Abstract
Aim:
To explore the mechanisms involved in ox-LDL transcytosis across endothelial cells and the role of caveolae in this process.
Methods:
An in vitro model was established to investigate the passage of oxidized low density lipoprotein (ox-LDL) through a tight monolayer of human umbilical vein endothelial cells (HUVEC) cultured on a collagen-coated filter. Passage of DiI-labeled ox-LDL through the monolayer was measured using a fluorescence spectrophotometer. The uptake and efflux of ox-LDL by HUVEC were determined using fluorescence microscopy and HPLC.
Results:
Caveolae inhibitors – carrageenan (250 μg/mL), filipin (5 μg/mL), and nocodazole (33 μmol/L)–decreased the transport of ox-LDL across the monolayer by 48.9%, 72.4%, and 79.8% as compared to the control group. In addition, they effectively decreased ox-LDL uptake and inhibited the efflux of ox-LDL. Caveolin-1 and LOX-1 were up-regulated by ox-LDL in a time-dependent manner and decreased gradually after depletion of ox-LDL (P<0.05). After treatment HUVEC with ox-LDL and silencing caveolin-1, NF-κB translocation to the nucleus was blocked and LOX-1 expression decreased (P<0.05).
Conclusion:
Caveolae can be a carrier for ox-LDL and may be involved in the uptake and transcytosis of ox-LDL by HUVEC.
Similar content being viewed by others
Introduction
The development of atherosclerotic lesions is associated with the accumulation of lipid deposits in the arterial intima and strongly depends on the level of ox-LDL in blood plasma1. Atherosclerotic plaque, the most common lesion, is characterized by a thickening of the arterial intima and is typically composed of a lipid core with an overlying fibrous cap. Increasing evidence indicates that ox-LDL is a highly toxic lipoprotein. Concentrated ox-LDL in the subendothelium contributes to the accumulation of cholesterol in atherosclerotic plaques2, 3. However, the mechanism underlying the passage of ox-LDL through the endothelium is poorly understood. Vascular endothelium controls the trafficking of lipoproteins between the vessel lumen and the arterial wall, and the control may be disturbed in arteriosclerotic blood vessels. Understanding the mechanisms of lipoprotein translocation, especially from arterial wall to vessel lumen, will be beneficial to the therapy of cardiovascular disorders. Caveolae are distinctive, flask-shaped invaginations of the plasma membrane generated in the Golgi complex. These organelles have a characteristic lipid composition and are associated with a 22 kDa protein called caveolin-1. Zhang reported that two ox-LDL receptors, CD36 and the related receptor SR-B1, localize to caveolae4, 5. Several human studies have indicated transcytosis of macromolecules by means of caveolae in endothelial cells6, 7, 8, 9, 10, 11. We hypothesized that ox-LDL may internalize and transcytose in this way. Bruneau described a model to study the passage of macromolecules through a monolayer of INT-407 cells12. We used this model to investigate the characteristics and mechanism of ox-LDL passage. LOX-1 is the major receptor for ox-LDL in endothelial cells13. The expression of LOX-1 is modulated by ox-LDL14. Caveolin-1 is the main structural protein of caveolae, and it plays a crucial role in the formation of these invaginations of the plasma membrane. Caveolin-1 is a cholesterol-binding protein. Hu and Wu demonstrated the correlation between caveolin-1 and cellular cholesterol level15, 16. Our previous studies demonstrated that caveolin-1 is involved in cellular cholesterol accumulation, vascular smooth muscle cell foam formation, intercellular cholesterol efflux, and vascular remodeling17, 18, 19. These findings indicate that caveolae and caveolin-1 may play an important role in cellular cholesterol homeostasis. The objective of this study was to explore the mechanisms involved in cholesterol transcytosis and the role of caveolin-1 in ox-LDL translocation. In this study, we used three caveolae inhibitors: carrageenan, filipin and nocodazole. Studies have shown that carrageenan abolishes ox-LDL binding to caveolae20; filipin treatment disrupts the structural integrity of caveolae in the plasma membrane and decreases the number of visible caveolae on the endothelial surface21; and nocodazole blocks the movement of caveolae by microtubule depolymerization22. We demonstrated the mediation of uptake and transcellular transport of ox-LDL by caveolae in HUVEC. HUVEC function as a post-house in ox-LDL translocation between blood plasma and subendothelium. The whole prossess avoids accumulation of ox-LDL in HUVEC.
Materials and methods
Chemicals and materials
The Transwell culture system used for permeability studies consisted of 24 mm diameter polycarbonate inserts with 0.4 μm pores (Costar, Cambridge, MA). Dulbecco's modified Eagle's medium (DMEM), penicillin, streptomycin, and trypsin-EDTA were obtained from GIBCO (Grand Island, NY). Carrageenan, nocodazole, filipin, DiI (1,1′-Dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine perchlorate) and cholesterol standard were purchased from Sigma Aldrich (St Louis, MO, USA). Ox-LDL was obtained from the Institute of Clinical Pharmacology of Sun Yat-sen University (Guangdong, China). Costar Transwell culture wells were obtained from Fisher Scientific (Springfield, NJ). Primary antibodies for caveolin-1 and NF-κB were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and for LOX-1 from Chemicon International. Secondary antibodies were obtained from Molecular Probes (Eugene, OR). Enhanced chemiluminescence (ECL) reagents for detection on Western blots were purchased from Amersham Biosciences (Arlington Heights, IL). SiRNA for transfection treatments were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Lipofectamine reagent for transfection of siRNA was obtained from Invitrogen Life Technologies (Grand Island, NY).
Cells and culture
HUVEC were obtained from the Institute of Pharmacology of Central South University (Hunan, China) as cryopreserved cells. After thawing, cells were cultured in 75 cm2 culture flasks and maintained in DMEM. Cell medium was supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin solution, and 30 μg of endothelial cell growth supplement per mL. For permeability studies, HUVEC were seeded in 24-mm Transwell- Clear inserts at a density of 4×104 cells per insert and allowed to reach confluency for 3 days. Intercellular junction integrity of the endothelial monolayer was assessed by microscopy and by measuring permeability to bovine serum albumin as described elsewhere23.
MTT assay
HUVECs were seeded in 96-well plates at 103 cells/well and allowed to adhere for 8 h. The subconfluent cells were then exposed to medium containing 40 μg/mL ox-LDL for different amounts of time. At various times, the cell survival fraction was calculated from the methyl thiazolyl tetrazolium (MTT) assay. Briefly, 10 μL MTT solution in 5 mg/mL of phosphate-buffered saline was added to each well, and the plates were incubated for 4 h, 50 μL of DMSO was added. Plates were then shaken until the crystals were dissolved and then they were analyzed on an enzyme linked immunosorbent assay plate reader at 490 nm to determine the absorbance of the samples.
Lipoprotein labeling
The ox-LDL was labeled with the fluorescent probe DiI by a modification of the method described by Fisher24. Briefly, a stock solution of DiI was prepared by dissolving 30 mg DiI in 1 mL dimethyl sulfoxide, and an appropriate volume was added to the ox-LDL solution. The concentration of ox-LDL was adjusted to 1 mg protein per mL to yield a final concentration of 150 μg DiI per mg ox-LDL protein. The mixture was protected from light and incubated at 37 °C for 18 h to provide DiI-labeled ox-LDL (DiI-ox-LDL), which was subsequently layered with an NaBr solution (density=1.1 g/mL) and re-isolated by ultracentrifugation (100 000×g, 18 h, 20 °C), dialyzed against phosphate-buffered saline (18 h, 4 °C), and sterilized by filtration (Nalgene polyether sulfone filter, 0.22 μm; Nalge Nunc International, Naperville, IL). The final protein concentration was determined by the Bradford method25. The DiI-ox-LDL was stored at 4 °C in the dark and used within 7 days. The fluorescence density and intensity of DiI-labeled ox-LDL inside the cell were observed by fluorescent microscopy.
Transendothelial ox-LDL permeability
Transendothelial permeability of ox-LDL in the HUVEC monolayer was determined by using Transwell filter units. HUVEC monolayers were washed and incubated for 1 h with carrageenan, filipin, or nocodazole. DiI-Ox-LDL was placed in the apical reservoir at a final concentration of 40 μg/mL. After 24 h incubation, samples were taken from the apical and basolateral compartments and relative fluorescent units of apical and basolateral samples were measured in a fluorescence spectrophotometer at 399- and 610-nm excitation and emission wavelengths, respectively. Values were expressed as the percentage of apical DiI-Ox-LDL per well that crossed the Transwell membrane.
HPLC assay
HPLC (high performance liquid chromatography) analysis was conducted as described previously26. Cells were washed with PBS 3 times. The appropriate volume (usually 1 mL) of 0.5% NaCl was added to 50–200 μg cellular proteins per mL. Cells were sonicated using an ultrasonic processor for 2 min. The protein concentration in cell solution was measured using the BCA kit. An aliquot of cell solution (0.1 mL containing 5–20 μg protein) was used to measure the free cholesterol, and another aliquot was used for total cholesterol detection. Free cholesterol was dissolved in isopropanol (1 mg cholesterol/mL) and stored at −20°C as stock solution. Cholesterol standard calibration solution ranging from 0 to 40 μg cholesterol per mL was obtained by diluting the cholesterol stock solution in the cell lysis buffer. Each sample (0.1 mL cholesterol standard calibration solution or cell solution) was supplemented with 10 μL reaction mixture including 500 mmol/L MgC12, 500 mmol/L Tris-HCl (pH 7.4), 10 mmol/L dithiothreitol, and 5% NaCl. Cholesterol oxidase (0.4 U) in 10 μL 0.5% NaCl was added to each tube for free cholesterol determination, or 0.4 U cholesterol oxidase plus 0.4 U of cholesterol esterase for total cholesterol measurement. The total reaction solution in each tube was incubated at 37°C for 30 min, and 100 μL methanol:ethanol (1:1) was added to stop the reaction. Each solution was kept cold for 30 min to allow protein precipitation and then was centrifuged at 1500 r/min for 10 min at 15 °C. Supernatant (10 μL) was applied onto a System Chromatographer (PerkinElmer Inc) including a PerkinElmer series 200 vacuum degasser, a pump, a PerkinElmer series 600 LINK, a PerkinElmer series 200 UV/vis detector and a Discovery C-18 HLPC column (Supelco Inc). The column was eluted using isopropanol:n-heptane:acetonitrile (35:13:52) at a flow rate of 1 mL/min for 8 min. Absorbance at 216 nm was monitored. Data were analyzed with TotalChrom software from PerkinElmer.
Ox-LDL binding and internalization
HUVEC were seeded in glass chamber slides at 100 cells/mm2 and incubated with DMEM containing 0.2% FCS for 24 h prior to the experiment. Binding experiments were performed as previously described27. HUVEC were incubated with 40 μg/mL DiI-Ox-LDL at 4 °C for 30 min. After binding, medium was removed, and cells were incubated at 37 °C for 4 h in the absence or presence of the different compounds tested. Cells were washed in DMEM-BSA containing heparin 100 U/mL for 15 min at 4°C with constant shaking, fixed at room temperature for 10 min in PBS containing 3% paraformaldehyde and 2% sucrose, and washed twice with PBS. Finally, fluorescent photomicrographs were taken with an Olympus Vanox AHBT3 microscope with an excitation filter for rhodamine.
siRNA transfection
Short-interfering RNA (siRNA) specific for human caveolin-1 (Santa Cruz Biotechnology) and nonsilencing control siRNA were synthesized by the Biology Engineering Corporation in Shanghai, China. HUVEC (2×106 cells/well) were transfected using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, real-time RT-PCR was performed. In comparison to the control siRNA, the siRNA of caveolin-1 suppressed the expression of caveolin-1 proteins by 82% according to Western blot analysis.
Nuclear extraction
HUVEC were incubated with ox-LDL (40 μg/mL) for 24 h after transfection. Cells were harvested for extraction of nuclear proteins after incubation. Cells were rinsed with cold PBS, scraped, collected by centrifugation, re-suspended in 300 μL lysis buffer (50 mmol/L KCl, 0.5% IGEPAL CA-630, 25 mmol/L HEPES, 10 μg/mL leupeptin, 20 μg/mL aprotinin, 125 μmol/L dithiothreitol (DTT), and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF)), transferred to a 1.5 mL Eppendorf tube, and kept on ice for 4 min. Nuclei were collected by centrifugation (10 000 r/min, 10 min at 4 °C) and washed in 300 μL washing buffer (50 mmol/L KCl, 25 mmol/L HEPES, 10 μg/mL leupeptin, 20 μg/mL aprotinin, 125 μmol/L DTT, and 1 mmol/L PMSF). Nuclei were pelleted (10 000 r/min, 1 min at 4 °C), and re-suspended in 30–100 μL extraction buffer (500 mmol/L KCl, 25 mmol/L HEPES, 10% glycerol, 10 μg/mL leupeptin, 20 μg/mL aprotinin, 125 μmol/L DTT, and 1 mmol/L PMSF) for 20 min. The suspension was centrifuged (14 000 r/min, 2 min at 4 °C). Protein concentration was measured using the Bradford assay25.
Western blot analysis
Cells were harvested and protein extracts were prepared as previously described15. They were then subjected to Western blot analysis [10% SDS-polyacrylamide gel electrophoresis (PAGE); 30 μg protein per lane] using rabbit anti-LOX-1, mouse anti-NF-κB, mouse anti-caveolin-1 and β-actin-specific antibodies. The proteins were visualized using a chemiluminescence method (ECL Plus Western Blotting Detection System; Amersham Biosciences, Foster City, CA, USA).
Statistical analysis
Data are expressed as means±SD. Results were analyzed by one-way ANOVA and Student's t-test, using SPSS 13.0 software. Statistical significance was obtained when P values were less than 0.05.
Results
Caveolae inhibitors inhibited transcytosis of ox-LDL across endothelium
Previous studies reported the potential role of caveolae in macromolecule transport across alveolar endothelium. To determine whether caveolae are responsible for receptor-mediated transport of ox-LDL across endothelial cells, we treated the HUVEC monolayers with caveolae inhibitors before ox-LDL incubation, as described above. The cell viability of HUVEC was not significantly changed during our experiments (Figure 1). Table 1 shows that carrangeen decreased the transport of ox-LDL across the monolayer by 48.9%, filipin decreased the transport of ox-LDL across the monolayer by 72.4%, and nocodazole decreased the transport of ox-LDL across the monolayer by 79.8%. These results suggest that caveolae and caveolin-1 play a crucial role in transcytosis.
Effect of ox-LDL on cell viability in HUVECs. HUVECs were seeded in 96-well plates at 103 cells/well and allowed to adhere for 8 h. The subconfluent cells were then exposed to medium containing 40 μg/mL ox-LDL. MTT assay was performed at 0, 6, 12, and 24 h time point. Each experiment was performed in triplicate and bars represent mean±SD. n=3.
Caveolae are involved in endocytic trafficking of ox-LDL
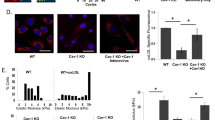
The physiological consequences of caveolae inhibitors on the uptake of ox-LDL through an LOX-1 dependent mechanism were studied. HUVEC were incubated with DiI-ox-LDL for 30 min at 4 °C. After removal of unbound ox-LDL, the three caveolae inhibitors were added, and the bound lipoprotein fraction was left to internalize for 4 h at 37 °C. Fluorescent photomicrographs were taken after internalization. As shown in Figure 2, carrageenan, nocodazole and filipin significantly decreased ox-LDL uptake and cholesterol loading of HUVEC. These observations suggest that caveolae are involved in ox-LDL uptake.
Effects of caveolae-specific inhibitors on DiI-ox-LDL uptake by HUVECs. HUVECs were incubated with DiI-ox-LDL (40 μg/mL) for 30 min at 4 °C. After binding, medium was removed, and cells were incubated at 37 °C for 4 h in the absence or presence of the different caveolae inhibitors. Then HUVECs were washed, fixed, and photographed with an inverted fluorescence microscope (magnification×400). (A) HUVEC were incubated with ox-LDL, (B) HUVEC incubated with DiI-ox-LDL, (C) HUVEC were incubated with DiI-ox-LDL for 30 min at 4 °C, then incubated with carrageenan (250 μg/mL) for 4 h, (D) HUVEC were incubated with DiI-ox-LDL for 30 min at 4 °C, then incubated with filipin (5 μg/mL) for 4 h, (E) HUVEC were incubated with DiI-ox-LDL for 30 min at 4 °C, then incubated with nocodazole (33 μmol/L) for 4 h.
Caveolae play a crucial role in the efflux of ox-LDL
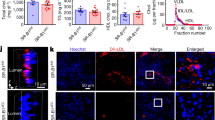
As described in the Introduction, we hypothesized that caveolae are involved in transcytosis of ox-LDL. If that is the case, efflux of ox-LDL is also expected to be slow when caveolae are inhibited. To examine whether the inhibition of caveolae decreased ox-LDL efflux, the cholesterol content in the medium and cellular cholesterol was determined after treatment with inhibitors. We incubated HUVEC with ox-LDL for 24 h. After lipid loading, HUVEC were washed twice with PBS and incubated for another 6, 12, and 24 h in an ox-LDL depleted medium in the absence or presence of the caveolae inhibitors. After incubation, the cholesterol content in the medium and cellular cholesterol were analyzed by HPLC. Caveolae inhibitors significantly reversed the decrease in cellular cholesterol and the increase in the cholesterol content in the medium (Figure 3). These data suggest that caveolae are involved in the efflux of ox-LDL by HUVEC.
Effects of caveolae-specific inhibitors on ox-LDL efflux by HUVECs. HUVECs were incubated with ox-LDL (40 μg/mL) for 24 h. After lipid loading, HUVECs were washed twice with PBS, and incubated for another 6, 12, and 24 h in an ox-LDL depleted medium with or without the caveolae inhibitors. After incubation, the cholesterol in the cytoplasm (A) and cholesterol in medium (B) were analyzed by HPLC. Similar results were obtained in six independent experiments. Data are mean±SD. bP<0.05 vs corresponding groups at 0 h.
Caveolin-1 participates in the uptake of ox-LDL in HUVEC
Our experiments show that caveolin-1 and LOX-1 were up-regulated by ox-LDL in a time-dependent manner and decreased gradually after depletion of ox-LDL (Figure 4). We attempted to determine if caveolin-1 plays a role in LOX-1 expression. We treated HUVEC with ox-LDL and silenced caveolin-1. NF-κB translocation to the nucleus was blocked and LOX-1 expression decreased (Figure 5). This study suggests that caveolin-1 participates in the regulation of LOX-1.
Effects of ox-LDL treatment and ox-LDL depletion on expression of caveolin-1 and LOX-1 in HUVECs. HUVECs in 75-cm2 flasks were incubated with ox-LDL (40 μg/mL) for 0, 6, 12, and 24 h, followed by incubation with ox-LDL-depleted medium for 0, 6, 12, and 24 h. Total cell lysates were prepared at the various time points indicated. For each sample, 20 μg of lysate protein was used. Western immunoblotting assays using antibody against human β-actin and caveolin-1 and LOX-1 were conducted. Results were reproduced in three independent experiments. (A) HUVECs were incubated with ox-LDL for 0, 6, 12, and 24 h. (B) HUVECs were incubated with ox-LDL-depleted medium for 6, 12, and 24 after uptake of lipid by HUVEC.
Effect of silencing caveolin-1 on NF-κB translocation and LOX-1 expression in lipid-loaded HUVECs. HUVECs transfected with control siRNA or caveolin-1 siRNA were incubated with or without ox-LDL. Lysates were prepared after incubation for 24 h to determine the expression of NF-κB and LOX-1. Total cellular proteins from HUVECs transfected with control siRNA or siRNA for caveolin-1 were immunoblotted with the anti-caveolin-1, LOX-1, and β-actin antibodies, and nuclear proteins were immunoblotted with the anti-NF-κB. Similar results were obtained in six independent experiments. Data are mean±SD. bP<0.05 vs corresponding groups transfected with control siRNA and treated with ox-LDL. eP<0.05 vs corresponding groups transfected with control siRNA but not treated with ox-LDL.
Discussion
Although great advances have been made in understanding vascular lipoprotein cholesterol transport, only recently has attention focused on cholesterol-rich plasma membrane microdomains and their interaction with pathways of intracellular cholesterol transport and metabolism. Since 1955, caveolae have been recognized as morphologically distinct “small pockets, caves, or recesses” (also termed “flask-shaped”) at the cell surface plasma membrane28. The majority of cholesterol uptake and efflux occurs via these specialized plasma membrane microdomains. Caveolin-1 is the main structural protein component of caveolae: it is localized at the cytoplasmic face of the caveolae and does not cross the caveolae membrane9. Caveolin-1 is postulated to organize formation of caveolae microdomains and to regulate caveolae-related signaling events, such as protein kinase C, Src-like kinases, Ras, and endothelial NO synthase. Ox-LDL has been implicated as a causal factor in the pathogenesis of atherosclerosis, including transformation of macrophages to foam cells.
The focus of the present article is the role of caveolae and caveolin-1 and their mediation of ox-LDL uptake/efflux and transcytotic trafficking. Our study demonstrated the uptake of ox-LDL by HUVEC, and ox-LDL passed across the endothelium. These processes were mediated via caveolae, which are blocked by two caveolae inhibitors, filipin and nocodazole. We also demonstrated that caveolin-1 plays a role in promoting uptake of ox-LDL by HUVEC. A previous study demonstrated lipid accumulation within macrophages as a major cause of macrophage foam cell formation. In the present study, ox-LDL increased the uptake of lipid in HUVEC but no foam cell formation was observed. It seems that HUVEC function as a post-house in ox-LDL translocation between blood plasma and subendothelium: this process may prevent lipid from accumulating in HUVEC.
Recently, much emphasis has been placed on ox-LDL as a major toxic lipoprotein to atherogenesis. The uptake of ox-LDL by target cells in the subendothelial space causes transformation of macrophages to foam cells and smooth muscle cells proliferation29. Caveolae play an important role in the regulation of endocytosis and transcytosis in endothelial cells. Based on the data from our study, ox-LDL recognized LOX-1 of endothelial cells, internalized it into the cells, and then passed through the endothelium. Caveolae thus functioned as a carrier for ox-LDL uptake and transcytosis. This concept was supported by two inhibitors, filipin and nocodazole, which inhibited ox-LDL uptake and transcytosis across the epithelium.
Caveolin-1 is an important structural protein in caveolae and its highest expression level is in endothelial cells30. Our study demonstrated that ox-LDL upregulated caveolin-1 in a time-dependent manner. In addition, caveolin-1 increased LOX-1 expression and promoted translocation of NF-κB, which is an oncogene protein regulating transcription of a variety of cellular genes, including LOX-1. Thus caveolin-1 may promote ox-LDL uptake by HUVEC, and NF-κB may play a role in this pathway.
In summary, lipid accumulation did not occur while incubating HUVEC with ox-LDL. Ox-LDL binding in HUVEC was internalized into cells by caveolae and transcytosed across endothelial cells with the movement of caveolae. Caveolae, working as a carrier, played an indispensable role during the whole process. Caveolin-1 may regulate the expression of LOX-1 and ox-LDL uptake.
Author contribution
Duan-fang LIAO and Chao-ke TANG designed research; Shao-wei SUN and Xu-yu ZU performed research; Xiao-yong LEI and Qin-hui TUO contributed new analytical tools and reagents; Lin-xi CHEN and Kai LI analyzed data and Shao-wei SUN wrote the paper.
References
Takenaka T, Takahashi K, Kobayashi T, Oshima E, Iwasaki S, Suzuki H . Oxidized low density lipoprotein (ox-LDL) as a marker of atherosclerosis in hemodialysis (HD) patients. Clin Nephrol 2002; 58: 33–7.
Lusis A . Atherosclerosis. Nature 2000; 407: 233–41.
Orem C, Orem A, Uydu H, Celik S, Erdol C, Kural B . The effects of lipid-lowering therapy on low-density lipoprotein auto-antibodies: relationship with low-density lipoprotein oxidation and plasma total antioxidant status. Coron Artery Dis 2002; 13: 65–71.
Zhang J, Crandall I . Expression of both N- and C-terminal GFP tagged huCD36 and their discrepancy in oxLDL and pRBC binding on CHO cells. Lipids Health Dis 2007; 6: 24.
Zhang J, Chu W, Crandall I . Lipoprotein binding preference of CD36 is altered by filipin treatment. Lipids Health Dis 2008; 7: 23.
Hu G, Vogel SM, Visintine DJ, Malik AB, Minshall RD . Activation of endothelial cell ICAM-1 stimulates caveolae-mediated transcytosis. FASEB J 2006; 20: A1166–d-67.
Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB . Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. PNAS 2004; 101: 7699–704.
Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD . G{beta}{gamma} activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem 2004; 279: 48055–62.
Predescu SA, Predescu DN, Malik AB . Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 2007; 293: L823–42.
Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD . Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem 2004; 279: 20392–400.
McIntosh DP, Tan XY, Oh P, Schnitzer JE . Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: A pathway to overcome cell barriers to drug and gene delivery. PNAS 2002; 99: 1996–2001.
Bruneau N, Nganga A, Bendayan M, Lombardo D . Transcytosis of pancreatic bile salt-dependent lipase through human Int407 intestinal cells. Exp Cell Res 2001; 271: 94–108.
Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997; 386: 73–7.
Li D, Mehta JL . Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol 2000; 20: 1116–22.
Hu Y, Ma X, Li X, Liu X, Xiao J, Mo Z, et al. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis 2009; 204: e35–43.
Wu C, Wang S, Kuan I, Tseng W, Chen M, Wu J, et al. OxLDL upregulates caveolin-1 expression in macrophages: role for caveolin-1 in the adhesion of oxLDL-treated macrophages to endothelium. J Cell Biochem 2009; 107: 460–72.
Luo D, Cheng J, Xiong Y, Li J, Xia C, Xu C, et al. Static pressure drives proliferation of vascular smooth muscle cells via caveolin-1/ERK1/2 pathway. Biochem Biophys Res Commun 2010; 391: 1693–7.
Qin L, Qin X, Wang Z, Zhu B, Liao D . Effect of pravastatin on cholesteryl esters in foam cells and the relation with caveolin-1. Sheng Li Xue Bao 2006; 58: 47–52.
Yuan H, Kuang S, Zheng X, Ling H, Yang Y, Yan P, et al. Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells. Acta Pharmacol Sin 2008; 29: 555–63.
Bruneau N, Richard S, Silvy F, Verine A, Lombardo D . Lectin-like ox-LDL receptor is expressed in human INT-407 intestinal cells: involvement in the transcytosis of pancreatic bile salt-dependent lipase. Mol Biol Cell 2003; 14: 2861–75.
Xu Y, Henning RH, van der Want J, van Buiten A, van Gilst W, Buikema H . Disruption of endothelial caveolae is associated with impairment of both NO- as well as EDHF in acetylcholine-induced relaxation depending on their relative contribution in different vascular beds. Life Sci 2007; 80: 1678–85.
Mundy DI, Machleidt T, Ying Y-s, Anderson RGW, Bloom GS . Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci 2002; 115: 4327–39.
Hennig B, Shasby DM, Fulton AB, Spector AA . Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis 1984; 4: 489–97.
Fisher TS, Surdo PL, Pandit S, Mattu M, Santoro JC, Wisniewski D, et al. Effects of pH and low density lipoprotein (LDL) on PCSK9-dependent LDL receptor regulation. J Biol Chem 2007; 282: 20502–12.
Bradford M . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–54.
Hao X, Cao D, Hu Y, Li X, Liu X, Xiao J, et al. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis 2009; 203: 417–28.
Fernandez-Borja M, Bellido D, Vilella E, Olivecrona G, Vilaro S . Lipoprotein lipase-mediated uptake of lipoprotein in human fibroblasts: evidence for an LDL receptor-independent internalization pathway. J Lipid Res 1996; 37: 464–81.
Anderson R . The caveolae membrane system. Annu Rev Biochem 1998; 67: 199–225.
Hu B, Li D, Sawamura T, Mehta J . Oxidized LDL through LOX-1 modulates LDL-receptor expression in human coronary artery endothelial cells. Biochem Biophys Res Commun 2003; 307: 1008–12.
Frank PG, Woodman SE, Park DS, Lisanti MP . Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 2003; 23: 1161–8.
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Sciences Foundation of China (No 30971170) and the National Major Basic Research Program of China (973 Program) (No 2006CB503808).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, Sw., Zu, Xy., Tuo, Qh. et al. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol Sin 31, 1336–1342 (2010). https://doi.org/10.1038/aps.2010.87
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.87
Keywords
This article is cited by
-
Caveolin-1 is a primary determinant of endothelial stiffening associated with dyslipidemia, disturbed flow, and ageing
Scientific Reports (2022)
-
Bile salt–dependent lipase promotes the barrier integrity of Caco-2 cells by activating Wnt/β-catenin signaling via LRP6 receptor
Cell and Tissue Research (2021)
-
Regulation of blood vascular permeability in the skin
Inflammation and Regeneration (2017)
-
Ezetimibe suppresses cholesterol accumulation in lipid-loaded vascular smooth muscle cells in vitro via MAPK signaling
Acta Pharmacologica Sinica (2014)
-
Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway
Genes & Nutrition (2013)