Abstract
Aim:
To investigate the effects of exhaustive swimming exercise on P2X1 receptor- and α1-adrenoceptor-mediated vasoconstriction of different types of arteries in rats.
Methods:
Male Wistar rats were divided into 2 groups: the sedentary control group (SCG) and the exhaustive swimming exercise group (ESEG). The rats in the ESEG were subjected to a swim to exhaustion once a day for 2 weeks. Internal carotid, caudal, pulmonary, mesenteric arteries and aorta were dissected out. Isometric vasoconstrictive responses of the arteries to α,β-methylene ATP (α,β-MeATP) or noradrenaline (NA) were recorded using a polygraph.
Results:
The exhaustive swimming exercise did not produce significant change in the EC50 values of α,β-MeATP or NA in vasoconstrictive response of most of the arteries studied. The exhaustive swimming exercise inhibited the vasoconstrictive responses to P2X1 receptor activation in the internal carotid artery, whereas it reduced the maximal vasoconstrictive responses to α1-adrenoceptor stimulation in the caudal, pulmonary, mesenteric arteries and aorta. The rank order of the reduction of the maximal vasoconstriction was as follows: mesenteric, pulmonary, caudal, aorta.
Conclusion:
Exhaustive swimming exercise differentially affects the P2X1 receptor- and α1-adrenoceptor-regulated vasoconstriction in internal carotid artery and peripheral arteries. The ability to preserve purinergic vasoconstriction in the peripheral arteries would be useful to help in maintenance of the basal vascular tone during exhaustive swimming exercise.
Similar content being viewed by others
Introduction
Regularly engaging in moderate exercise provides many well-established health benefits, including the prevention of, or a reduction in, the deleterious effects of pathological conditions such as hypertension, coronary artery disease, atherosclerosis, diabetes mellitus, and osteoporosis1, 2, 3. However, the beneficial effects of exercise are lost with overexertion, which causes the production of free radicals4 and subsequent damage to lipids, proteins, and DNA. Exhaustive exercise has been shown to lead to tissue damage in animals5, 6. It has also been reported that exhaustive exercise resulted in significant impairment of regional left ventricular systolic and diastolic function in rats7. In the right ventricle of the human heart, tissue Doppler measurements of systolic and early diastolic function have been found to decrease significantly after prolonged strenuous exercise compared to pre-training values8.
Functional changes in the vasculature of animals subjected to physical exercise have attracted considerable attention for many years. Acute exercise significantly attenuates α-adrenoceptor-mediated vasoconstriction of the thoracic aorta isolated from rabbits9, and chronic exercise also decreases adrenergic agonist-induced vasoconstriction in the isolated thoracic aorta and carotid artery of spontaneously hypertensive rats10. It has also been reported that sedentary (control) and exercise-trained rats show no difference in the maximum levels of vasoconstriction induced by noradrenaline (NA) or phenylephrine in the isolated thoracic aorta11. Moderate levels of exercise did not alter either the myogenic regulation of the arterial diameter stimulated by increased transmural pressure or the smooth muscle responses to a thromboxane agonist in control and db/db mice12. Chronic treadmill running significantly enhanced myogenic vasoconstriction in the coronary resistance arteries isolated from female pigs13. Potential reasons for this discrepancy might be differences among arteries studied, the laboratory animals used and the exercise modes employed.
It is well known that the sympathetic and purinergic co-transmission involving NA and adenosine 5′-triphosphate (ATP) exists in a variety of blood vessels. The neurogenic vasoconstriction induced by electrical field stimulation consists of a purinergic (prazosin-resistant) component and an adrenergic (prazosin-sensitive) component in different blood vessels. P2X and P2Y receptors are widely distributed throughout the cardiovascular system and are important in the regulation of vascular tone14. The P2X1 purinoceptor is the primary P2X subtype expressed on most vascular smooth muscle cells15, and is responsible for purinergic arterial contraction16, 17. α,β-Methylene ATP (α,β-MeATP) is considered to be a useful reagent to investigate P2X1 receptor-mediated vasoconstriction18. It has been reported that rats with diet-induced obesity have enhanced sympathetic nerve-mediated vasoconstriction via upregulation of purinergic and adrenergic neurotransmission19. Vidal et al20, however, have indicated that α,β-MeATP significantly inhibits vasoconstrictive responses to electrical field stimulation in the tail arteries of spontaneously hypertensive rats, but does not inhibit these responses in the tail arteries of normal rats. Therefore, the purpose of this study was to examine whether there is similar or differential inhibition of α1-adrenoceptor- and P2X1 purinoceptor-mediated vasoconstriction in the aorta and the internal carotid, caudal, pulmonary and mesenteric arteries in healthy rats after exhaustive swimming exercise.
Materials and methods
Animals
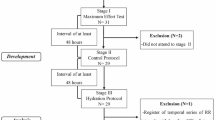
Male Wistar rats weighing 300–350 g (aged 12–13 weeks) were housed one per cage in a temperature-controlled room (24±1 °C) with a 12 h light/dark cycle and received approximately 50% of their daily food intake6 and tap water ad libitum. The commercial standard chow was purchased from Hebei Medical University. Rats were randomly divided into two groups: a sedentary control group (SCG) and an exhaustive swimming exercise group (ESEG). All animals used in this study received humane care in compliance with institutional animal care guidelines. All procedures performed were approved by the Local Institutional Committee.
Chemicals
[–]-Noradrenaline bitartrate (NA), α,β-methylene adenosine 5′-triphosphate lithium salt (α,β-MeATP), desmethylimipramine hydrochloride, deoxycorticosterone acetate, yohimbine hydrochloride, propranolol hydrochloride and acetylcholine hydrochloride were obtained from the Sigma Chemical Company, USA. The above reagents were dissolved in distilled water except for deoxycorticosterone acetate which was dissolved in 1,2-propanediol. The final concentration of 1,2-propanediol in the tissue bath did not affect the vascular responses to NA.
Training protocols
To familiarize the rats with water immersion and reduce water-induced stress, the rats of the ESEG were made to swim in an apparatus holding no less than a water depth of 60 cm for 15 min daily at 8:30 am for 6 days/week during the first week. After the rats had adapted to the swimming exercise, the animals were subjected to a swim to exhaustion with a weight equivalent to 3% of their body weight tied to their tails21 for two weeks. The training, began from 8:30 am to 11:30 am22, was conducted daily for 6 days/week by the same person. Exhaustion was defined by two criteria: the rats remained below the water surface for 10 s, and the rats showed a lack of a “righting reflex” when they were placed on a flat surface21. Simultaneously, the rats of the SCG were kept in a small chamber holding a water depth of 10 cm. The water temperature was maintained at 34-36 °C23.
Arterial preparations
Rats of the ESEG and SCG were anesthetized by subcutaneous injection of urethane (1.5 g/kg) 24 h after the last exhaustive swimming session then sacrificed by cutting the femoral artery, resulting in exsanguination. After the chest and abdomen were opened by a midline incision, the thoracic aorta, right pulmonary artery and superior mesenteric artery were carefully removed. An anterior midline incision was made on the neck to expose the carotid artery. The left and right internal carotid arteries were identified from their origin at the common carotid artery bifurcation to their entry points into the skull and were isolated. The caudal artery was surgically exposed from the ventral side then dissected from surrounding tissues and removed. These isolated arteries were maintained in ice-cold oxygenated Krebs-Henseleit (K-H) solution (133 mmol/L NaCl, 4.7 mmol/L KCl, 1.35 mmol/L NaH2PO4, 16.3 mmol/L NaHCO3, 0.61 mmol/L MgSO4, 7.8 mmol/L glucose and 2.52 mmol/L CaCl2). The vascular endothelium of each artery was removed by gently rubbing the lumen with a scored polythene cannula, the external diameter of which was slightly smaller than the internal diameter of the blood vessel. A ring segment (4 mm long) without endothelium was mounted horizontally in a 10-mL organ bath, and the isometric tension was recorded by a polygraph (ERT-884, Youlin Electron Co, Kaifeng, China). Preloads were applied to the preparations of internal carotid artery (1.0 g), caudal artery (0.75 g), pulmonary artery (1.0 g), mesenteric artery (1.0 g), and aorta (2.0 g)24. The preparations were allowed to equilibrate for 1 h in K-H solution. The solution was maintained at 37 °C and aerated with 95% O2 and 5% CO2 (pH 7.4). Successful removal of the arterial endothelium was confirmed by the loss of the relaxation response to acetylcholine (ACh, 1 μmol/L) in pre-contracted arterial rings treated with NA.
Experimental protocols
Before performing the following procedures, a cumulative dose-response curve for NA (0.0001–100 μmol/L) was constructed for each of the arterial preparations to observe the vasoconstrictive responsiveness, followed by further equilibration for 1 h. After the isolated arterial experiments were performed, a dose-response curve for KCl (10–120 mmol/L) was constructed in the arterial preparations, and the wet weight of each preparation was recorded.
Vasoconstrictive responses to NA in the aorta and the internal carotid, caudal, pulmonary, and mesenteric arteries
In the arterial preparations used to construct a second cumulative dose-response curve for NA, desmethylimipramine (0.1 μmol/L), deoxycorticosterone (5 μmol/L), yohimbine (0.3 μmol/L) and propranolol (1 μmol/L) were added to the organ bath for 30 min to block neuronal or extra-neuronal uptake of NA and to block α2- and β-adrenoceptors, respectively. The second cumulative dose-response curve for NA (0.0001–100 μmol/L) was constructed for each arterial preparation of the aorta and the internal carotid, caudal, pulmonary, and mesenteric arteries to observe α1-adrenoceptor-regulated vasoconstriction.
Vasoconstrictive responses to α,β-MeATP in the aorta and the internal carotid, caudal, pulmonary, and mesenteric arteries
Because α,β-MeATP rapidly desensitizes its own receptors, a single concentration of α,β-MeATP at 0.1, 1.0, 10, or 100 μmol/L was added to the organ bath (each arterial preparation was exposed to α,β-MeATP only once) for each arterial preparation, and the resultant responses of several preparations exposed to different concentrations were grouped together to form a dose-response curve25.
Statistical analysis
Vasoconstrictive responses to NA and α,β-MeATP were expressed as the maximal changes in tension (g) and were further normalized to wet tissue weight (g/mg tissue). Values presented here are the mean±SEM. Two-way ANOVA was used to evaluate any differences between the two sets of dose-response curves. If the F statistic was significant, it was compared to the individual datum with its respective control value by Bonferroni's test. We compared the EC50 values of the agonists, the maximal vasoconstriction to KCl and the wet tissue weight of arterial preparations between the two groups using an unpaired t-test. P values less than 0.05 were considered to be statistically significant. The data were analyzed using GraphPad Prism version 5.00 (San Diego, California, USA).
Results
Effect of exhaustive swimming exercise on wet tissue weight and the vasoconstrictive response to KCl
The wet weight of the mesenteric arterial ring segments in the SCG was 0.73±0.02 mg, which was significantly lower than that in the ESEG (0.85±0.02 mg) (P<0.01, Figure 1A). The values of the wet weights of the internal carotid, caudal, pulmonary, and aortic arterial ring segments in the SCG were not significantly different from those in the ESEG (P>0.05, Figure 1A). The maximal vasoconstrictive responses to KCl in the mesenteric and caudal arteries in the SCG were 1.72±0.05 g/mg tissue and 3.27±0.10 g/mg tissue, respectively. These responses were much greater than those of the ESEG; the vasoconstrictive responses in the mesenteric and caudal arteries were 1.46±0.06 g/mg tissue and 2.85±0.10 g/mg tissue, respectively (P<0.01, Figure 1B). There were no significant differences in the maximal vasoconstrictive responses to KCl of the internal carotid artery, pulmonary artery and the aorta between the SCG and the ESEG rats (P>0.05, Figure 1B). However, the range of EC50 values for KCl in the 5 types of arteries subsequently exposed to the second administration of NA ranged from 17.23 to 31.74 mmol/L, and the EC50 values of KCl in the SCG were not significantly different from those in the ESEG (Table 1).
Tissue wet weight (A) and vasoconstriction to 120 mmol/L KCl (B) in the rat mesenteric (Mes), caudal (Cau), pulmonary (Pul), internal carotid (Int) arteries and aorta (Aor) from the sedentary control group (SCG, n=29−34) and the exhaustive swimming exercise group (ESEG, n=28−35). Data were expressed as mean±SEM. cP<0.01 vs SCG.
The vasoconstrictive responsiveness of the aorta and the internal carotid, caudal, pulmonary, and mesenteric arteries to NA
Before analyzing the difference in the vasoconstrictive responses to either α1-adrenoceptor stimulation or P2X1 purinoceptor stimulation between the two rat groups, we examined the vasoconstrictive responsiveness of the selected arteries individually. In the rats of the SCG, there were no significant differences in the vasoconstrictive responses to the first exposure to NA in the internal carotid, caudal, pulmonary, mesenteric artery or aorta between the preparations subsequently exposed to the second administration of NA and those subsequently exposed to α,β-MeATP (P>0.05, Figure 2A–6A). The same results were observed in the rats from the ESEG (P>0.05, Figure 2B–6B).
A comparison of the vasoconstrictive responses to noradrenaline between the preparations exposing to the second administration of noradrenaline (treatment with NA; n=10, left; n=9, right) and those exposing to α,β-methylene ATP (treatment with α,β-MeATP; n=24, left; n=24, right) in the rat pulmonary arteries from the sedentary control group (A) and the exhaustive swimming exercise group (B). Data were expressed as mean±SEM.
A comparison of the vasoconstrictive responses to noradrenaline between the preparations exposing to the second administration of noradrenaline (treatment with NA; n=9, left; n=8, right) and those exposing to α,β-methylene ATP (treatment with α,β-MeATP; n=20, left; n=20 right) in the rat internal carotid arteries from the sedentary control group (A) and the exhaustive swimming exercise group (B). Data were expressed as mean±SEM.
Effect of exhaustive swimming exercise on the vasoconstrictive responses to NA in the aorta and the internal carotid, caudal, pulmonary, and mesenteric arteries
A second exposure of the 5 selected arteries to NA (0.0001–100 μmol/L) produced vasoconstrictive responses in a dose-dependent manner in the rats of the SCG and the ESEG. The exhaustive swimming exercise significantly decreased the vasoconstrictive response to NA in the pulmonary and caudal arterial preparations (P<0.01, Figure 7A and 8A), reaching a maximal inhibition of 13.68% in the pulmonary artery and 9.48% in the caudal artery. In the mesenteric arterial preparation, the inhibition of vasoconstrictive response to NA by exhaustive swimming exercise was more potent, reaching a maximal inhibition of 21.02% (P<0.01, Figure 9A). Exhaustive swimming exercise significantly inhibited the vasoconstrictive responses to NA in the aorta, but the maximal response was not affected (Figure 10A). The vasoconstrictive response to NA in the internal carotid arterial preparation was not significantly affected by exhaustive swimming exercise (P>0.05, Figure 11A). The range of the negative log(EC50) values (where the EC50 values are expressed as mol/L) for the 5 types of arteries given a second treatment with NA was 6.70–7.58. In the thoracic aorta, the negative log(EC50) value of NA in the SCG (7.43±0.04) was slightly larger than that in the ESEG (7.11±0.10) (P<0.01); however, the EC50 values of NA in the SCG were not significantly different from those in the ESEG in the other 4 types of arteries (Table 1).
A comparison of the vasoconstrictive responses to the second administration of noradrenaline (A) or to α,β-methylene ATP (B) in the rat pulmonary arteries between the sedentary control group (SCG; n=10, A; n=5−7, B) and the exhaustive swimming exercise group (ESEG; n=9, A; n=5−7, B). Mean±SEM. Statistical significance was analyzed by two-way ANOVA in A: cP<0.01 vs SCG.
A comparison of the vasoconstrictive responses to the second administration of noradrenaline (A) or to α,β-methylene ATP (B) in the rat caudal arteries between the sedentary control group (SCG; n=11, A; n=5, B) and the exhaustive swimming exercise group (ESEG; n=15, A; n=5, B). Data were expressed as mean±SEM. Statistical significance was analyzed by two-way ANOVA in A: cP<0.01 vs SCG.
A comparison of the vasoconstrictive responses to the second administration of noradrenaline (A) or to α,β-methylene ATP (B) in the rat mesenteric arteries between the sedentary control group (SCG; n=11, A; n=5−6, B) and the exhaustive swimming exercise group (ESEG; n=9, A; n=5−6, B). Data were expressed as mean±SEM. cP<0.01 vs SCG.
A comparison of the vasoconstrictive responses to the second administration of noradrenaline (A) or to α,β-methylene ATP (B) in the rat thoracic aorta between the sedentary control group (SCG; n=11, A; n=5−6, B) and the exhaustive swimming exercise group (ESEG; n=8, A; n=5−6, B). Data were expressed as mean±SEM. cP<0.01 vs SCG.
A comparison of the vasoconstrictive responses to the second administration of noradrenaline (A) or to α,β-methylene ATP (B) in the rat internal carotid arteries between the sedentary control group (SCG; n=9, A; n=5, B) and the exhaustive swimming exercise group (ESEG; n=8, A; n=5, B). Data were expressed as mean±SEM. cP<0.01 vs SCG.
Effect of exhaustive swimming exercise on vasoconstrictive responses to α,β-MeATP in the aorta and the internal carotid, caudal, pulmonary, and mesenteric arteries
α,β-MeATP (0.1–100 μmol/L) produced vasoconstriction in the internal carotid, caudal, pulmonary, and mesenteric arteries, as well as the aorta, in a dose-dependent manner in the rats of the SCG and the ESEG. The vasoconstrictive responses to α,β-MeATP in the caudal artery, pulmonary artery, mesenteric artery and the aorta were not significantly affected by exhaustive swimming exercise (P>0.05, Figure 7B–10B). However, exhaustive swimming exercise significantly decreased the vasoconstrictive response to α,β-MeATP in the internal carotid arterial preparations, reaching a maximum inhibition of 50.38% (P<0.01, Figure 11B). The range of the negative log(EC50) values for α,β-MeATP in the 5 types of arteries was 4.71–6.19, and the EC50 values of α,β-MeATP in the SCG were not significantly different from those in the ESEG (Table 1).
Discussion
The co-transmission of NA and ATP is well established in the sympathetic innervation of a variety of blood vessels in animals. However, in this study, we discovered that exhaustive swimming exercise did not affect the EC50 values of NA or α,β-MeATP, but this type of exercise significantly decreased the P2X1 receptor-mediated vasoconstriction in the rat internal carotid artery. Conversely, the vasoconstrictive response to α1-adrenoceptor, but not P2X1 receptor, activation was inhibited in the caudal artery, pulmonary artery, mesenteric artery and the aorta obtained from rats subjected to the exhaustive swimming exercise.
Before observing the vasoconstrictive responses to α1-adrenoceptor or P2X1 receptor stimulation, we examined the vasoconstrictive responsiveness of each artery. In the rats of the SCG and the ESEG, there were no significant differences in the vasoconstrictive responses to the first exposure to NA (Figure 2–6) in the internal carotid, caudal, pulmonary, and mesenteric artery along with the aorta. No significant differences in vasoconstrictive responsiveness between the preparations that were either exposed to a second administration of NA or to α,β-MeATP were observed, suggesting that the 5 types of arteries obtained from the SCG rats and the ESEG rats were comparable in the present study. Because the EC50 values of KCl treatment were significantly greater in different types of arteries treated with α,β-MeATP than NA24, and the maximum vasoconstrictive response to KCl in the rat mesenteric and caudal arteries in the SCG were greater than the response in the ESEG, we expressed the vasoconstrictive responses to NA and α,β-MeATP as g/mg tissue. Concomitantly, we found the exhaustive swimming exercise did not alter the EC50 values of KCl.
It has been reported that long-term swimming exercise significantly reduces the vasoconstrictive response to phenylephrine in the rat mesenteric artery and thoracic aorta when the endothelium is intact, but not in the endothelium-denuded arteries26, 27. Chronic exercise also enhances endothelium-mediated vasodilatation in the endothelium-intact aorta and mesenteric artery, but this exercise does not affect NA-induced vasoconstriction in the endothelium-denuded arteries isolated from spontaneously hypertensive rats28. Moreover, exercise training increases acetylcholine-induced relaxation and eNOS protein levels in the porcine pulmonary artery29, 30, but not in the pulmonary artery from hypertensive rats31. Therefore, we removed the vascular endothelium of the isolated mesenteric, pulmonary, caudal and internal carotid arteries from the rat to directly observe the changes in α1-adrenoceptor- and P2X1 receptor-mediated vascular smooth muscle contractions because several studies suggest that exercise affects not only the vasoconstriction of vascular smooth muscles32, 33, but also the function of the vascular endothelium26, 27.
NA induces vasoconstriction and vasodilatation via α-adrenoceptors and β-adrenoceptors. Furthermore, Carter et al34 reported that though α2-adrenoceptors do not play a large role in NA-mediated vasoconstriction of the thoracic aorta in normotensive rats, there is an increased role of these receptors in hypertensive rats. Prior to measuring vasoconstrictive responsiveness after a second treatment with NA in this study, we added several reagents (propranolol, yohimbine, desmethylimipramine and deoxycorticosterone) to the organ bath to block β-adrenoceptors and α2-adrenoceptors, as well as neuronal and extra-neuronal uptake of NA. This allows for the measurement of vasoconstriction after a second exposure of each arterial preparation to NA to be attributed to α1-adrenoceptors alone.
Though it is widely accepted that the P2X1 receptor seems to be the most important P2X subtype in the vascular smooth muscle, this concept is not observed in functional studies. Nori et al35 observed the coexpression of three P2X receptor mRNAs (P2X1, P2X2, and P2X4) in the rat vascular smooth muscle. However, it was reported that the P2X4 receptor did not couple to a vasomotor response36, and the P2X2 receptor was mainly expressed in nerves and arterial endothelial cells and only found at low levels in smooth muscle cells37. Recently, Wallace et al38 investigated the expression of P2X receptors in the tail and mesenteric arteries of rats aged 4, 6, and 12 weeks by using immunohistochemistry. P2X1 receptor-specific immunoreactivity was associated with the smooth muscle layer of both arteries from all rats of all three ages, and the P2X4 receptor was weakly expressed in the smooth muscle layer of the tail artery in 4- and 6-week-old rats along with the mesenteric artery of 4- and 12-week-old rats. Immunoreactivity of the other subtypes of the P2X receptor family was not detected in the smooth muscle layer of arteries in 6- and 12-week-old rats38. In our study, we used 12–13-week-old rats (300–350 g), and the vascular endothelium of the regional arteries was removed. This age of rats combined with the use of α,β-MeATP (which is inactive as an agonist at the recombinant P2X2 purinoceptor39) allows us to assume that the P2X1 purinoceptors are primarily involved in the vasoconstrictive response to α,β-MeATP.
This study was the first investigation of measuring the changes in the P2X1 receptor-regulated vasoconstriction of regional arteries from rats subjected to the exhaustive swimming exercise. We also compared the effects of exhaustive swimming exercise on both α1-adrenoceptor- and P2X1 receptor-mediated vasoconstriction in the isolated internal carotid, caudal, pulmonary and mesenteric arteries along with the aorta. Our study clearly showed that the vasoconstrictive responses to α1-adrenoceptor stimulation in the aorta and the caudal, pulmonary and mesenteric arteries from the exhaustively exercised rats were significantly smaller than those from the normal rats, and a rank order of the decrease in the maximal vasoconstriction was determined as follows: mesenteric artery, pulmonary artery, caudal artery, aorta. Similar results were reported in the endothelium-denuded aorta32 and mesenteric artery33 from normal rats subjected to physical exercise. Moreover, we found that the P2X1 receptor-mediated vasoconstriction in all 5 arterial subtypes was not affected by exhaustive exercise. Using an isolated perfused splenic artery, Yang et al40 observed two peaks of vasoconstriction in response to periarterial nerve stimulation: an initial transient constriction induced by the purinergic ligand ATP and a second peak response consisting primarily of an adrenergic component. Our recent study demonstrated that the maximal vasoconstriction mediated by P2X1 receptors reached at least 40% of those mediated by α1-adrenoceptors in the rat internal carotid, mesenteric and pulmonary arteries. P2X1 receptor-mediated vasoconstriction even reached 80% in the caudal artery24, which suggested that purinergic transmission can be useful to maintain the basal vascular tone and could be important to the hemodynamic control in rats subjected to exhaustive swimming exercise. Sugawara et al41 also suggested that a reduction in α-adrenoceptor-mediated vascular tone contributes to improved arterial compliance in healthy adults who engage in endurance exercise.
In contrast to the rat caudal artery, pulmonary artery, mesenteric artery and the aorta, the vasoconstrictive response to α1-adrenoceptor stimulation in the rat internal carotid artery was not significantly affected by exhaustive swimming exercise. However, the vasoconstrictive response to P2X1 receptor stimulation in the internal carotid artery from the exhaustively exercised rats was significantly reduced. The reason for this difference is still unclear based on the data from the present study. Normally, the brain is entirely dependent upon glucose as an energy substrate as implied by cerebral metabolic ratio, which is close to 6.042. After exhaustive exercise, the cerebral metabolic ratio drops to 1.7, suggesting a link between brain metabolism and central fatigue43.
It was reported recently that the ratio of ischemic-to-nonischemic blood flow decreases from 6 to 24 months of age, and the capillary density of nonischemic and ischemic muscle also decreases in an age-dependent manner in wild-type mice44. Swimming training was shown to reduce the formation of abnormal vessels and promoted collateral artery formation in the ischemic limbs of aged wild-type mice44. We plan to observe and clarify the effect of exhaustive swimming on vasoconstriction mediated by purinoceptors or adrenoceptors in aged rats in the near future. Maiorana et al45 reported that chronic heart failure patients engaging in aerobic exercise had no changes in the wall thickness and the wall-to-lumen ratio of the brachial artery, while patients engaging in resistance exercise had a significantly reduced brachial artery wall thickness and wall-to-lumen ratio. The method of exhaustive swimming used in our study does not fall within the categories of either aerobic exercise or resistance exercise. It is unclear whether aerobic exercise affects the vasoconstriction mediated by purinoceptors and adrenoceptors in the same manner as resistance exercise does in normal or heart failure model animals.
Overall, the results of the present study showing the different effects of exhaustive swimming exercise on the vasoconstriction mediated by α1-adrenoceptors and P2X1 receptors between rat internal carotid artery and the peripheral arteries could help to explain that stress or injury can mediate different vascular responses in animals and humans and could be associated with tissue or organ functions. In conclusion, the exhaustive swimming exercise differentially affects the vasoconstriction regulated by P2X1 receptors and α1-adrenoceptors in the rat internal carotid artery and the peripheral arteries, and the ability to preserve purinergic vasoconstriction of the peripheral arteries is useful in maintaining the basal vascular tone during exhaustive swimming exercise.
Author contribution
Lei-ming REN and Yi-ling WU designed the research. Lu LI, Tao WU, Cong WEI, and Jian-ke HAN performed the research. Zhen-hua JIA analyzed the data.
A comparison of the vasoconstrictive responses to noradrenaline between the preparations exposing to the second administration of noradrenaline (treatment with NA; n=11, left; n=15, right) and those exposing to α,β-methylene ATP (treatment with α,β-MeATP; n=20, left; n=20, right) in the rat caudal arteries from the sedentary control group (A) and the exhaustive swimming exercise group (B). Mean±SEM.
A comparison of the vasoconstrictive responses to noradrenaline between the preparations exposing to the second administration of noradrenaline (treatment with NA; n=11, left; n=9, right) and those exposing to α,β-methylene ATP (treatment with α,β-MeATP; n=22, left; n=22, right) in the rat mesenteric arteries from the sedentary control group (A) and the exhaustive swimming exercise group (B). Data were expressed as mean±SEM.
A comparison of the vasoconstrictive responses to noradrenaline between the preparations exposing to the second administration of noradrenaline (treatment with NA; n=11, left; n=8, right) and those exposing to α,β-methylene ATP (treatment with α,β-MeATP; n=23, left; n=22, right) in the rat thoracic aorta from the sedentary control group (A) and the exhaustive swimming exercise group (B). Data were expressed as mean±SEM.
References
Kingwell BA . Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J 2000; 14: 1685–96.
Sutoo D, Akiyama K . Regulation of brain function by exercise. Neurobiol Dis 2003; 13: 1–14.
Larson EB, Wang L . Exercise, aging, and Alzheimer disease. Alzheimer Dis Assoc Disord 2004; 18: 54–6.
Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T, et al. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol 1992; 263: R992–5.
Banerjee AK, Mandal A, Chanda D, Chakraborti S . Oxidant, antioxidant and physical exercise. Mol Cell Biochem 2003; 253: 307–12.
Fehrenbach E, Northoff H . Free radicals, exercise, apoptosis, and heat shock proteins. Exerc Immunol Rev 2001; 7: 66–89.
Huang CC, Lin TJ, Chen CC, Lin WT . Endurance training accelerates exhaustive exercise-induced mitochondrial DNA deletion and apoptosis of left ventricle myocardium in rats. Eur J Appl Physiol 2009; 107: 697–706.
Aslani A, Babaee Bigi MA, Moaref AR, Aslani A . Effect of extreme exercise on myocardial function as assessed by tissue Doppler imaging. Echocardiography 2009; 26: 1036–40.
Howard MG, DiCarlo SE, Stallone JN . Acute exercise attenuates phenylephrine-induced contraction of rabbit isolated aortic rings. Med Sci Sports Exerc 1992; 24: 1102–7.
Chen Hi H, Chiang IP, Jen CJ . Exercise training increases acetylcholine-stimulated endothelium-derived nitric oxide release in spontaneously hypertensive rats. J Biomed Sci 1996; 3: 454–60.
Graham DA, Rush JW . Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol 2004; 96: 2088–96.
Moien-Afshari F, Ghosh S, Elmi S, Khazaei M, Rahman MM, Sallam N, et al. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol 2008; 295: H1470–80.
Korzick DH, Laughlin MH, Bowles DK . Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol 2004; 96: 1425–32.
Boarder MR, Hourani SM . The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci 1998; 19: 99–107.
Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, et al. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 1996; 16: 2495–507.
Galligan JJ, Hess MC, Miller SB, Fink GD . Differential localization of P2 receptor subtypes in mesenteric arteries and veins of normotensive and hypertensive rats. J Pharmacol Exp Ther 2001; 296: 478–85.
Vial C, Evans RJ . P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol 2002; 62: 1438–45.
Erlinge D, Burnstock G . P2 receptors in cardiovascular regulation and disease. Purinergic Signal 2008; 4: 1–20.
Haddock RE, Hill CE . Sympathetic overdrive in obesity involves purinergic hyperactivity in the resistance vasculature. J Physiol 2011; 589: 3289–307.
Vidal M, Hicks PE, Langer SZ . Differential effects of alpha-beta-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn Schmiedebergs Arch Pharmacol 1986; 332: 384–90.
Thomas DP, Marshall KI . Effects of repeated exhaustive exercise on myocardial subcellular membrane structures. Int J Sports Med 1988; 9: 257–60.
Peijie C, Hongwu L, Fengpeng X, Jie R, Jie Z . Heavy load exercise induced dysfunction of immunity and neuroendocrine responses in rats. Life Sci 2003; 72: 2255–62.
Sun B, Wang JH, Lv YY, Zhu SS, Yang J, Ma JZ . Proteomic adaptation to chronic high intensity swimming training in the rat heart. Comp Biochem Physiol Part D Genomics Proteomics 2008; 3: 108–17.
Li L, Jia ZH, Chen C, Wei C, Han JK, Wu YL, et al. Physiological significance of P2X receptor-mediated vasoconstriction in five different types of arteries in rats. Purinergic Signal 2011; 7: 221–9.
Wihlborg AK, Slätt J, Sun X, Zhao XH, Malmsjö M, Bergman J, et al. 2,2′-Nitrophenylisatogen potentiates P2X1 receptor mediated vascular contraction and blood pressure elevation. Drug Dev Res 2003; 59: 82–7.
Jansakul C, Hirunpan P . Effects of exercise training on responsiveness of the mesenteric arterial bed to phenylephrine and KCl in male rats. Br J Pharmacol 1999; 127: 1559–66.
Jansakul C . Effect of swimming on vascular reactivity to phenylephrine and KCl in male rats. Br J Pharmacol 1995; 115: 587–94.
Yen MH, Yang JH, Sheu JR, Lee YM, Ding YA . Chronic exercise enhances endothelium-mediated dilation in spontaneously hypertensive rats. Life Sci 1995; 57: 2205–13.
Johnson LR, Parker JL, Laughlin MH . Chronic exercise training improves ACh-induced vasorelaxation in pulmonary arteries of pigs. J Appl Physiol 2000; 88: 443–51.
Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH . Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol 2001; 90: 1102–10.
Goret L, Reboul C, Tanguy S, Dauzat M, Obert P . Training does not affect the alteration in pulmonary artery vasoreactivity in pulmonary hypertensive rats vasoreactivity in pulmonary hypertensive rats. Eur J Pharmacol 2005; 527: 121–8.
Izawa T, Morikawa M, Mizuta T, Nagasawa J, Kizaki T, Oh-ishi S, et al. Decreased vascular sensitivity after acute exercise and chronic exercise training in rat thoracic aorta. Res Commun Mol Pathol Pharmacol 1996; 93: 331–42.
Chies AB, de Oliveira AM, Pereira FC, de Andrade CR, Correa FM . Phenylephrine-induced vasoconstriction of the rat superior mesenteric artery is decreased after repeated swimming. J Smooth Muscle Res 2004; 40: 249–58.
Carter RW, Begaye M, Kanagy NL . Acute and chronic NOS inhibition enhances alpha(2)- adrenoreceptor-stimulated RhoA and Rho kinase in rat aorta. Am J Physiol Heart Circ Physiol 2002; 283: H1361–9.
Nori S, Fumagalli L, Bo X, Bogdanov Y, Burnstock G . Coexpression of mRNAs for P2X1, P2X2, and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J Vasc Res 1998; 35: 179–85.
Ralevic V, Burnstock G . Receptors for purines and pyrimidines. Pharmacol Rev 1998; 50: 413–92.
Hansen MA, Dutton JL, Balcar VJ, Barden JA, Bennett MR . P2X (purinergic) receptor distributions in rat blood vessels. J Auton Nerv Syst 1999; 75: 147–55.
Wallace A, Knight GE, Cowen T, Burnstock G . Changes in purinergic signalling in developing and ageing rat tail artery: importance for temperature control. Neuropharmacology 2006; 50: 191–208.
Brake AJ, Wagenbach MJ, Julius D . New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 1994; 371: 519–23.
Yang XP, Chiba S . Effects of a selective neuropeptide Y Y(1) receptor antagonist BIBP 3226 on double peaked vasoconstrictor responses to periarterial nerve stimulation in canine splenic arteries. Br J Pharmacol 2000; 130: 1699–705.
Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, et al. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol 2009; 135: 346–52.
Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH . A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol 2004; 554: 571–8.
Volianitis S, Fabricius-Bjerre A, Overgaard A, Stromstad M, Bjarrum M, Carlson C, et al. The cerebral metabolic ratio is not affected by oxygen availability during maximal exercise in humans. J Physiol 2008; 586: 107–12.
Cheng XW, Kuzuya M, Kim W, Song HZ, Hu L, Inoue A, et al. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation 2010; 122: 707–16.
Maiorana AJ, Naylor LH, Exterkate A, Swart A, Thijssen DH, Lam K, et al. The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension 2011; 57: 56–62.
Acknowledgements
This work was supported by a grant from the National Program on Key Basic Research Project of China (973 Program) (No 2005CB523301).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, L., Wu, T., Wei, C. et al. Exhaustive swimming differentially inhibits P2X1 receptor- and α1-adrenoceptor-mediated vasoconstriction in isolated rat arteries. Acta Pharmacol Sin 33, 221–229 (2012). https://doi.org/10.1038/aps.2011.148
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.148
Keywords
This article is cited by
-
Comparison of mRNA Expression of P2X Receptor Subtypes in Different Arterial Tissues of Rats
Biochemical Genetics (2020)
-
(−)Doxazosin is a necessary component for the hypotensive effect of (±)doxazosin during long-term administration in conscious rats
Acta Pharmacologica Sinica (2014)