induced significant decreases in uterine wet weights. In OVX rabbits, a significant increase in uterine weight was observed in the OVX+E2 group, no estrogenic stimulatory effect was found in the OVX+Ral group, when paired with E2, raloxifene antagonized the E2-induced increase in uterine wet weight to a level similar as that of vehicle control. Mean±SEM. n=10 animals per treatment group. cP<0.01 relative to sham group; fP<0.01 relative to vehicle control; iP<0.01 relative to OVX+E2 group. Veh, vehicle; E2, estradiol valerate; Ral, raloxifene; ASA, aspirin.
In the OVX rabbit model, 12-week treatment with E2 (OVX+E2 group) demonstrated a significant increase in whole-blood platelet aggregation compared to the OVX+Veh group (83.8%±6.94% vs 63.1%±8.45%, P<0.05). Aspirin (5 mg·kg−1·d−1) decreased the whole-blood platelet aggregation significantly compared to the vehicle control (P<0.01). When aspirin was co-administered with E2 in OVX+E2+ASA group, the stimulation in platelet aggregation induced by E2 was reduced to almost the same levels as in the OVX+Veh group (64.5%±7.25% vs 63.1%±8.45%, P>0.05) (Figure 3).
Platelet aggregation response of E2/raloxifene/aspirin in mature OVX rabbits. After 12 weeks of treatment, whole-blood platelet aggregation (%) in response to E2 (0.1 mg·kg−1·d−1), raloxifene (10 mg·kg−1·d−1), aspirin (5 mg·kg−1·d−1), raloxifene paired with E2, aspirin and E2, aspirin paired with E2 and combined raloxifene were determined. A significant increase in platelet aggregation was observed in the OVX+E2 and OVX+E2+Ral groups, but not in the OVX+Ral group. Aspirin decreased the platelet aggregation significantly as compared to the vehicle control. When co-administered with E2, the E2-induced increase in platelet aggregation was reduced to a level with no difference to vehicle control. Mean±SEM. n=10 animals per treatment group. bP<0.05, cP<0.01 relative to vehicle control; eP<0.05 relative to OVX+E2 group. Veh, vehicle; E2, estradiol valerate; Ral, raloxifene; ASA, aspirin.
The serum estradiol level was much higher in the estrogen-treated groups including OVX+E2, OVX+E2+Ral, OVX+E2+ASA, and OVX+E2+Ral+ASA, than in the OVX+Veh group (P<0.01). Dyslipidemia was improved in the estrogen and/or raloxifene-treated animals (OVX+E2, OVX+E2+Ral, OVX+E2+ASA, OVX+E2+Ral+ASA, and OVX+Ral groups) compared to the vehicle-treated animals (OVX+Veh group). When raloxifene was co-administered with E2, an approximate 15% greater reduction in total cholesterol and a 20% greater reduction in LDL-C relative to E2 alone were observed. There were no significant differences in body weight gain before and after treatments (Table 1).
It has been reported that ovariectomy could induce an increase in MCP-1 level29, and in this study, a significant increase in MCP-1 level was observed after ovariectomy. In OVX rabbits, although reduction in MCP-1 level was not as profound as those associated with E2 (approximately a 40% reduction vs control, P<0.01), a significant decrease in MCP-1 level was observed with raloxifene (approximately a 20% reduction vs control, P<0.05) compared with the OVX+Veh group. When raloxifene was used in combination with E2 in the OVX+E2+Ral group, a 20% greater reduction in MCP-1 level relative to E2 alone was observed (Figure 4).
Effects of E2/raloxifene/aspirin on MCP-1 level in mature OVX rabbits. After 12 weeks of treatment, serum MCP-1 levels (mmol/L) in response to E2 alone, raloxifene alone, aspirin alone, raloxifene paired with E2, aspirin paired with E2 and combined raloxifene, aspirin and E2 were determined. A significant increase in MCP-1 level was observed after ovariectomy in the OVX+Veh group compared with the sham group, and both E2 (0.1 mg·kg−1·d−1) and raloxifene (10 mg·kg−1·d−1) decreased the MCP-1 level significantly compared to the vehicle control. When raloxifene was paired with E2, MCP-1 level was reduced by approximately 20% relative to E2 alone. Mean±SEM. n=10 animals per treatment group. cP<0.01 relative to sham group; eP<0.05, fP<0.01 relative to vehicle control; hP<0.05 relative to OVX+E2 group. Veh, vehicle; E2, estradiol valerate; Ral, raloxifene; ASA, aspirin.
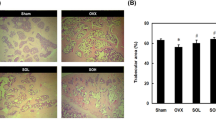
Histopathological analysis demonstrated that the size of atherosclerotic lesions in the aorta increased significantly after ovariectomy. In the OVX rabbit model, 12-week treatment with E2 decreased the size of atherosclerotic lesions significantly (approximately a 40% reduction vs control, P<0.01). Similar results were observed with raloxifene (approximately a 20% reduction vs control, P<0.05). A slight reduction was observed with aspirin although the difference was not statistically significant compared with vehicle control (P=0.052). When raloxifene and aspirin were co-administered with E2, an approximate 20% greater reduction relative to E2 alone was seen (Figure 5).
Effects of E2/ raloxifene/aspirin on atherosclerotic lesion in mature OVX rabbits. After 12 weeks of treatment, atherosclerotic lesions in the aorta (%) in response to E2, raloxifene, aspirin, raloxifene paired with E2, aspirin paired with E2 and combined raloxifene, aspirin and E2 were determined by Oil Red O staining (n=10 animals per treatment group). (A) Oil Red O staining on photographs of aorta. Oil Red O positive area corresponds to lipid deposition. (B) Quantitative analysis of the percentage of Oil Red O positive area (% vessel wall staining positively±SEM). A significant increase in the size of atherosclerotic lesions (Oil Red O staining) was observed after ovariectomy compared with sham-operated animals. In OVX rabbits, although the reduction was not as profound as that associated with E2, a significant decrease was observed in the OVX+Ral group (P<0.05) compared with OVX+Veh group. A mild reduction was observed with aspirin, although the difference was not significant. Further reductions relative to E2 alone were observed with E2/raloxifene (approximately 10% vs E2 alone) and E2/raloxifene/aspirin (approximately 20% vs E2 alone). cP<0.01 relative to sham group; eP<0.05, fP<0.01 relative to vehicle control; hP<0.05 relative to OVX+E2 group. Veh, vehicle; E2, estradiol valerate; Ral, raloxifene; ASA, aspirin.
Discussion
The purpose of this series of in vivo studies was to establish the proof-of-concept for a novel paradigm of HRT. This concept was based on the hypothesis that the combination of raloxifene and aspirin with estrogen would result in a physiological profile that was distinct from estrogen alone, and in particular, an improved pharmacological profile compared with estrogen alone.
The key physiological responses that require close monitoring in the evaluation of estrogen use are breast and uterine tissue stimulation, as estrogen functions directly through the ERs, which are abundant in those tissues. In previous studies, ER-antagonistic activity in the breast and uterus has been found after raloxifene treatment14, 15, 27. In the present study, serum estradiol levels increased significantly in all the estrogen-treated animals, including animals treated with E2 alone, E2 plus raloxifene, and E2 plus aspirin, as well as combined E2, raloxifene and aspirin, compared to vehicle controls in the OVX rabbits. However, maximal breast and uterine stimulations were observed with E2 alone and E2 plus aspirin. In contrast, raloxifene alone did not stimulate the uterus and mammary glands, and when raloxifene was co-administered with E2, the stimulation of the breast and uterus was reduced to vehicle control levels.
Platelets contribute to thrombosis in several ways. They provide the membrane surface for the generation of thrombin, express membrane receptors that affect platelet-platelet and platelet-vessel wall interactions. In low doses, aspirin has an antiplatelet effect by inhibiting the production of thromboxane. Platelets contain both ERα and ERβ30, 31, but the effect of estrogen on the platelet aggregation has been controversial32, 33. In the current study, significant stimulation in platelet aggregation was observed with E2 alone and E2 plus raloxifene, but not with raloxifene alone in the OVX rabbits. As expected, aspirin reduced the platelet aggregation significantly compared with vehicle control, and addition of aspirin to E2 decreased the platelet aggregation to the vehicle control levels.
Due to the fact that raloxifene antagonized the stimulatory effect of E2 on the breast and uterus and aspirin reduced the stimulation of E2 in platelet aggregation, an obvious question that arose was whether the combinatory use of raloxifene and aspirin with E2 would unfavorably antagonize the positive effects of E2, such as lowering levels of LDL-C and MCP-1 and inhibiting atherosclerotic lesions1, 2, 3, 4, 5.
It has been well-documented that a high level of LDL-C and a low level of HDL-C are risk factors for atherosclerosis, and MCP-1, a potent chemoattractant for monocytes, has been suggested to be an especially important mediator of atherogenic signals34, 35. Raloxifene has been confirmed to have estrogen-agonist effects on lipid profiles14, 15 , and an in vitro study has shown that raloxifene could downregulate the expression of MCP-1 in human coronary artery smooth muscle cells, although the degree of this inhibition was less than when induced by estrogen36. In the present study, ovariectomy induced a significant decrease in serum estradiol level and increased total cholesterol, LDL-C, and MCP-1 levels, as well as decreased HDL-C levels. However, significant reductions in total cholesterol, LDL-C, and MCP-1 levels and an increase in HDL-C level were found with E2 treatment alone and raloxifene alone compared with the vehicle control, and further effects were observed with their co-administration, which suggested that raloxifene reinforced the positive effects of E2. As well as its anticoagulant properties, aspirin has cardioprotective effects that include inhibiting the proliferation of vascular smooth muscle cells and improving endothelium-dependent vascular relaxation37. In the present study, reductions in the sizes of atherosclerotic lesions were found by Oil Red O staining assay with E2 alone, raloxifene alone, E2/raloxifene, E2/aspirin, and E2/raloxifene/aspirin compared with vehicle controls in the OVX rabbits. The maximal reduction was observed with E2/raloxifene/aspirin, indicating an additive effect of the combination therapy.
The mechanism by which a target cell simultaneously responds to a mixture of drugs is not entirely clear. Hypothetically, the different molecules would compete for the available pool of receptors in the target cell, and simple receptor kinetics would dictate the dominant effector molecule. The final physiological response would be attributed to a blend of all of the activities of these molecules. In the current study, raloxifene was shown to abrogate the stimulatory effects of E2 on the uterus and breast effectively, while aspirin reduced the stimulatory effect of E2 on platelet aggregation, and both E2 and raloxifene demonstrated positive agonistic effects on the lipid and MCP-1 profiles, and combined raloxifene, aspirin and E2 was the most efficacious treatment in reducing atherosclerotic lesions in the aorta.
The ideal postmenopausal estrogen therapy is expected to reproduce the beneficial effects of estrogen without producing the adverse effects; however, no ideal postmenopausal estrogen therapy has been found to date. The present study demonstrated for the first time that the combination of raloxifene, aspirin and E2 might be an attractive novel paradigm of HRT. This finding may prompt additional research with the aim of improving quality of life for peri- and postmenopausal women.
Author contribution
Hong HE and Fa-lin YANG designed the research and wrote the paper; Ke-qing HU performed the research and analysed the data; Hong HE, Fa-lin YANG, Xin WANG, Zi-mo LIU, Qin HU, and Ji-fu LI contributed to perform the research and analyse the data.
References
Ross R . Atherosclerosis-an inflammatory disease. N Eng J Med 1999; 340: 115–26.
Blum A, Cannon Ro III . Effects of estrogens and selective oestrogen receptor modulators on serum lipoproteins and vascular function. Curr Opin Lipidol 1998; 9: 575–86.
Störk S, Baumann K, Clemens S, Angerer P . The effect of 17β-estradiol on MCP-1 serum levels in postmenopausal women. Cardiovasc Rev 2002; 53: 642–9.
Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, et al. Inhibition of coronary artery atherosclerosis by 17β-estradiol in ovariectomized monkeys: lack of an effect of added progesterone. Arteriosclerosis 1990; 10: 1051–7.
Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. WHI and WHI-CACS Investigators. Estrogen therapy and coronary artery calcification. N Engl J Med 2007; 356: 2591–602.
Barrett-Connor E . Hormone replacement and cancer. Br Med Bull 1992; 48: 345–55.
Cauley, JA, Lucas FL, Kuller FH, Vogt MT, Browner WS, Cummings SR . Bone mineral density and risk of breast cancer in older women: the study of osteoporotic fractures. JAMA 1996; 276: 1404–8.
Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA 1998; 280: 605–13.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA 2002; 288: 321–33.
Hanke H, Hanke J, Bruck B, Brehme U, Gugel N, Finking G, et al. Inhibition of the protective effect of estrogen by progesterone in experimental atherosclerosis. Atherosclerosis 1996; 121: 129–38.
Adams MR, Register TC, Golden DL, Wagner JD, Williams JK . Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogen on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol 1997; 17: 217–21.
He H, Yang F, Liu X, Zeng X, Hu Q, Zhu Q, et al. Sex hormone ratio changes in men and postmenopausal women with coronary artery disease. Menopause 2007; 14: 385–90.
Palacios S . The future of the new selective estrogen receptor modulators. Menopause Int 2007; 13: 27–34.
Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol and uterine endometrium in postmenopausal women. N Engl J Med 1997; 337: 1641–7.
Cummings Sr, Eckert S, Krueger K, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281: 2189–97.
Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, et al. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the raloxifene use for heart trial: results of subgroup analyses by age and other factors. Circulation 2009; 119: 922–30.
Davies GC, Huster WJ, Lu Y, Plouffe L, Lakshmanan M . Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol 1999; 93: 558–65.
Cohen FJ, Lu Y . Characterization of hot flashes reported by healthy postmenopausal women receiving raloxifene or placebo during osteoporosis prevention trials. Maturitas 2000; 34: 65–73.
Stovall DW, Utian WH, Gass M, Qu Y, Muram D, Wong M, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause 2007; 14: 510–7.
Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132: 689–96.
Varas-Lorenzo C, Garcia-Rodriguez LA, Cattaruzzi C, Troncon MG, Agostinis L, Perez-Gutthann S . Hormone replacement therapy and the risk of hospitalization for venous thromboembolism: a population-based study in southern Europe. Am J Epidemiol 1998; 147: 387–90.
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004; 291: 1701–12.
Peter FW, Franken RJ, Wang WZ, Anderson GL, Schuschke DA, O'Shaughnessy MM, et al. Effect of low dose aspirin on thrombus formation at arterial and venous microanastomoses and on the tissue microcirculation. Plast Reconstr Surg 1997; 99: 1112–21.
Pulmonary Embolism Prevention (PEP) trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355: 1295–302.
Finking G, Brehme U, Bruck B, Wehrmann M, Hanke S, Kamenz J, et al. Does anti-atherogenic estradiol valerate treatment cause adverse effects on liver and uterus in NZW rabbits? Vet Hum Toxicol 1998; 40: 136–40.
Bjarnason NH, Haarbo J, Byrjalsen I, Kauffman RF, Knadler MP, Christiansen C . Raloxifene reduces atherosclerosis: studies of optimized raloxifene doses in ovariectimized, cholesterol-fed rabbits. Clin Endocrinol 2000; 52: 225–33.
Al-Jamal JH, Dubin NH . The effect of raloxifene on the uterine weight response in immature mice exposed to 17beta-estradiol, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, and methoxychlor. Am J Obstet Gynecol 2000; 182: 1099–102.
Friedewald WT, Levy RI, Fredrikson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Pervin S, Singh R, Rosenfeld ME, Navab M, Chaudhuri G, Nathan L . Estradiol suppresses MCP-1 expression in vivo. Implications for atherosclerosis. Arterioscler Thromb Vasc Biol 1998; 18: 1838–45.
Jayachandran M, Miller VM . Human platelets contain estrogen receptor α, caveolin-1 and estrogen receptor associated proteins. Platelets 2003; 14: 75–81.
Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, et al. Human megakaryocytes and platelets contain the estrogen receptor β and androgen receptor (AR): testosterone regulates AR expression. Blood 2000; 95: 2289–96.
Elam MB, Limscomb GE, Chesney CM, Terragno DA, Terragno NA . Effect of synthetic estrogen on platelet aggregation and vascular release of PGI2-like material in the rabbit. Prostaglandins 1980; 20: 1039–51.
Nakano Y, Oshima T, Matsuura H, Kajiyama G, Kambe M . Effect of 17β-estradiol on inhibition of platelet aggregation in vitro is mediated by an increase in no synthesis. Arterioscler Thromb Vasc Biol 1998; 18: 961–7.
Tanaka E, Shimokawa H, Kamiuneten H, Eto Y, Matsumoto Y, Morishige K, et al. Disparity of MCP-1 mRNA and protein expressions between the carotid artery and the aorta in WHHL rabbits. Arterioscler Thromb Vasc Biol 2003; 23: 244–21.
Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard E, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A 1991; 88: 5252–6.
Seli E, Selam B, Mor G, Kayisli UA, Pehlivan T, Arici A . Estradiol regulate monocyte chemotactic protein-1 in human coronary artery smooth muscle cells: a mechanism for its antiatherogenic effect. Menopause 2001; 8: 296–301.
Awtry EH, Loscalzo J . Aspirin. Circulation 2000; 101: 1206–18.
Acknowledgements
This work was supported by Grant from the Natural Science Foundation of Shandong Province, Ji-nan, Shandong, China (#Y2005C50), Grant-in-Aid for China-Japan Sasagawa Researchers, from the Ministry of Health, Beijing, China (#083). The authors are grateful to Chun-xi LIU and Zhi YANG for their excellent technical contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Fl., Hu, Kq., Wang, X. et al. Combination of raloxifene, aspirin and estrogen as novel paradigm of hormone replacement therapy in rabbit model of menopause. Acta Pharmacol Sin 32, 1031–1037 (2011). https://doi.org/10.1038/aps.2011.87
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.87